Back to Journals » Drug Design, Development and Therapy » Volume 13
Design, synthesis, and biological study of 4-[(2-nitroimidazole-1H-alkyloxyl)aniline]-quinazolines as EGFR inhibitors exerting cytotoxicities both under normoxia and hypoxia
Authors Cheng W, Wang S, Yang Z, Tian X, Hu Y
Received 20 March 2019
Accepted for publication 22 July 2019
Published 28 August 2019 Volume 2019:13 Pages 3079—3089
DOI https://doi.org/10.2147/DDDT.S209481
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Prof. Dr. Tin Wui Wong
Weiyan Cheng,1,2,* Suhua Wang,1,2,* Zhiheng Yang,1,2 Xin Tian,1,2 Yongzhou Hu3
1Department of Pharmacy, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, People’s Republic of China; 2Henan Key Laboratory of Precision Clinical Pharmacy, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, China; 3Zhejiang Province Key Laboratory of Anti-Cancer Drug Research, College of Pharmaceutical Sciences, Zhejiang University, Hangzhou 310058, People’s Republic of China
Correspondence: Xin Tian
Department of Pharmacy, The First Affiliated Hospital of Zhengzhou University, 1 Jianshedong Road, Zhengzhou 450052, People’s Republic of China
Email [email protected]
Yongzhou Hu
Zhejiang Province Key Laboratory of Anti-Cancer Drug Research, College of Pharmaceutical Sciences, Zhejiang University, 388 Yuhangtang Road, Hangzhou 310058, People’s Republic of China
Email [email protected]
*These authors contributed equally to this work
Purpose: In order to get novel EGFR inhibitors exerting more potency in tumor hypoxia than in normoxia.
Methods: A series of 4-[(2-nitroimidazole-1H-alkyloxyl)aniline]-quinazolines were designed and synthesized, and their in vitro cytotoxicity and EGFR inhibitory activity were evaluated. Molecule docking study was performed for the representative compound.
Results: The structure-activity relationship (SAR) studies revealed that compounds bearing both meta-chloride and para-(2-nitroimidazole-1H-alkyloxy) groups on the aniline displayed potent inhibitory activities both in enzymatic and cellular levels. The most promising compound 16i potently inhibited EGFR with an IC50 value of 0.12 μM. Meanwhile, it manifested more potent cytotoxicity than the positive control lapatinib under tumor normoxia and hypoxia conditions (IC50 values of 1.59 and 1.09 μM against A549 cells, 2.46 and 1.35 μM against HT-29 cells, respectively). The proposed binding model of 16i in complex with EGFR was displayed by the docking results.
Conclusion: This study provides insights for developing hypoxia-activated kinase inhibitors.
Keywords: EGFR inhibitor, hypoxia, tumor, 4-anilinoquinazoline, 2-nitroimidazole
Introduction
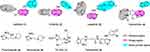
EGFR is a trans-membrane protein that belongs to the HER family of receptor tyrosine kinases,1 its overexpression has been confirmed in many solid carcinomas, which include non-small-cell lung carcinoma,2 colon carcinoma,3 ovarian carcinoma,4 head and neck carcinoma,5 and so on. On the other hand, hypoxia is unavoidable in solid carcinomas, it makes carcinomas resistant to radiotherapy and chemotherapy.6 In particular, EGFR is massively overexpressed when carcinomas are under hypoxia.7,8 The dual functions of EGFR overexpression and tumor hypoxia exhibit a great challenge to carcinoma treatment. In other words, if a drug can inhibit EGFR, and also be activated under carcinoma hypoxia, it may exert a stronger effect. Recently, many small molecule EGFR inhibitors have been successfully developed and introduced onto the market, these drugs include gefitinib (1),9 erlotinib (2),10 lapatinib (3),11 osimertinib (4),12 etc (Figure 1). In addition, reagents targeting carcinoma-hypoxia have been massively explored, including the 2-nitroimidazole derivative pimonidazole (5),13 nimorazole (6),14 and so on (Figure 1). Moreover, compounds that have been combined with hypoxia-activated groups and other functional moieties have been widely reported.15,16,17 For example, the hypoxia-activated prodrug TH-302 (7, Figure 1) is 270-fold more effective in carcinoma hypoxia than in normoxia;18 the hypoxia-activated EGFR inhibitor tarloxotinib (8, Figure 1) has been advanced to a phase II clinical study due to its excellent potency.19
EGFR inhibitors compete with ATP to bind to kinase, blocking signal transduction and playing an inhibitory role.20 Three main interaction regions, the solvent region, the hinge region, and the backpocket region, are involved in the inhibitor-EGFR kinase bindings (Figure 1).21 In carcinoma hypoxic environment, 2-nitroimidazole can be reduced to hydroxylamine intermediates through four-electron reduction. The intermediates are further reduced to free radicals under hypoxia, and covalently bind to nucleophilic proteins, amino acids, and other components to target tumors. In normoxia, the presence of oxygen prevents the 2-nitroimidazole group from being reduced and activated, and it exists in the prototype state, thus ensuring its safety in normal tissue (Figure 2).22
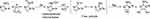 |
Figure 2 Reductive activation mechanism of 2-nitroimidazole in carcinoma hypoxic environment. |
In order to get novel EGFR inhibitors that exert more potency in tumor hypoxia than in normoxia, we designed a series of compounds by combining 2-nitroimidazole group and 4-anilinoquinazolines (Figure 3). It has been confirmed that the aniline group of 4-anilinoquinazoline was positioned in the backpocket region of EGFR kinase, and this space was enough to accommodate bulky moieties,23 so we incorporated 2-nitroimidazole group into the aniline position herein (Figure 3). Meanwhile, different kinds of side chains at C-7 and C-8 of the quinazolines were chosen, referencing the structures of gefitinib and erlotinib. Through investigation of the aniline substituents and the styles of the side chain, the structure-activity relationships (SARs) of these compounds were studied.
 |
Figure 3 Our design strategy for EGFR inhibitors. |
Methods and materials
Chemistry
Melting points were determined by a B-540 Büchi melting-point apparatus, nuclear magnetic resonance (NMR) spectra were recorded on BRUKER AVIII 500 MHz or BRUKER AVII 400 MHz spectrometer (500 or 400 MHz for 1H NMR, 100 MHz for 13C NMR). Mass spectra were obtained on the Finnigan LCQ DecaXP ion trap mass spectrometer.
Procedure for the synthesis of substituted nitrobenzene (10a-d)
Dihalide alkane (30 mmol) was added to a stirred solution of substituted phenol (9a-c) (10 mmol) and K2CO3 (5 mmol) in DMF (10 mL). The reaction mixture was heated to 60°C and stirred overnight. After the reaction was completed, the mixture was cooled to room temperature (rt) and filtered, the filtrate was removed in vacuo. Finally, silica gel column chromatography was used to purify the residue, and pure 10a-d were obtained.
1-(2-Bromoethoxy)-2-chloro-4-nitrobenzene (10a): white solid. Yield: 64%, mp: 56°C–58°C. 1H NMR (500 MHz, CDCl3) δ 8.33–8.30 (m, 1H), 8.16 (dd, J =9.0, 2.5 Hz, 1H), 6.99 (d, J =9.0 Hz, 1H), 4.45 (t, J =6.5 Hz, 2H), 3.73 (t, J =6.5 Hz, 2H). ESI-MS m/z: 280 [M+1]+.
1-(3-Bromopropoxy)-2-chloro-4-nitrobenzene (10b): white solid. Yield: 71%, mp: 52°C–54°C. 1H NMR (500 MHz, CDCl3) δ 8.30 (d, J =2.5 Hz, 1H), 8.17 (dd, J =9.0, 2.5 Hz, 1H), 7.02 (d, J =9.0 Hz, 1H), 4.30 (t, J =5.5 Hz, 2H), 3.67 (t, J =6.0 Hz, 2H), 2.46–2.39 (m, 2H). ESI-MS m/z: 296 [M+1]+.
1-(2-Chloroethoxy)-3-nitrobenzene (10c): white solid. Yield: 59%, mp: 57°C–59°C (lit. 59°C–60°C).24
1-(2-Bromoethoxy)-4-nitrobenzene (10d): white solid. Yield: 51%, mp: 59°C–61°C (lit. 62°C–64°C).25
Procedure for the synthesis of substituted aniline (11a-d)
To a solution of 10a-d (10 mmol) in methanol (20 mL), and 10% Pd/C was added. Then 10a-d were reduced by hydrogen at atmospheric pressure for about 2 hours and the catalyst was removed by filtration. The filtrate was evaporated to afford 11a-d in the yields >93%.
4-(2-Bromoethoxy)-3-chloroaniline (11a): brown solid. Yield: 95%, mp: 68°C–70°C. 1H NMR (500 MHz, CDCl3) δ 6.83 (d, J =8.5 Hz, 1H), 6.73 (d, J =3.0 Hz, 1H), 6.52 (dd, J =8.5, 3.0 Hz, 1H), 4.24 (t, J =6.5 Hz, 2H), 3.62 (t, J =6.5 Hz, 2H), 3.53 (br, 2H). ESI-MS m/z: 252 [M+1]+.
4-(3-Bromopropoxy)-3-chloroaniline (11b): brown solid. Yield: 93%, mp: 62°C–64°C. 1H NMR (500 MHz, CDCl3) δ 6.80 (d, J =8.5 Hz, 1H), 6.74 (d, J =3.0 Hz, 1H), 6.54 (dd, J =8.5, 3.0 Hz, 1H), 4.07 (t, J =5.5 Hz, 2H), 3.65 (t, J =6.5 Hz, 2H), 3.25 (br, 2H), 2.34–2.28 (m, 2H). ESI-MS m/z: 266 [M+1]+.
3-(2-Chloroethoxy)-aniline (11c): colorless oil. Yield: 96%. 1H NMR (500 MHz, CDCl3) δ 7.07 (t, J =8.0 Hz, 1H), 6.38–6.32 (m, 2H), 6.30 (t, J =2.5 Hz, 1H), 4.19 (t, J =6.0 Hz, 2H), 3.79 (t, J =6.0 Hz, 2H), 3.58 (br, 2H). ESI-MS m/z: 172 [M+1]+.
4-(2-Bromoethoxy)-aniline (11d): white solid. Yield: 100%, mp: 89°C–91°C. 1H NMR (500 MHz, CDCl3) δ 6.79–6.75 (m, 2H), 6.67–6.61 (m, 2H), 4.21 (t, J =6.5 Hz, 2H), 3.59 (t, J =6.5 Hz, 2H), 3.44 (br, 2H). ESI-MS m/z: 216 [M+1]+.
Procedure for the synthesis of 2-nitroimidazole derivative (12a-d)
To a stirred solution of substituted aniline (11a-d) (10 mmol) and powdered K2CO3 (5 mmol) in DMF (10 mL), 2-nitroimidazole (1.36g, 12 mmol) was added. The reaction mixture was heated to 70°C and stirred for about 10 hours. After the reaction was completed, the mixture was cooled to rt and filtered, the filtrate was removed in vacuo. Then methylene chloride was used to dissolve the obtained residue, which was further washed with water. Finally, the organic solvent was dried over sodium sulfate and concentrated to obtain crude 12a-d, which were further purified by column chromatography to get pure materials.
3-Chloro-4-(2-(2-nitro-1H-imidazol-1-yl)ethoxy)aniline (12a): yellow solid. Yield: 55%, mp: 127°C–129°C. 1H NMR (500 MHz, CDCl3) δ 7.38 (d, J =1.0 Hz, 1H), 7.17 (d, J =1.0 Hz, 1H), 6.70 (d, J =3.0 Hz, 1H), 6.67 (d, J =8.5 Hz, 1H), 6.50 (dd, J =8.5, 3.0 Hz, 1H), 4.85–4.80 (m, 2H), 4.31–4.26 (m, 2H), 2.75 (br, 2H). ESI-MS m/z: 283 [M+1]+.
3-Chloro-4-(3-(2-nitro-1H-imidazol-1-yl)propoxy)aniline (12b): yellow solid. Yield: 53%, mp: 136°C–138°C. 1H NMR (500 MHz, CDCl3) δ 7.17 (s, 1H), 7.11 (s, 1H), 6.76 (d, J =2.5 Hz, 1H), 6.72 (d, J =8.5 Hz, 1H), 6.53 (dd, J =8.5, 3.0 Hz, 1H), 4.71 (t, J =6.5 Hz, 2H), 3.89 (t, J =5.5 Hz, 2H), 3.53 (br, 2H), 2.36–2.30 (m, 2H). ESI-MS m/z: 297 [M+1]+.
3-(2-(2-Nitro-1H-imidazol-1-yl)ethoxy)aniline (12c): brown solid. Yield: 58%, mp: 51°C–53°C. 1H NMR (500 MHz, CDCl3) δ 7.23 (d, J =0.5 Hz, 1H), 7.13 (d, J =1.0 Hz, 1H), 7.02 (t, J =8.0 Hz, 1H), 6.30 (dd, J =8.0, 2.0 Hz, 1H), 6.22 (dd, J =8.0, 2.5 Hz, 1H), 6.15 (t, J =2.0 Hz, 1H), 4.77 (dd, J =12.5, 8.0 Hz, 2H), 4.27 (dd, J =10.5, 5.5 Hz, 2H), 4.04–3.10 (br, 2H). ESI-MS m/z: 249 [M+1]+.
4-(2-(2-Nitro-1H-imidazol-1-yl)ethoxy)aniline (12d): brown solid. Yield: 48%, mp: 48°C–50°C. 1H NMR (500 MHz, CDCl3) δ 7.24 (d, J =1.0 Hz, 1H), 7.14 (t, J =3.0 Hz, 1H), 6.69–6.62 (m, 2H), 6.62–6.57 (m, 2H), 4.77 (dd, J =14.5, 10.0 Hz, 2H), 4.25 (dd, J =13.5, 8.5 Hz, 2H), 3.47 (br, 2H). ESI-MS m/z: 249 [M+1]+.
Procedure for the synthesis of 4-(3-((4-chloro-6-methoxyquinazolin-7-yl)oxy)propyl)morpholine (13)
Compound 13 was prepared following the published synthesis route.26 Briefly, commercially available 6-methoxy-7-(3-morpholinopropoxy)quinazolin-4(3H)-one (2.0 g, 6.3 mmol) was mixed with SOCl2 (15 mL) and DMF (0.2 mL), then the mixture was heated at reflux temperature for 5 hours. The volatiles were removed under reduced pressure. The residue was dissolved in CH2Cl2 (50 mL) and the organic layer was washed with aqueous NaHCO3 solution and brine, and dried over Na2SO4, filtered and evaporated to give the crude product 13, which was purified by silica gel column chromatography to get pure intermediate 13 as a white solid. Yield: 48%, mp: 111°C–113°C. 1H NMR (CDCl3, 500 MHz) δ 8.86 (s, 1H), 7.39 (s, 1H), 7.35 (s, 1H), 4.30 (t, 2H, J =6.4 Hz), 4.05 (s, 3H), 3.85 (m, 4H), 2.33–2.94 (m, 6H), 2.20 (m, 2H). ESI-MS m/z: 338 [M+1]+.
Procedure for the synthesis of 4-chloro-6,7-bis(2-methoxyethoxy)quinazoline (14)27
Compound 14 was prepared in the same way as compound 13, but the was changed from 6-methoxy-7-(3-morpholinopropoxy)quinazolin-4(3H)-one to 6,7-bis(2-methoxyethoxy)quinazolin-4(3H)-one. White solid. Yield: 52%, mp: 105°C–107°C. 1H NMR (400 MHz, CDCl3) δ 8.78 (s, 1H), 7.35 (s, 1H), 7.25 (s, 1H), 4.30–4.23 (m, 4H), 3.91–3.74 (m, 4H), 3.43 (s, 3H), 3.42 (s, 3H). ESI-MS m/z: 313 [M+1]+.
Procedure for the synthesis of 4-(3-((4-chloro-7-methoxyquinazolin-6-yl)oxy)propyl)morpholine (15)28
Compound 15 was prepared in the same way as compound 13, but the start material was changed from 6-methoxy-7-(3-morpholinopropoxy)quinazolin-4(3H)-one to 7-methoxy-6-(3-morpholinopropoxy)quinazolin-4(3H)-one. White solid. Yield: 63%, mp: 119°C–121°C. 1H NMR (400 MHz, CDCl3) δ 8.78 (s, 1H), 7.31 (s, 1H), 7.25 (s, 1H), 4.30–4.14 (m, 2H), 3.98 (s, 3H), 3.75–3.61 (m, 4H), 2.62–2.48 (m, 2H), 3.47–2.30 (m, 4H), 2.12–2.03 (m, 2H). ESI-MS m/z: 338 [M+1]+.
Procedure for the synthesis of the target compounds (16a-l)
To a stirred solution of 2-nitroimidazole derivative (12a-d) (1.0 mmol) in isopropyl alcohol (10 mL), compound 13, 14 or 15 (1.0 mmol) was added. The mixture was reacted in reflux temperature and stirred for about 2 hours. After completion, the mixture was washed with saturated NaHCO3 solution and CH2Cl2, then the CH2Cl2 solvent was dried by anhydrous Na2SO4, which was concentrated to obtain crude products 16a-l. They were further purified by column chromatography to get pure materials.
N-(3-Chloro-4-(2-(2-nitro-1H-imidazol-1-yl)ethoxy)phenyl)-6-methoxy-7-(3-morpholinopropoxy)quinazolin-4-amine (16a): yellow solid. Yield: 83%, mp: 203°C–205°C. 1H NMR (500 MHz, DMSO-d6) δ 9.41 (s, 1H), 8.42 (s, 1H), 7.91 (d, J =2.5 Hz, 1H), 7.77 (s, 1H), 7.70 (s, 1H), 7.67 (dd, J =9.0, 2.5 Hz, 1H), 7.20 (s, 1H), 7.16 (d, J =10.0 Hz, 2H), 4.87 (t, J =5.0 Hz, 2H), 4.43 (t, J =5.0 Hz, 2H), 4.16 (t, J =6.5 Hz, 2H), 3.93 (s, 3H), 3.61–3.54 (m, 4H), 2.47–2.30 (m, 6H), 1.98–1.91 (m, 2H). 13C NMR (100 MHz, DMSO-d6) δ 156.6, 154.1, 153.3, 149.6, 149.5, 147.3, 145.4, 134.2, 128.8, 128.1, 124.2, 122.5, 121.2, 114.4, 109.1, 108.3, 102.4, 68.0, 67.2, 66.7 (2), 56.7, 55.2, 53.8 (2), 49.0, 26.1. ESI-MS m/z: 584 [M+1]+.
N-(3-Chloro-4-(3-(2-nitro-1H-imidazol-1-yl)propoxy)phenyl)-6-methoxy-7-(3-morpholinopropoxy)quinazolin-4-amine (16b): yellow solid. Yield: 81%, mp: 92°C–94°C. 1H NMR (500 MHz, DMSO-d6) δ 9.42 (s, 1H), 8.43 (s, 1H), 7.92 (d, J =2.5 Hz, 1H), 7.78 (s, 1H), 7.67 (dd, J =9.0, 2.5 Hz, 1H), 7.65 (d, J =1.0 Hz, 1H), 7.19–7.14 (m, 3H), 4.60 (t, J =7.0 Hz, 2H), 4.16 (t, J =6.5 Hz, 2H), 4.09 (t, J =6.0 Hz, 2H), 3.94 (s, 3H), 3.57 (t, J =4.5 Hz, 4H), 2.44 (t, J =7.0 Hz, 2H), 2.40–2.34 (m, 4H), 2.33–2.26 (m, 2H), 2.00–1.89 (m, 2H). 13C NMR (100 MHz, DMSO-d6) δ 156.7, 154.1, 153.3, 150.1, 149.5, 147.4, 145.2, 134.0, 128.4, 128.2, 124.3, 122.6, 121.4, 114.5, 109.1, 108.4, 102.5, 67.2, 66.7 (2), 66.6, 56.7, 55.2, 53.8 (2), 47.3, 29.8, 26.1. ESI-MS m/z: 598 [M+1]+.
6-Methoxy-7-(3-morpholinopropoxy)-N-(3-(2-(2-nitro-1H-imidazol-1-yl)ethoxy)phenyl)quinazolin-4-amine (16c): yellow solid. Yield: 84%, mp: 149°C–151°C. 1H NMR (500 MHz, DMSO-d6) δ 9.38 (s, 1H), 8.44 (s, 1H), 7.80 (s, 1H), 7.71 (d, J =1.0 Hz, 1H), 7.43 (dd, J =8.0, 1.5 Hz, 1H), 7.39 (t, J =2.0 Hz, 1H), 7.26 (t, J =8.0 Hz, 1H), 7.18 (d, J =1.0 Hz, 1H), 7.16 (s, 1H), 6.65 (dd, J =8.0, 2.0 Hz, 1H), 4.82 (t, J =5.0 Hz, 2H), 4.36 (t, J =5.0 Hz, 2H), 4.17 (t, J =6.5 Hz, 2H), 3.94 (s, 3H), 3.57 (t, J =4.5 Hz, 4H), 2.44 (t, J =7.0 Hz, 2H), 2.41–2.32 (m, 4H), 1.99–1.90 (m, 2H). 13C NMR (100 MHz, DMSO-d6) δ 158.4, 156.7, 154.1, 153.2, 149.5, 147.5, 145.4, 141.3, 129.7, 128.6, 128.1, 115.5, 109.4, 109.3, 109.2, 108.3, 102.5, 67.2, 66.7, 66.7 (2), 56.8, 55.2, 53.8 (2), 49.1, 26.1. ESI-MS m/z: 550 [M+1]+.
6-Methoxy-7-(3-morpholinopropoxy)-N-(4-(2-(2-nitro-1H-imidazol-1-yl)ethoxy)phenyl)quinazolin-4-amine (16d): yellow solid. Yield: 79%, mp: 152°C–154°C. 1H NMR (500 MHz, DMSO-d6) δ 9.36 (s, 1H), 8.35 (s, 1H), 7.79 (s, 1H), 7.71 (d, J =1.0 Hz, 1H), 7.64–7.57 (m, 2H), 7.19 (d, J =1.0 Hz, 1H), 7.13 (s, 1H), 6.94–6.87 (m, 2H), 4.81 (t, J =5.0 Hz, 2H), 4.35 (t, J =5.0 Hz, 2H), 4.15 (t, J =6.5 Hz, 2H), 3.92 (s, 3H), 3.57 (t, J =4.0 Hz, 4H), 2.46–2.41 (m, 2H), 2.41–2.33 (m, 4H), 2.01–1.85 (m, 2H). 13C NMR (100 MHz, DMSO-d6) δ 157.0, 154.6, 153.9, 153.5, 149.3, 147.2, 145.5, 133.4, 128.6, 128.1, 124.7 (2), 114.8 (2), 109.1, 108.3, 102.6, 67.2, 67.0, 66.7 (2), 56.7, 55.2, 53.8 (2), 49.1, 26.1. ESI-MS m/z: 550 [M+1]+.
N-(3-Chloro-4-(2-(2-nitro-1H-imidazol-1-yl)ethoxy)phenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine (16e): yellow solid. Yield: 84%, mp: 92°C–94°C. 1H NMR (500 MHz, DMSO-d6) δ 9.39 (s, 1H), 8.42 (s, 1H), 7.90 (s, 1H), 7.80 (s, 1H), 7.70 (s, 1H), 7.66 (dd, J =9.0, 2.0 Hz, 1H), 7.20–7.16 (m, 3H), 4.87 (t, J =4.5 Hz, 2H), 4.43 (t, J =4.5 Hz, 2H), 4.28–4.23 (m, 4H), 3.79–3.69 (m, 4H), 3.35(s, 3H), 3.33(s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 156.7, 154.1, 153.3, 149.7, 148.6, 147.3, 145.4, 134.2, 128.8, 128.1, 124.2, 122.5, 121.3, 114.5, 109.2, 108.8, 103.8, 70.6, 70.6, 68.9, 68.5, 68.0, 58.9, 58.8, 49.0. ESI-MS m/z: 559 [M+1]+.
N-(3-Chloro-4-(3-(2-nitro-1H-imidazol-1-yl)propoxy)phenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine (16f): yellow solid. Yield: 89%, mp: 90°C–92°C. 1H NMR (500 MHz, DMSO-d6) δ 9.41 (br, 1H), 8.43 (s, 1H), 7.91 (d, J =2.5 Hz, 1H), 7.82 (s, 1H), 7.69–7.63 (m, 2H), 7.19 (s, 1H), 7.18 (d, J =1.0 Hz, 1H), 7.16 (d, J =9.0 Hz, 1H), 4.60 (t, J =7.0 Hz, 2H), 4.26 (t, J =9.0 Hz, 4H), 4.09 (t, J =6.0 Hz, 2H), 3.76 (t, J =9.0 Hz, 2H), 3.73 (t, J =9.0 Hz, 2H), 3.35 (s, 3H), 3.33 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 156.7, 154.1, 153.4, 150.2, 148.6, 147.3, 145.1, 133.9, 128.4, 128.2, 124.3, 122.6, 121.4, 114.5, 109.3, 108.8, 103.9, 70.6, 70.5, 68.9, 68.5, 66.6, 58.9, 58.8, 47.3, 29.8. ESI-MS m/z: 573 [M+1]+.
6,7-Bis(2-methoxyethoxy)-N-(3-(2-(2-nitro-1H-imidazol-1-yl)ethoxy)phenyl)quinazolin-4-amine (16g): yellow solid. Yield: 81%, mp: 183°C–185°C. 1H NMR (400 MHz, DMSO-d6) δ 9.39 (s, 1H), 8.46 (s, 1H), 7.86 (s, 1H), 7.73 (d, J =1.2 Hz, 1H), 7.44 (dd, J =8.0, 1.2 Hz, 1H), 7.41 (t, J =2.0 Hz, 1H), 7.27 (t, J =8.0 Hz, 1H), 7.22 (s, 1H), 7.20 (d, J =1.2 Hz, 1H), 6.66 (dd, J =8.0, 2.0 Hz, 1H), 4.84 (t, J =5.2 Hz, 2H), 4.38 (t, J =5.2 Hz, 2H), 4.32–4.24 (m, 4H), 3.81–3.72 (m, 4H), 3.37 (s, 3H), 3.35 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 158.2, 156.3, 154.7, 153.3, 149.0, 147.1, 140.1, 129.9 (2), 128.3, 127.2, 114.8, 110.0, 109.1, 108.5, 108.1, 102.7, 71.0, 70.5, 69.3, 68.4, 66.2, 59.3, 59.3, 49.4. ESI-MS m/z: 525 [M+1]+.
6,7-Bis(2-methoxyethoxy)-N-(4-(2-(2-nitro-1H-imidazol-1-yl)ethoxy)phenyl)quinazolin-4-amine (16h): white solid. Yield: 83%, mp: 196°C–198°C. 1H NMR (500 MHz, DMSO-d6) δ 9.34 (s, 1H), 8.35 (s, 1H), 7.82 (s, 1H), 7.71 (d, J =1.0 Hz, 1H), 7.62–7.58 (m, 2H), 7.19 (d, J =1.0 Hz, 1H), 7.16 (s, 1H), 6.94–6.88 (m, 2H), 4.81 (t, J =5.0 Hz, 2H), 4.35 (t, J =5.0 Hz, 2H), 4.29–4.21 (m, 4H), 3.78–3.74 (m, 2H), 3.74–3.69 (m, 2H), 3.35 (s, 3H), 3.33 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 157.0, 154.6, 154.0, 153.5, 148.4, 147.2, 145.5, 133.3, 128.6, 128.1, 124.7 (2), 114.8 (2), 109.2, 108.7, 104.0, 70.6, 70.6, 68.9, 68.5, 67.0, 58.9, 58.8, 49.1. ESI-MS m/z: 525 [M+1]+.
N-(3-Chloro-4-(2-(2-nitro-1H-imidazol-1-yl)ethoxy)phenyl)-7-methoxy-6-(3-morpholinopropoxy)quinazolin-4-amine (16i): yellow solid. Yield: 65%, mp: 132°C–134°C. 1H NMR (500 MHz, DMSO-d6) δ 9.56 (s, 1H), 8.46 (s, 1H), 7.96 (d, J =2.5 Hz, 1H), 7.91 (s, 1H), 7.77–7.69 (m, 2H), 7.27–7.13 (m, 3H), 4.90 (t, J =5.0 Hz, 2H), 4.45 (t, J =5.0 Hz, 2H), 4.23 (t, J =5.5 Hz, 2H), 3.94 (s, 3H), 3.88–3.60 (m, 4H), 3.44–3.22 (m, 6H), 2.24–2.04 (m, 2H). 13C NMR (100 MHz, DMSO-d6) δ 156.8, 154.9, 153.2, 149.7, 148.4, 147.0, 145.4, 134.2, 128.8, 128.1, 124.3, 122.6, 121.2, 114.4, 109.2, 107.6, 104.0, 68.0, 67.4, 64.7, 56.4 (2), 54.6, 52.3, 49.0 (2), 24.4. ESI-MS m/z: 584 [M+1]+.
N-(3-Chloro-4-(3-(2-nitro-1H-imidazol-1-yl)propoxy)phenyl)-7-methoxy-6-(3-morpholinopropoxy)quinazolin-4-amine (16j): yellow solid. Yield: 59%, mp: 107°C–109°C. 1H NMR (500 MHz, CDCl3) δ 8.63 (s, 1H), 7.90 (d, J =2.5 Hz, 1H), 7.70 (br, 1H), 7.57 (dd, J =9.0, 2.5 Hz, 1H), 7.35 (s, 1H), 7.25 (s, 1H), 7.18 (s, 1H), 7.13 (d, J =0.5 Hz, 1H), 6.91 (d, J =9.0 Hz, 1H), 4.74 (t, J =6.5 Hz, 2H), 4.26 (t, J =6.5 Hz, 2H), 4.04–3.96 (m, 5H), 3.83 (t, J =4.5 Hz, 4H), 2.74 (t, J =7.0 Hz, 2H), 2.70–2.61 (m, 4H), 2.44–2.36 (m, 2H), 2.24–2.16 (m, 2H). 13C NMR (100 MHz, DMSO-d6) δ 156.7, 154.9, 153.3, 150.2, 148.7, 147.3, 145.4, 133.9, 128.4, 128.2, 124.4, 122.7, 121.4, 114.4, 109.2, 107.8, 103.2, 67.6, 66.6 (2), 66.5, 56.3, 55.4, 53.9 (2), 47.3, 29.8, 26.3. ESI-MS m/z: 598 [M+1]+.
7-Methoxy-6-(3-morpholinopropoxy)-N-(3-(2-(2-nitro-1H-imidazol-1-yl)ethoxy)phenyl)quinazolin-4-amine (16k): yellow solid. Yield: 58%, mp: 60°C–62°C. 1H NMR (400 MHz, CDCl3,): 8.61 (s, 1H, CH), 7.82 (br, 1H), 7.49 (t, J =2.0 Hz, 1H), 7.35–7.27 (m, 4H), 7.25 (s, 1H), 7.15 (d, J =1.2 Hz, 1H), 4.84 (t, J =9.6 Hz, 2H), 4.39 (t, J =9.6 Hz, 2H), 4.24 (t, J =6.8 Hz, 2H), 3.99 (s, 3H), 3.79 (t, J =9.2 Hz, 4H), 2.69 (t, J =7.2 Hz, 2H), 2.62 (t, J =9.2 Hz, 4H), 2.17–2.15 (m, 2H). 13C NMR (100 MHz, DMSO-d6) δ 158.4, 156.7, 154.9, 153.2, 148.7, 147.5, 145.5, 141.2, 129.7, 128.6, 128.1, 115.6, 109.4 (2), 109.3, 107.8, 103.3, 67.7, 66.7, 66.6 (2), 56.3, 55.4, 53.9 (2), 49.1, 26.3. ESI-MS m/z: 550 [M+1]+.
7-Methoxy-6-(3-morpholinopropoxy)-N-(4-(2-(2-nitro-1H-imidazol-1-yl)ethoxy)phenyl)quinazolin-4-amine (16l): yellow solid. Yield: 55%, mp: 212°C–214°C. 1H NMR (400 MHz, CDCl3) δ 8.58 (s, 1H), 7.61–7.56 (m, 2H), 7.40 (s, 1H), 7.27 (d, J =1.2 Hz, 2H), 7.24 (s, 1H), 7.22 (s, 1H), 7.17 (d, J =1.2 Hz, 1H), 6.93–6.84 (m, 2H), 4.87–4.82 (m, 2H), 4.44–4.31 (m, 2H), 4.23 (t, J =6.8 Hz, 2H), 4.00 (s, 3H), 3.79–3.75 (m, 4H), 2.65 (t, J =5.6 Hz, 2H), 2.60–2.54 (m, 4H), 2.19–2.10 (m, 2H). 13C NMR (100 MHz, DMSO-d6) δ 156.6, 154.3, 154.2, 153.0, 148.1, 146.8, 145.0, 132.9, 128.2, 127.7, 124.4 (2), 114.37 (2), 108.8, 107.3, 102.8, 67.2, 66.5, 66.2 (2), 55.8, 55.0, 53.5 (2), 48.7, 25.9. ESI-MS m/z: 550 [M+1]+.
In vitro enzymatic activity assay
Activity of kinases was determined using Z’-Lyte Kinase Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the instructions.29 The compounds were first tested the inhibition percentage at the concentration of 1.0×10−6 mol/L. If the inhibition percentage was higher than 50%, these compounds were further chosen to test their IC50 values. For the IC50 values, ten concentration gradients from 5.1×10−11 to 1.0×10−6 mol/L were set for the tested compounds. The experiments were performed according to the instructions of the manufacturer.
Cytotoxicity assay
The cytotoxicity assay was performed as described previously.16,17 Two human cancer cell lines (A549 and HT-29) were purchased from Cell Bank of China Science Academy (Shanghai, People's Republic of China). The cells were cultured in RPMI-1640 (Thermo Fisher Scientific) medium with heat-inactivated 10% FBS, penicillin (100 units/mL), and streptomycin (100 mg/mL) and incubated at 37°C. The cytotoxic activity in vitro was measured using sulforhodamine B (SRB) assay. All the compounds were dissolved in DMSO at the concentration 10.0 mg/mL and were then diluted to the appropriate concentrations. Cells were plated in 96-well plates (5×103 per well) for 24 hours and subsequently treated with different concentrations of all tested compounds under normoxia or hypoxia for 72 hours, respectively. Cells were then washed with PBS and fixed with 10% (w/v) trichloroacetic acid at 4°C for 1 hour. After washing, the cells were stained for 30 minutes with 0.4% SRB dissolved in 1% acetic acid. Then the cells were washed with 1% acetic acid five times, and protein-bound dye was extracted with 10 mmol unbuffered Tris base. The absorbance was measured at 515 nm using a multiscan spectrum (Thermo Electron Co., Vantaa, Finland). The inhibition rate on cell proliferation of each well was calculated as (A515 control cells - A515 treated cells)/A515 control cells ×100%, and the IC50 values were determined by Logit method.
Molecular modeling
According to the docking mode of compound 16i with EGFR, the co-crystal structure of EGFR/lapatinib was chosen as the template (PDB ID: 1XKK). The docking procedure was carried out by using C-DOCKER protocol within Discovery Studio 2.1. The detailed process has been described previously, and the pictures were prepared using Pymol.16,17
Results and discussion
Synthesis
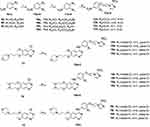
Synthesis of the target compounds 16a-l followed the general pathway shown in Scheme 1. The start materials 9a-c reacted with α, ω-dihalide alkane in the presence of potassium carbonate to afford 10a-d, then they were reduced with hydrogen to generate 11a-d. Coupling 11a-d with 2-nitroimidazole in the presence of potassium carbonate to obtain intermediates 12a-d. In addition, compounds 13, 14, and 15 were prepared following the published synthesis route.26,27,28 Finally, condensing 12a-d with 13 in isopropanol to furnish the target compounds 16a-l. Compounds 16e-h and 16i-l were obtained in a similar way by condensing 12a-d with compound 14 and 15, respectively.
Enzymatic activity
The inhibitory activity of compound 16a-l against EGFR was summarized in Table 1. Half of the tested compounds (16a, 16b, 16e, 16f, 16i, and 16j) manifested potent activities, with the IC50 values ranging from 0.12–0.40 μM. As expected, the meta-chloride substituent on aniline was crucial to the EGFR inhibitory activity. For example, compounds 16d, 16h, and 16l, bearing no chloride group on the aniline, only exhibited the inhibited rate lower than 50% at 1.0 μM. While meta-chloride-bearing compounds 16a, 16e, and 16i displayed the IC50 values of 0.19, 0.23, and 0.12 μM, respectively. The bindings of lapatinib/EGFR indicate that the chloride atom of lapatinib takes up a small cavity comprised by Leu788, Thr790, and Ala743,23 it seems that the chloride atom of this series exerts similar effects. The position of linker impacts activities as well, compounds with 2-nitroimidazole-1H-alkoxy moieties at the meta- position exhibited decreased enzymatic inhibitory activities compared to those at para- positions (16c vs 16d, 16g vs 16h, and 16k vs 16l, inhibition% at 1.0 μM: 24% vs 42%, 28% vs 44%, and 30% vs 45%, respectively). The length of the alkyloxyl linker between aniline and 2-nitroimidazole also had obvious impact on the enzymatic inhibitory activities. The compromised EGFR inhibitory activities of compounds 16b, 16f, and 16j (IC50: 0.33, 0.40, and 0.18 μM, respectively) compared with 16a, 16e, and 16i (IC50: 0.19, 0.23, and 0.12 μM, respectively) indicated that shorter linkers (n=1) were more favorable than longer ones (n=2), probably because longer linkers restricted the molecules from adapting the kinase. Moreover, the types and positions of groups adjusting physicochemical properties affected activities as well. Compounds with 6-morpholinopropoxyl-7-methoxy side chains (16i-l) exhibited more potent EGFR inhibitory activities than those with 6, 7-dimethoxylethoxy (16e-h) or 6-methoxyl-7-morpholinopropoxy (16a-d) ones.
 |
Table 1 EGFR inhibitory activity and cytotoxicity of compounds 16a-l |
In vitro cytotoxicity
The in vitro cytotoxicities of compounds 16a-l were evaluated on human non-small-cell lung cancer A549 cells and human colorectal adenocarcinoma HT-29 cells under normoxic and hypoxic conditions (Table 1). Seven of all the 12 new compounds (16a, 16b, 16e, 16f, 16i, 16j, and 16l) exhibited good anti-proliferation activities. The SARs analysis indicated that both the introduction of 2-nitroimidazole-1H-alkoxy moieties at the aniline- para-position and the 3ʹ-chloro substitution were favorable in terms of cytotoxicity (16a >16c, 16e >16g, and 16i >16k), and the results were consisted with the enzymatic inhibitory activities. Almost all of these compounds manifested comparable to more potent cytotoxicities against A549 and HT-29 cells under normoxia compared to under hypoxia. Although the difference in cytotoxicity was not obvious between hypoxia and normoxia, considering EGFR expression was significantly up-regulated under hypoxia, which could partly offset the cytotoxicity generated in that circumstance,17 it was speculated that the 2-nitroimidazole moieties underwent a bio-reductive activated process and exerted cytotoxicity especially under tumor hypoxia. Particularly, the most potent compound, 16i, exhibited corresponding IC50 values of 1.59 and 1.09 μM under normoxia and hypoxia against A549 cells, 2.46 and 1.35 μM under normoxia and hypoxia against HT-29 cells, which were superior to the positive control lapatinib.
Molecular docking study
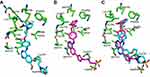
Figure 4, compound 16i bound in the ATP-binding pocket of EGFR, which is similar to that of lapatinib. The 2-nitroimidazole group of 16i was surrounded by the residues of Thr854, Phe856, Met766, and Leu777. Two hydrogen bonds were formed between the two oxygen atoms of 2-nitroimidazole and the residues of Arg776 and Leu777 of EGFR. In addition, the 3ʹ-chloro group of the aniline was inserted in a pocket formed by the residues of Ala743, Thr790, and Leu788 (Figure 4A). Although compounds with two carbon linkers between aniline and 2-nitroimidazole moiety exhibited good EGFR inhibitory activity, this linker still appeared crowed and repulsed the 4-anilinoquinazoline group to the more “out” positione than lapatinib (Figure 4C). This caused the quinazoline group to not form hydrogen bonds with Thr790 or Thr854, which was observed in lapatinib-EGFR complex (Figure 4B). We speculate that the loss of these important interactions led compound 16i to display weaker EGFR inhibitory activity than lapatinib (IC50: 0.12 μM vs 0.011 μM, Table 1). These results provide guidance for the further optimization of this series of compounds.Conclusion
For the purpose of getting novel EGFR inhibitors and taking advantage of the carcinoma hypoxia circumstance, a series of small molecule inhibitors was designed and synthesized by incorporating 2-nitroimidazole group into the aniline of the 4-anilinoquinazolines. The SARs studies revealed that the introduction of chloride at the meta- position and the 2-nitroimidazole-1H-alkoxy moiety at para- position of the aniline were crucial to the EGFR inhibitory activity. Meanwhile, the optimal alkyl linker between aniline and 2-nitroimidazole moiety was two carbons in these compounds. The anti-proliferative activities were consistent with the EGFR inhibitory activities, and almost all the new compounds exhibited more potent cytotoxicities under hypoxia than normoxia. In particular, the most promising compound, 16i, exhibited good EGFR inhibitory activity (IC50 value of 0.12 μM) and more potent cytotoxicities (A549 cells: IC50 values of 1.59 and 1.09 μM under normoxia and hypoxia, respectively; HT-29 cells: IC50 values of 2.46 and 1.35 μM under normoxia and hypoxia, respectively) than lapatinib. Moreover, the docking study confirmed the binding models between 16i and EGFR. These results provide new insights for development of EGFR inhibitors exerting activities both under normoxia and hypoxia.
Acknowledgments
The authors are grateful for the support of the National Natural Science Foundation of China (81703417), the Major Science and Technology Project of Henan Province (161100310100), and Henan Medical Science and Technology Research Project (2018020039). Dr Weiyan Cheng is grateful for the support of the Young Scholar Fund from The First Affiliated Hospital of Zhengzhou University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chong CR, Janne PA. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat Med. 2013;19(11):1389–1400. doi:10.1038/nm.3388
2. Oronsky B, Ma P, Reid TR, et al. Navigating the “no man’s land” of TKI-failed EGFR-mutated non-small cell lung cancer (NSCLC): a review. Neoplasia. 2018;20(1):92–98. doi:10.1016/j.neo.2017.11.001
3. Stintzing S, Tejpar S, Gibbs P, et al. Understanding the role of primary tumour localisation in colorectal cancer treatment and outcomes. Eur J Cancer. 2017;84:69–80. doi:10.1016/j.ejca.2017.07.016
4. Cortez AJ, Tudrej P, Kujawa KA, et al. Advances in ovarian cancer therapy. Cancer Chemoth Pharm. 2018;81(1):17–38. doi:10.1007/s00280-017-3501-8
5. Matsumoto Y, Sakurai H, Kogashiwa Y, et al. Inhibition of epithelial-mesenchymal transition by cetuximab via the EGFR-GEP100-Arf6-AMAP1 pathway in head and neck cancer. Head Neck. 2017;39(3):476–485. doi:10.1002/hed.24626
6. Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11(6):393–410. doi:10.1038/nrc3064
7. Wang T, Niki T, Goto A, et al. Hypoxia increases the motility of lung adenocarcinoma cell line A549 via activation of the epidermal growth factor receptor pathway. Cancer Sci. 2007;98(4):506–511. doi:10.1111/j.1349-7006.2007.00428.x
8. Nishi H, Nishi KH, Johnson AC. Early growth response-1 gene mediates up-regulation of epidermal growth factor receptor expression during hypoxia. Cancer Res. 2002;62(3):827–834.
9. Rawluk J, Waller CF. Gefitinib. Recent Results Cancer Res. 2018;211:235–246. doi:10.1007/978-3-319-91442-8_16
10. Lyseng-Williamson KA. Erlotinib: a pharmacoeconomic review of its use in advanced non-small cell lung cancer. Pharmacoeconomics. 2010;28(1):75–92. doi:10.2165/10482880-000000000-00000
11. Medina PJ, Goodin S. Lapatinib: a dual inhibitor of human epidermal growth factor receptor tyrosine kinases. Clin Ther. 2008;30(8):1426–1447. doi:10.1016/j.clinthera.2008.08.008
12. Greig SL. Osimertinib: first global approval. Drugs. 2016;76(2):263–273. doi:10.1007/s40265-015-0533-4
13. Wack LJ, Monnich D, Yaromina A, et al. Correlation of FMISO simulations with pimonidazole-stained tumor xenografts: a question of O2 consumption? Med Physics. 2016;43(7):4113. doi:10.1118/1.4951728
14. Hassan Metwally MA, Jansen JA, Overgaard J. Study of the population pharmacokinetic characteristics of nimorazole in head and neck cancer patients treated in the DAHANCA-5 trial. Clin Oncol. 2015;27(3):168–175. doi:10.1016/j.clon.2014.11.024
15. Wei H, Li D, Yang X, et al. Design and synthesis of vandetanib derivatives containing nitroimidazole groups as tyrosine kinase inhibitors in normoxia and hypoxia. Molecules. 2016;21:12. doi:10.3390/molecules21121693
16. Cheng W, Yuan Y, Qiu N, et al. Identification of novel 4-anilinoquinazoline derivatives as potent EGFR inhibitors both under normoxia and hypoxia. Bioorg Med Chem. 2014;22(24):6796–6805. doi:10.1016/j.bmc.2014.10.038
17. Cheng W, Zhu S, Ma X, et al. Design, synthesis and biological evaluation of 6-(nitroimidazole-1H-alkyloxyl)-4-anilinoquinazolines as efficient EGFR inhibitors exerting cytotoxic effects both under normoxia and hypoxia. Eur J Med Chem. 2015;89:826–834. doi:10.1016/j.ejmech.2014.11.010
18. Duan J, Jiao H, Kaizerman J, et al. Potent and highly selective hypoxia-activated achiral phosphoramidate mustards as anticancer drugs. J Med Chem. 2008;51:2412–2420. doi:10.1021/jm701028q
19. Nepali K, Lee H, Liou J. Nitro-group-containing drugs. J Med Chem. 2019;62:2851–2893. doi:10.1021/acs.jmedchem.8b00147
20. Cheng W, Zhou J, Tian X, et al. Development of the third generation EGFR tyrosine kinase inhibitors for anticancer therapy. Curr Med Chem. 2016;23:3343–3359.
21. Wang C, Gao H, Dong J, et al. Insight into the medicinal chemistry of EGFR and HER-2 inhibitors. Curr Med Chem. 2014;21(11):1336–1350.
22. Ikeda Y, Hisano H, Nishikawa Y, et al. Targeting and treatment of tumor hypoxia by newly designed prodrug possessing high permeability in solid tumors. Mol Pharm. 2016;13(7):2283–2289. doi:10.1021/acs.molpharmaceut.6b00011
23. Wood ER, Truesdale AT, McDonald OB, et al. A unique structure for epidermal growth factor receptor bound to GW572016 (Lapatinib): relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Res. 2004;64(18):6652–6659. doi:10.1158/0008-5472.CAN-04-1168
24. Frearson PM, Stern ES. Some new analogues of pethidine. Part III. 1-aryloxy-alkylnorpethidines, and close analogues. J Chem Soc. 1958:3065–3067.
25. Liu H, Ji M, Luo XM, et al. New p-methylsulfonamido phenylethylamine analogues as class III antiarrhythmic agents: design, synthesis, biological assay, and 3D-QSAR analysis. J Med Chem. 2002;45(14):2953–2969. doi:10.1021/jm010574u
26. Ple PA, Green TP, Hennequin LF, et al. Discovery of a new class of anilinoquinazoline inhibitors with high affinity and specificity for the tyrosine kinase domain of c-Src. J Med Chem. 2004;47(4):871–887. doi:10.1021/jm030317k
27. Marzaro G, Guiotto A, Pastorini G, et al. A novel approach to quinazolin-4(3H)-one via quinazoline oxidation: an improved synthesis of 4-anilinoquinazolines. Tetrahedron. 2010;66(4):962–968. doi:10.1016/j.tet.2009.11.091
28. Lueth A, Lowe W. A novel synthesis of EGFR-tyrosine-kinase inhibitors with 4-(indol-3-yl)quinazoline structure. J Heterocyclic Chem. 2008;45(3):703–708. doi:10.1002/jhet.5570450311
29. Zhou W, Liu XF, Tu ZC, et al. Discovery of pteridin-7(8H)-one-based irreversible inhibitors targeting the epidermal growth factor receptor (EGFR) kinase T790M/L858R mutant. J Med Chem. 2013;56(20):7821–7837. doi:10.1021/jm401045n
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.