Back to Journals » International Journal of Nanomedicine » Volume 15
Design of a Novel Theranostic Nanomedicine (III): Synthesis and Physicochemical Properties of Tumor-Targeting Cisplatin Conjugated to a Hydrophilic Polyphosphazene
Authors Patil BR, Kang SY, Jung DH, Avaji PG, Jun YJ, Lee HJ, Sohn YS
Received 22 October 2019
Accepted for publication 14 January 2020
Published 12 February 2020 Volume 2020:15 Pages 981—990
DOI https://doi.org/10.2147/IJN.S235618
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Basavaraj R Patil,1 Su Yeon Kang,2 Da Hee Jung,2 Prakash G Avaji,1 Yong Joo Jun,1 Hwa Jeong Lee,2 Youn Soo Sohn1
1C & Pharm, Ewha Womans University, Seoul 03760, Republic of Korea; 2Graduate School of Pharmaceutical Sciences, Ewha Womans University, Seoul 03760, Republic of Korea
Correspondence: Youn Soo Sohn
C & Pharm, Ewha Womans University, Room 304, University-Industry Cooperation Building, 52 Ewhayeodae-Gil, Seodaemun-Gu, Seoul 03760, Republic of Korea
Tel +82 70 7008 2120
Fax +82 2 362 8813
Email [email protected]
Hwa Jeong Lee
Graduate School of Pharmaceutical Sciences, Ewha Womans University, 52 Ewhayeodae-Gil, Seodaemun-Gu, Seoul 03760, Republic of Korea
Tel +82 2 3277 3409
Fax +82 2 3277 2851
Email [email protected]
Purpose: A new theranostic nanomedicine involving anticancer-active cisplatin moiety was designed to study its tumor-targeting properties as well as its drug efficacy and toxicity.
Methods: A cisplatin carrier polymer was prepared by grafting equimolar polyethylene glycol of a molecular weight of 550 (PEG550) and aminoethanol to the poly(dichlorophosphazene) backbone. Cisplatin was conjugated to the carrier polymer using cis-aconitic acid as a linker.
Results: The cisplatin-loaded polyphosphazene, named “Polycisplatin” was found to be amphiphilic in aqueous solution and self-assembled into nanoparticles with an average particle size of 18.6 nm in diameter. The time-dependent organ distribution study of Cy5.5-labeled Polycisplatin in the A549-tumor-bearing mice exhibited a high tumor selectivity of Polycisplatin by EPR effect despite the relatively small particle size. In order to compare the in vivo efficacy of Polycisplatin and cisplatin, their xenograft trials were performed using nude mice against the human gastric cell line MKN-28. Polycisplatin exhibited slightly less tumor suppression effect compared with cisplatin at the same dose of 1.95 mg Pt/kg, which is the maximum tolerate dose of cisplatin, but at the higher double dose of 3.9 mg Pt/kg, Polycisplatin exhibited a little better efficacy than cisplatin. Furthermore, mice treated with cisplatin at the dose of 1.95 mg Pt/kg exhibited severe body weight decrease by about 25%, while mice treated with Polycisplatin did not show serious body weight decrease even at its double dose of 3.9 mg Pt/kg. Furthermore, kidney indicators including kidney index, BUN, and creatinine values measured displayed that Polycisplatin is much less nephrotoxic than cisplatin.
Conclusion: Nanoparticular Polycisplatin was successfully prepared by conjugating cisplatin to a hydrophilic polyphosphazene carrier polymer using the acid-cleavable cis-aconitic acid. Polycisplatin nanoparticles exhibit excellent tumor-targeting properties by EPR effect. The xenograft trials exhibited excellent antitumor efficacy and reduced systemic toxicity of Polycisplatin.
Keywords: cisplatin, polyphosphazene, platinum drug, drug delivery, nanomedicine
Introduction
Cisplatin (cis-diamminedichloroplatinum(II)) is one of the most widely used first-line chemotherapeutic agents for a broad range of cancers. Cisplatin continues to be in widespread clinical use for various solid tumors such as ovarian, testicular, bladder, head and neck, and non-small cell lung cancers1,2 but its usefulness is limited due to its high toxicity and various side effects along with its cross-resistance.3,4 Therefore, recently versatile efforts have been focused to search an ideal prodrug of cisplatin by using a variety of drug delivery systems. For example, Kataoka group5 incorporated cisplatin into polymeric micelles formed from a block copolymer composed of polyethylene glycol and poly(glutamic acid) (NC-6004), and Shen et al6 prepared a cisplatin-loaded nanoparticles from PDEA (poly[2-(N,N-diethylamino) ethyl methacrylate]. There are many other attempts to deliver cisplatin by using polymeric micelles,7,8 microspheres or nanoparticles9–13 which are designed to take advantage of the enhanced permeability and retention (EPR) effect to target the tumor tissue for efficient delivery of the anticancer agent. Among the above several attempts, the long-circulating polymeric micelles NC-6004, liposomal cisplatin formulations SPI-077 and L-NDDP entered clinical trials. However, it seems clear from their animal experiments that we need to improve further drug efficacy by more efficient tumor targeting and rapid drug releasing in tumor tissue along with overcoming its drug resistance.
We have designed and prepared a new nanoparticular polyphosphazene–cisplatin conjugate by linking cisplatin to a hydrophilic polyphosphazene carrier polymer using acid-cleavable cis-aconitic anhydride as a linker14,15 to improve drug availability in tumor tissue and minimize drug toxicity. Furthermore, such polymeric cisplatin is expected to overcome cisplatin cross-resistance by endocytosis mechanism,16 but we could not test the cross-resistance of our Polycisplatin compound in the present study because of domestic unavailability of the cisplatin-resistant cell lines at the present time.
Materials and Methods
Materials
Methoxy poly(ethylene glycol) with a number average molecular weight of 550 (MPEG550), ethanolamine, sodium hydride, silver sulfate, sodium diethyldithiocarbamate, phosphonitrilic chloride trimer and aluminium chloride were procured from Sigma-Aldrich. Anhydrous tetrahydrofuran was procured from Merck. Potassium tetrachloroplatinate(II) (Kojima Chemicals), potassium iodide (Junsei Chemicals) and ammonium hydroxide (Daejung Chemicals) were used as received. Phosphonitrilic chloride trimer was purified by sublimation. cis-Diamminediaquoplatinum(II) sulfate was prepared from cis-diamminediiodoplatinum(II) as reported previously.17 Poly(dichlorophosphazene) was prepared by thermal polymerization of phosphonitrilic chloride trimer in the presence of AlCl3 catalyst as detailed in our earlier publication.18 Human alveolar basal epithelial carcinoma cell line (A549) and human gastric cancer cell line (MKN-28) were purchased from Korean Cell Line Bank (Seoul, Korea). BALB/c nude mice (male, 5 weeks, 20 ± 2 g) and ICR mice (male, 5 weeks, 20 ± 2 g) were purchased from Orient Bio Inc. (Gyeonggi-do, Korea)
Methods
1H and proton decoupled 31P, NMR spectra were measured using Varian 500 MHz NMR spectrophotometer (Varian, Inc., Palo Alto, CA, USA). Phosphoric acid was the internal standard for 31P, NMR spectra. The infrared spectra were recorded on the PerkinElmer Frontier FT-IR spectrometer. The particle size distribution of the polyphosphazene carrier polymer and Polycisplatin were measured by the dynamic light scattering (DLS) method using a Malvern Zetasizer Nano-ZS analyzer (Malvern Instruments, UK). The Pt content of Polycisplatin was determined by Agilent 1260 Infinity HPLC-UV system using diethyldithiocarbamate (DDTC) as the complexing agent according to the literature method.19 The TEM image of Polycisplatin was measured in aqueous solution (20 mg/mL) using 200 kV Tecnai F20 (FEI, Eindhoven, the Netherlands).
Synthesis of Polycisplatin [NP(MPEG550)(AE)(AA)Pt(NH3)2]n
Polyphosphazene carrier polymer, [NP(MPEG550)(AE)]n, was prepared by the procedure described in our previous report.20 The solution of [NP(MPEG550)(AE)]n (1.0 g, 1.5 mmol) in water was cooled to 0°C and cis-aconitic acid anhydride (AA) (0.234 g, 1.5 mmol) was slowly added to this solution. Aqueous NaOH solution was added dropwise to maintain this reaction mixture at pH 8.0. The reaction mixture was stirred for 5 h at 0–4°C.
The solution of [(NH3)2(H2O)2Pt]SO4 (0.272 g, 0.75 mmol) dissolved in 25 mL of distilled water was added dropwise with stirring to the carrier polymer [NP(MPEG550)(AE)(AA)]n (1.5 mmol) solution in water. The pH of the solution was maintained above 7 throughout the reaction course. This reaction mixture was stirred for 2 h at RT and then dialyzed in distilled water using a cellulose membrane (MWCO: 10 kDa). The dialyzed solution was freeze-dried finally to obtain the cisplatin-loaded polyphosphazene named “Polycisplatin”. Yield: 70%. FTIR (cm−1): 2866.4s, 1562.2m, 1453w, 1349.1m, 1290.3m, 1198.8w, 1097.6sh, 1039.8m, 947.5m, 847m, 614.9m, 523.5s, 493.3m. The platinum content (4.91%) of Polycisplatin was determined by modified HPLC–UV method detailed in the literature.19 Briefly, a known amount of Polycisplatin was dissolved in 0.5 M HCl solution and stirred so as to cleave the AA-Pt bond and release the cisplatin. The cisplatin was then treated with 100 µL of (10%) DDTC in 0.1 N NaOH and incubated in a water bath at 37°C for 30 min. The resultant Pt-DDTC complex formed was extracted with chloroform by vigorous vortex of the solution mixture, and then the chloroform layer collected was evaporated under vacuum. The resultant residue was reconstituted with 20 µL of acetonitrile and the reconstituted sample was injected into the HPLC for Pt analysis on an Agilent Eclipse plus C18 column (5 µm, 4.6 × 150 mm) maintained at ambient temperature (25°C). The water: methanol: acetonitrile (30:40:30 v/v/v) mobile phase was employed with a fixed flow rate of 1.0 mL/min. Eluent was monitored using a UV/VIS detector set at 254 nm. The amount of Pt loaded on the carrier polymer (mass/mass %) was found to be 4.91%.
Ex vivo Imaging Study of Cy5.5-Labeled Polycisplatin
BALB/C nude mice (5 weeks old), weighing between 18 and 20 g, were housed in a specific pathogen-free room kept at a temperature of 23°C ± 3°C and relative humidity of 50% ± 5%. All mice were accessible to sterilized standard chow diet and water. All animal study protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC number 16–062) of Ewha Womans University, Seoul, Korea. Animal care was performed according to the Institute for Laboratory Animal Research (ILAR) guidelines.
Human alveolar basal epithelial carcinoma cell line (A549) was grown in Roswell Park Memorial Institute MI 1640 (RPMI 1640) media containing 10% fetal bovine serum at 37°C in a humidified 5% CO2 atmosphere. The A549 cells were suspended in serum-free RPMI 1640 media and inoculated subcutaneously at a density of 5 × 106 cells per 150 µL into the right flank region of each mouse. After 2 weeks, when the tumor was grown to 200–250 mm3, the Cy5.5-labeled Polycisplatin was injected intravenously into the tail vein of the tumor-bearing mice. Cy5.5-labeled Polycisplatin was prepared following the same procedure for Cy5.5-labeled Polyplatin.20 Briefly, some terminal amine groups of AE of the carrier polymer [NP(MPEG550)(AE)(AA)]n are required for Cy5.5 labeling and imaging study. Coupling agent HBTU (7 mg, 1.8×10−3 mmol) and triethylamine (0.5 mL) were added to a suspension of Polycisplatin (63 mg, 0.06 mmol) and Cy5.5 mono acid (1 mg, 9.69×10−4 mmol) in anhydrous DMF (5 mL) and the reaction mixture was stirred at room temperature for 18 hrs. The reaction mixture was diluted with 50 mL of ethanol and then centrifuged at 4,000 rpm in Vivaspin Polyethersulfone Ultrafiltration Spin Columns (MWCO: 50 kDa) and finally washed with water several times.
The animals were sacrificed at 12, 24 and 48 h after injection, and their liver, heart, lung, kidney, spleen, tumor, and muscle (from the left flank region) were harvested and weighed. The fluorescence intensity of each organ was measured immediately after sacrifice. The fluorescence images of harvested organs and tumors were obtained using IVISⓇ Lumina Series Ⅲ In vivo Imaging System (Perkin Elmer, CLS136334). To observe the fluorescence image, Living Image 4.4 program was used.
In vitro Drug Release Study
The time-dependent release of the cisplatin moiety (NH3)2Pt(II) from Polycisplatin in different buffer solutions of pH 5.4 and 7.4 was examined at 37°C under sink condition. Each 5 mL of the sample solutions (2 mg/mL) was placed in a cellulose tubing membrane with MWCO of 3,000 Da and suspended in 50 mL of respective buffer solutions under gentle stirring at 100 rpm. From each sample solution, 1 mL was withdrawn from the dialysate at predetermined time intervals over 14 days. Inductively coupled plasma mass spectrometry was employed to determine the amount of released platinum.
In vivo Nude Mouse Xenograft Trial
The in vivo antitumor efficacy of Polycisplatin and cisplatin (as a reference) was evaluated against a gastric tumor cell line MKN-28 using BALB/C nude mice (5 weeks old, 18–20 g). Animals were adapted under controlled temperature and humidity for 1 week prior to the experiments. MKN-28 cells were cultured in RPMI 1640 media containing 10% fetal bovine serum and 1% antibiotic–antimycotic agent (cell culture media) at 37°C in a humidified 5% CO2 atmosphere and were detached with 0.25% trypsin–EDTA solution. The assembled cells were centrifuged in cell culture media for 2 min.
The concentration of cells in media was calculated from the number of cells counted using a hemocytometer (catalog number 0650030; Paul Marienfeld, Lauda-Königshofen, Germany). The tumor cells were suspended in serum-free RPMI 1640 media at a density of 5 × 106 cells in 150 µL of media and inoculated subcutaneously into the right flank region of each mouse. When the average tumor volume reached about 100–150 mm3, the test drugs or normal saline as a control were injected intravenously via the tail vein of the mice according to a triple injection regimen on days 1, 5, and 9. In order to compare the antitumor efficacy of Polycisplatin and cisplatin, tumor size and weights of the mice were measured every 3 or 4 days. The mice were sacrificed on the 35th day from the first injection.
Estimation of Acute Toxicity of Polycisplatin
ICR mice (5 weeks old) weighing 20–23 g were subjected to a study to measure the lethal dose (LD50) of Polycisplatin to compare with that of cisplatin. The mice were randomly divided into six groups with four mice each. Different doses of Polycisplatin based on its platinum content (5, 10, 15 and 20 mg Pt/kg) and 6.5 and 13 mg Pt/kg of cisplatin for comparison were intravenously injected. Also, one group of four mice was intravenously injected with saline as a control. After injection, the changes in body weight and the survival rate were daily recorded over a period of 2 weeks. The survival rate of mice was calculated as (number of live mice/total number of mice treated) × 100%. The LD50 values of the Polycisplatin and cisplatin were calculated according to the Organisation for Economic Co-operation and Development guideline 425.
Kidney Toxicity Test
Using the kidney and plasma samples obtained from above in vivo xenograft experiments, the kidney indicators such as kidney index, BUN and creatinine values were estimated. The kidney index was calculated in the following Equation:
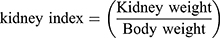
BUN values were estimated using BUN kit (Quanti ChormTM Urea Assay Kit, BioAssay systems). Each of plasma samples, distilled water (blank), and 5 μL of standard solution were mixed in 96-well plates in duplicate. Two hundred microliters of working reagent was added to each well. After tapping and incubation for 20 mins, optical density was measured at 520 nm. We calculated BUN using the following equation:
Creatinine values were obtained using Creatinine kit (QuantiChormTM Creatinine Assay Kit, BioAssay systems). Each of samples (plasma) and 30 μL of standard solution were mixed in 96-well plates in duplicate. Two hundred microliters of working reagent combined A and B to each well. After tapping, optical density of each sample was measured using Elisa Reader (Multiskan GO, Thermo Scientific) at 510 nm at 0 and 5 mins. We have calculated creatinine values using the following equation: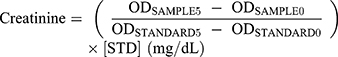
Results and Discussion
Synthesis and Physicochemical Properties of Polycisplatin
The anticancer-active cisplatin moiety, (NH3)2Pt(II) was successfully conjugated to a polyphosphazene carrier polymer using the cis-aconitic acid anhydride as a linker as shown in the following reaction Scheme 1, which is described in detail in the experimental section.
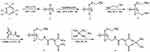 |
Scheme 1 Synthetic pathway for the polyphosphazene carrier polymer and its cisplatin conjugate, Polycisplatin. |
The final product of Polycisplatin (6) was obtained as a light yellow gel highly soluble in water and polar organic solvents such as ethanol, acetonitrile, methylene chloride, etc. In order to estimate its tumor-targeting properties by EPR (enhanced permeability and retention) effect,21,22 we have performed its morphology study in aqueous solution. We have measured the particle size of Polycisplatin in distilled water by dynamic light scattering (DLS) method using a Malvern Zetasizer Nano-ZS analyzer. The average diameter of Polycisplatin nanoparticles was measured to be 18.6 nm as shown in Figure 1A and the PDI value was measured to be 0.39. We have also scanned the TEM image of Polycisplatin, which appears as irregularly shaped nanoparticles as shown in Figure 1B.
 |
Figure 1 (A) Particle size distributions of Polycisplatin in saline. (B) TEM image of Polycisplatin in saline. |
Biodistribution and Targeting Properties of Polycisplatin
In order to evaluate the passive tumor-targeting properties of Polycisplatin, we have examined the time-dependent organ distributions of Cy5.5-labeled Polycisplatin in the A549-tumor-bearing mice by measuring the fluorescence intensities of their major organs and plasma as described earlier. The fluorescence images of organs including liver, heart, lung, kidney, spleen, muscle, and tumor, as well as plasma, harvested from mice at 12, 24, and 48 h after injection are displayed in Figure 2.
 |
Figure 2 Time-dependent distributions of Cy-Polycisplatin in major organs of A549 lung tumor-bearing mouse. |
As shown in Figure 3A, Cy-Polycisplatin was distributed dominantly in the tumor with the maximum value at 24 h after injection. The plasma concentration of Cy-Polycisplatin exhibited a time-dependent decrease, as shown in Figure 3B; however, strong fluorescence intensity was still detected at the last time point.
 |
Figure 3 Time-dependent quantitative fluorescence intensities of Cy-Polycisplatin distributed in the major organs of A549 tumor-bearing mice (A) and tumor-to-tissue ratios (TTRs) (B). |
A quantitative diagram of the fluorescence intensity detected in each organ as well as the TTR (tumor-to-tissue ratio) values representing the tumor selectivity are displayed in Figure 3B. The highest TTR value was observed approximately 11 to 12 hr post injection but decreased to 5–6 during 24–48 hr post injection. Thus, the present Polycisplatin nanoparticles (18.6 nm) exhibited excellent tumor-targeting properties by EPR effect21,22 in spite of their relatively small particle size.
In vitro Release of the Cisplatin Moiety (NH3)2Pt(II) from Polycisplatin
The time-dependent platinum contents released from Polycisplatin were measured using inductively coupled plasma atomic absorption spectrometry and displayed in Figure 4. At pH 7 the accumulated amount of platinum released from Polycisplatin after 1 day was nearly 50% of the total loaded platinum and the total accumulated amount of released platinum for 2 weeks was approximately 60%. The overall releasing pattern and total accumulated amount of platinum released at pH 5 are very similar to the above results at pH 7. It is known that cis-aconitic acid may be used as an acid-cleavable linker12 but it seems not working in the present system for an unclear reason.14,15 However, such a result is in consistent with the following xenograft results displaying that double dose of Polycisplatin exhibits almost the same efficacy at the double dose of cisplatin based on platinum metal.
 |
Figure 4 The release profiles of (NH3)2Pt(II) moiety from Polycisplatin in pH 5.0 and pH 7.0 buffer solutions. |
In vivo Antitumor Efficacy of Polycisplatin
To estimate the antitumor efficacy of Polycisplatin compared with cisplatin as a positive control, we performed xenograft trials using gastric MKN-28 cell-derived tumor-bearing nude mouse as described in detail in the experimental section. In this study, Polycisplatin and cisplatin as a reference were administered three times on days 1, 5, and 9 by intravenous injection at two different doses of 1.95 mg Pt/kg and 3.9 mg Pt/kg of Polycisplatin but reference cisplatin at a single dose of 1.95 mg Pt/kg, which is its maximum tolerate dose. To confirm the xenograft model, a negative control group was injected with saline solution. The tumor size and body weight of each mouse were measured every 3 or 4 days after injections. The results of the antitumor efficacy study and the body weight changes of the treated mice are displayed in Figures 5 and 6, respectively.
 |
Figure 5 The results of xenograft trials of Polycisplatin and cisplatin as reference against the gastric MKN-28 cell line. |
 |
Figure 6 The body weight changes of the mice treated with Polycisplatin (PCP) and cisplatin as reference against the gastric MKN-28 cell line. |
Tumor growth inhibition was observed in all drug-treated groups. Polycisplatin exhibited tumor suppression effect similar to that of cisplatin at the low dose of 1.95 mg Pt/kg but a little better effect than cisplatin at the higher dose. When cisplatin was injected into mice even at the low dose, the mean body weight was severely decreased by 25%, while body weights of mice injected with Polycisplatin at the low dose of 1.95 mg Pt/kg increased similarly to the saline group. The body weights of mice treated with the higher dose of 3.9 mg Pt/kg of Polycisplatin were a little reduced but recovered immediately. The excellent antitumor efficacy and reduced systemic toxicity of Polycisplatin seem to be due to the stability of the nanoparticles formed from amphiphilic cisplatin–polyphosphazene conjugate molecules.
Acute Toxicity of Polycisplatin
ICR mice (5 weeks old) were treated by intravenous injection at increasing doses of 5, 10, 15 and 20 mg Pt/kg of Polycisplatin and 6.5 and 13 mg Pt/kg of cisplatin to compare their LD50 values. After intravenous injection to mice, their aspects and changes in body weight were observed carefully and recorded daily for 14 days. The survival rates of each group consisting of four mice treated at different dosages are presented in Figure 7.
 |
Figure 7 Results of the acute toxicity experiment of Polycisplatin compared with cisplatin. |
In the case of cisplatin, all four mice survived at its lower dosage of 6.5 mg Pt/kg, but none of mice survived at the dose of 13 mg Pt/kg. According to the pharmaceutical paper,23 the LD50 value of cisplatin was estimated to be approximately 11 mg/kg equivalent to 7.12 mg Pt/kg. In case of Polycisplatin, no mortality was observed up to the dose of 2, 25, 100 mg/kg Polycisplatin. Only one of 4 mice survived at 200 mg/kg, 2 of 4 mice survived at 300 mg/kg but all the mice treated with 400 mg/kg Polycisplatin died within several minutes after injection. Thus, LD50 value of Polycisplatin was determined to be about 300 mg/kg (Pt 15 mg/kg) which is about 2 times higher compared with LD50 value of cisplatin, indicating that Polycisplatin has much lower acute toxicity compared to cisplatin.
Nephrotoxicity of Polycisplatin and Cisplatin
As shown in the above xenograft trials, there was a severe reduction in body weight of the mice treated with cisplatin even at the low dose of 1.95 mg Pt/kg compared to other groups treated with Polycisplatin at the doses of 1.95 and 3.9 mg Pt/kg. It is well known that cisplatin critically damages the kidney3,24 and therefore, we have measured the kidney toxicity indicators such as kidney index, BUN and creatinine values as above-mentioned, and the results are illustrated in Figure 8.
Cisplatin-treated group (b) shows an increased kidney weight/body weight ratio, a marker for the body weight loss and increased renal size compared with both Polycisplatin-treated groups (c) and (d). In other words, Polycisplatin has significantly lower kidney toxicity compared with cisplatin. Also, increased BUN and creatinine values have been reported to be nephrotoxic.3,23 The BUN and creatinine values of cisplatin-treated group (b) are much higher than that of the control group (a) whereas Polycisplatin-treated groups (c) and (d) show very low values similar to those of the control groups (a). In conclusion, Polycisplatin exhibited much lower nephrotoxicity compared with cisplatin.
Conclusion
In this study, nanoparticular Polycisplatin was successfully prepared by conjugating cisplatin to a hydrophilic polyphosphazene carrier polymer using the acid-cleavable cis-aconitic acid and thoroughly characterized by various techniques. In spite of relatively small size, polycisplatin nanoparticles exhibit excellent tumor-targeting properties by EPR effect. The xenograft trials exhibited excellent antitumor efficacy and reduced systemic toxicity of Polycisplatin. Polycisplatin exhibited a slightly less tumor suppression effect compared with cisplatin at the low dose of 1.95 mg Pt/kg but a little better effect at higher dose of 3.9 mg Pt/kg. The body weights of mice treated with cisplatin was severely decreased even at the low dose, while body weights of mice injected with Polycisplatin did not show serious body weight decrease even at its double dose. Polycisplatin is much less nephrotoxic than cisplatin as shown by kidney indicators including kidney index, BUN, and creatinine values.
Acknowledgments
This research work was supported by National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (2017R1A2B4007869).
Disclosure
The authors report no conflicts of interests in this work.
References
1. Reedijk J. New clues for platinum antitumor chemistry: kinetically controlled metal biding to DNA. Proc Natl Acad Sci USA. 2003;100:3611–3616. doi:10.1073/pnas.0737293100
2. Galanski M, Jakupec MA, Keppler BK. Update of the preclinical situation of anticancer platinum complexes: novel design strategies and innovative analytical approaches. Curr Med Chem. 2005;12:2075–2094. doi:10.2174/0929867054637626
3. McKeage MJ. Clinical toxicology of platinum-based cancer chemotherapeutic agents. In: Kelland LR, Farrell NP, editors. Platinum-Based Drugs in Cancer Therapy. Totowa: Human Press; 2000:251–275.
4. Brown R. Cisplatin resistance in ovarian cancer: mismatch repair and engagement of apoptosis. In: Kelland LR, Farrell NP, editors. Platinum-Based Drugs in Cancer Therapy. Totowa: Human Press; 2000:115–128.
5. Uchino H, Matsumura Y, Negishi T, et al. Cisplatin-incorporating polymeric micelles (NC-6004) can reduce nephrotoxicity and neurotoxicity of cisplatin in rats. Brit J Cancer. 2005;93:678–687. doi:10.1038/sj.bjc.6602772
6. Xu P, Van Kirk EA, Murdoch WJ, et al. Anticancer efficacies of cisplatin releasing pH-responsive nanoparticles. Biomacromolecules. 2006;7:829–835. doi:10.1021/bm050902y
7. Jadhav VB, Jun YJ, Song JH, et al. A novel micelle-encapsulated platinum(II) anticancer agent. J Control Release. 2010;147:144–150. doi:10.1016/j.jconrel.2010.07.101
8. Avaji PG, Joo HI, Park JH, et al. Synthesis and Properties of a new micellar polyphosphazene-platinum(II) conjugate drug. J Inorg Biochem. 2014;140:45–52. doi:10.1016/j.jinorgbio.2014.06.014
9. Duan X, He C, Kron SJ, Lin W. Nanoparticle formulations of cisplatin for cancer therapy. WIREs Nanomed Nanobiotech. 2016;8:776–791. doi:10.1002/wnan.1390
10. Haxtn KJ, Burt HM. Polymeric drug delivery of platinum-based anticancer agents. J Pharm Sci. 2009;98:2299–2316. doi:10.1002/jps.21611
11. Ma P, Xiao H, Li C, et al. Inorganic nanocarriers for platinum drug delivery. Mater Today. 2015;18:554–564. doi:10.1016/j.mattod.2015.05.017
12. Yu H, Tang Z, Li M, et al. Cisplatin loaded poly(L-glutamic acid)-g-methoxy poly(ethylene glycol) complex nanoparticles for potential cancer therapy: preparation, In vitro and in vivo evaluation. J Biomed Nanotechnol. 2016;12:69–78. doi:10.1166/jbn.2016.2152
13. Johnstone TC, Suntharalingam K, Lippard SJ. The next generation of platinum drugs: targeted Pt(II) agents, nanoparticle delivery, and Pt(IV) prodrugs. Chem Rev. 2016;116:3436–3486. doi:10.1021/acs.chemrev.5b00597
14. Kakinoki A, Kaneo Y, Ikeda Y, Tanaka T, Fujita K. Synthesis of poly(vinyl alcohol)-doxorubicin conjugates containing cis-aconityl acid –cleavable bond and its isomer dependent doxorubicin release. Biol Pharm Bull. 2008;31:103–110. doi:10.1248/bpb.31.103
15. Yoo HS, Lee EA, Park TG. Doxorubicin-conjugated biodegradable polymeric micelles having acid-cleavable linkages. J Control Release. 2002;82:17–27. doi:10.1016/S0168-3659(02)00088-3
16. Xue X, Hall MD, Zhang Q, Wang PC, Gottesman MM, Liang X-J. Nanoscale drug delivery platforms overcome platinum-based resistance in cancer cells due to abnormal membrane protein trafficking. ACS Nano. 2013;7:10452–10464. doi:10.1021/nn405004f
17. Singh MM, Szafran Z, Pike RM. Microscale synthesis of cis-diamminedihaloplatinum(II) and a corresponding trans isomer: A rapid and convenient method of preparing cisplatin - An anticancer drug. A laboratory experiment for inorganic and bioinorganic chemistry. J Chem Educ. 1990;67:A261–A262. doi:10.1021/ed067pA261
18. Sohn YS, Cho YH, Beak H, Jung OS. Synthesis and properties of low molecular weight polyphosphazenes. Macromolecules. 1995;28:7566–7568. doi:10.1021/ma00126a039
19. Kaushik KH, Sripuram VK, Bedada S, Reddy NY, Priyadarshini GI, Devarakonda KR. A simple and sensitive validated HPLC method for quantitative determination of cisplatin in human plasma. Clin Res Regul Aff. 2010;27:1–6. doi:10.3109/10601330903490462
20. Avaji PG, Park JH, Lee HJ, et al. Design of a novel theranostic nanomedicine: synthesis and physicochemical properties of a biocompatible polyphosphazene–platinum(II) conjugate. Int J Nanomed. 2016;11:837–851.
21. Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and EPR effect in macromolecular therapeutics. J Control Release. 2000;65:271–284. doi:10.1016/S0168-3659(99)00248-5
22. Torchilin VP. Drug targeting. Eur J Pharm Sci. 2000;11:S81–S91. doi:10.1016/S0928-0987(00)00166-4
23. Barcellona PS, Campana A, Rossi V, Corradino C, Silvestrini B, Angelini F. A comparative study of acute toxicity of drugs used during anticancer therapy in healthy and tumor-bearing mice. Arch Toxicol Suppl. 1984;7:90–93.
24. Lee CK, Park KK, Hwang JK, Lee SK, Chung WY. Extract of Prunus persica Flesh improves chemotherapeutic efficacy and protects against nephrotoxicity in cisplatin-treated mice. Phytother Res. 2009;23:999–1005. doi:10.1002/ptr.2740
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

