Back to Journals » Drug Design, Development and Therapy » Volume 14
Desflurane Preconditioning Protects Against Renal Ischemia–Reperfusion Injury and Inhibits Inflammation and Oxidative Stress in Rats Through Regulating the Nrf2-Keap1-ARE Signaling Pathway
Authors Zheng Y, Lu H, Huang H
Received 18 July 2019
Accepted for publication 16 March 2020
Published 3 April 2020 Volume 2020:14 Pages 1351—1362
DOI https://doi.org/10.2147/DDDT.S223742
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sukesh Voruganti
Yan Zheng,1,* Hui Lu,1,* Huiqiong Huang2
1Department of Anesthesiology, Xiamen Haicang Hospital, Xiamen 361000, People’s Republic of China; 2Department of Anesthesiology, Women and Children’s Hospital Affiliated to Xiamen University, Xiamen 361000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Huiqiong Huang
Department of Anesthesiology, Women and Children’s Hospital Affiliated to Xiamen University, No. 10 Zhenhai Road, Xiamen 361000, People’s Republic of China
Tel +86-13799799464
Email [email protected]
Objective: Kidney is sensitive to ischemia–reperfusion (I/R) injury because of its special structure and function. In this study, we aimed to explore the mechanism of desflurane (DFE) preconditioning effecting on renal I/R injury in rats.
Methods: Renal I/R injury rats model was constructed, and the expressions of serum renal function parameters (blood urea nitrogen (BUN) and serum creatinine (SCr)) and lipid peroxidation-related factors were detected using corresponding commercial kits to assess the degrees of renal functional damage and oxidative stress. Hematoxylin–-eosin (HE) staining and Masson trichrome staining were applied to measure the renal histologic damage. The expressions of inflammation-related factors were determined by ELISA assay. The cell apoptosis was analyzed using TUNEL, Western blot and immunohistochemistry (IHC). IHC was also used to detect the number of myeloperoxidase (MPO)-positive cells. The expressions of proteins associated with the Nrf2-Keap1-ARE pathway were assessed by Western blot and IHC.
Results: DFE preconditioning inhibited I/R injury-induced BUN and SCr increase and renal histologic injury in rats. Also, DFE suppressed the inflammation, apoptosis and oxidative stress caused by renal I/R injury in vivo. In addition, DFE preconditioning repressed peroxide-related factors (MDA, MPO and NO) expressions and promoted antioxidant-related factors (GSH, SOD, GPx and CAT) expressions. In addition, DFE promoted Nrf2-Keap1-ARE-related proteins including Nrf2, NQO1, HO-1, γ-GCS, GSR and GCLc expressions.
Conclusion: DFE preconditioning protected the kidney as well as inhibited the inflammation, cell apoptosis and oxidative stress in renal I/R injury rats by activating the Nrf2-Keap1-ARE signaling pathway.
Keywords: ischemia–reperfusion, kidney, animal model, desflurane, Nrf2-Keap1-ARE signaling pathway
Introduction
The recovery of blood supply after ischemia of tissues and organs is called ischemia–reperfusion (I/R).1 In most cases, I/R can restore the structure and function of tissues and organs. However, in some cases, I/R leads to the aggravation of structural and functional damage of tissues and organs, and this is I/R injury.2,3 Kidney is one of the organs sensitive to I/R injury because of its special structure and function.4 With the development of economy, people’s lifestyle has been changed, and the incidence of acute kidney injury (AKI) is gradually increasing. Although medical technology is improving, the mortality rate of AKI is still not decreasing. Recent study reports that there are nearly 13 million patients suffering from AKI every year in the worldwide, resulting in about 1.7 million deaths.5,6
Shock caused by various reasons, kidney transplantation for renal failure, angioplasty for renal artery malformation and contrast medium nephropathy after enhanced CT often lead to AKI, and renal I/R is still the risk factor of AKI.7 The main characteristics of renal I/R injury are oxidative stress and inflammation.8,9 It is commonly seen in disseminated intravascular coagulation, hemorrhage or toxic shock, kidney transplantation, partial nephrectomy, nephrolithotomy and other surgical procedures.10,11 The pathogenesis of acute I/R renal injury has not been fully elucidated so far. Therefore, actively exploring and studying the pathogenesis of AKI caused by I/R is helpful to provide the theoretical and practical basis for the AKI treatment.
Since ether was used as an anesthetic in 1846, inhalational anesthetics have been widely used in clinic.12,13 Inhaled anesthetics play an important role in the induction and maintenance of general anesthesia, because they are more controllable than intravenous anesthetics and have less impact on the body.14,15 Desflurane (DFE), a kind of halogenated inhalation anesthetics, can reduce the excitability of the nervous system.16 DFE exerts sedative and hypnotic effects by promoting the opening of inhibitory neurotransmitter receptors such as gamma absorptiometry aminobutyric acid (GABAA) and glycine receptor, inhibiting their inactivation and hyperpolarizing nerve cells.17 On the other hand, they act on the spinal cord and exhibit sedative and hypnotic effects by inhibiting the muscle action potential induced by spinal cord stimulation.18 Furthermore, DFE played important roles in some organs sensitive to I/R injury, such as lung I/R injury,19 heart I/R injry20 and forebrain I/R injury.21 Although some achievements have been made in the study of DFE in renal I/R injury, the mechanism of DFE in renal I/R injury can not be fully explained.
In the current study, the effects of DFE preconditioning on renal I/R injury and the underlying mechanism were explored. Firstly, we investigated that DFE preconditioning adjusted the renal serum function parameters induced by I/R. We consequently confirmed that DFE treatment reduced renal histological damage, inflammation, apoptosis, oxidative stress and lipid peroxidation caused by I/R injury. In addition, we demonstrated that DFE relieved I/R renal injury by regulating the Nrf2-Keap1-ARE signaling pathway. This study provided a new explanation and theoretical basis for the DFE application in renal I/R injury treatment.
Materials and Methods
Renal I/R Injury Animal Models and Treatment
Male Wistar rats (220 ± 20 g, 8 weeks), which were purchased from Shanghai Laboratory Animal Center (Shanghai, China), ate in 12/12 light/dark cycles and freely obtained water and food.
Next, the rats were randomly divided into four groups (n = 8): sham group, sham + DFE group, I/R group and I/R + DFE group. The methods of animal preparation22 and the DFE treatment23 were performed as previous research. For I/R groups, the rats were anaesthetized by sodium pentobarbital (80 mg/kg, Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) intraperitoneal injecting and then subjected to I/R experiments 2 weeks after right-sided nephrectomy. To avoid the effects on the renal function of a normal kidney, the single kidney model was employed. The renal pedicle was exposed through a lateral ventral incision. The right kidney was removed, and then a noninvasive s-clip was used to clamp the left renal pedicle for 60 min. The clip was removed and the color of the kidney was monitored. The renal pedicle recanalized the blood flow and the color of the kidney was observed: the change of the kidney color from dark purple to red indicated that the model of I/R injury was successfully established to confirm the blood reflux before suturing the incision. The same surgery was performed in sham groups except for renal artery occlusion. For DFE groups, 3 mins before rats renal reperfusion, sodium pentobarbital injection was discontinued and DFE was preoperatively inhaled at the concentration of the end of expiration was maintained at 1.0 minimum alveolar effective concentration (MAC) for 15 min. After natural awakening, the mice were placed in a relatively clean and thermostatic metabolic cage, fed with standard feed, drank freely, and collected their urine for 24 h. In the process of operation, all the animals were placed on a heating blanket at a temperature of 37°C.
After 24 hrs of reperfusion, rats were killed by air embolization and blood samples (1.3 mL/sample, 8 samples in each group) were collected by cardiac puncture to determine serum creatinine (SCr) and blood urea nitrogen (BUN). The serum SCr and BUN indexes were respectively measured using creatinine Assay Kit (Sigma-Aldrich, St. Louis, MO, USA) and urea assay kits (Shanghai Yuanye Science and Technology Co., Ltd., Shanghai, China) according to the manufacturer’s instruction, respectively. All animal experiments were performed according with protocols approved by the Institutional Animal Care and Use Committee of Women and Children’s Hospital Affiliated to Xiamen University and the guidelines of the National Institutes of Health for the Care and Use of Laboratory Animals.
Hematoxylin–Eosin (HE) Staining and Masson Staining
The kidney tissues (5 mm each) of rats were washed using PBS and then fixed in 4% paraformaldehyde buffer (Sinopharm chemical Reagent Co., Ltd, Shanghai, China) for 24 h. After fixing, the tissues were washed three times with distilled water, then washed two times with 50% alcohol (Sinopharm chemical Reagent Co., Ltd). The following steps were subsequently used to dehydrate: 70% alcohol (60 min), 80% alcohol (40 min), 95% alcohol (30 min), 100% alcohol (25 min), 100% alcohol II (25 min). Tissue sections were soaked in in xylene I (35 min) and xylene II (35 min) for transparent. The transparent tissue sections were placed in the dissolved paraffin and stored in the wax dissolving box for heat preservation. After the paraffin was completely immersed in the tissue sections, it was embedded and cooled and solidified. The embedded wax blocks were fixed on the slicer, sliced into slices (5 μm), ironed in heated water, then pasted on the slide, and dried in a 45°C incubator. After drying, the tissue sections were invaded into xylene I and xylene II to dewax for 10 mins each step. Tissues were immersed in absolute ethanol I, absolute ethanol II, 95% ethanol, 80% ethanol and 70% ethanol for 2 min, respectively, and then washed twice with PBS for 5 mins each time.
Tissue sections were stained with hematoxylin (Amresco, Pennsylvania, USA) for 3 min, washed with water for 3 min, separated with 1% hydrochloric alcohol for 2 s, washed with water for 2 min, soaked in 50%, 70%, 80% alcohol in turn for 2 min each, soaked in eosin (Amresco) for 5 s, washed with water for 3 min. Tissue sections were then invaded into 95% alcohol, absolute ethanol I and absolute ethanol II for 3 mins, respectively, and finally soaked into xylene I and xylene II for 5 mins, respectively. And then tissues sections were sealed with neutral gum (Solarbio, Beijing, China). Renal histology, acute tubular necrosis (ATN) value was detected under a light microscope (Olympus Corporation, Tokyo, Japan). The evaluation standards of ATN value were as follows: according to the percentage of damaged renal tubules in the sample, the higher the score, the more severe the damage (maximum score: 4 points): 0, the kidney is normal; 1, minimal necrosis (<5%); 2, mild necrosis (5–25%); 3, moderate necrosis (25–75%); 4, severe necrosis (>75%).
To determine the areas of fibrotic kidney lesions, the tissue sections were then stained with Masson trichrome staining (Sigma-Aldrich) according to the instructions provided by manufacture. Red-stained tissue indicated normal, and Blue-stained tissue suggested renal fibrotic tissue.
ELISA Assay
The kidney tissues of rats were homogenized ultrasonically on ice for 30 min, centrifuged at 11,000 r for 15 min (4°C) and then the supernatant was collected. The content of TNF-α, IL-1β, IL-I6 and L-10 in the supernatant was determined by ELISA kit (Sigma-Aldrich) in accordance with the manufacturer’s instruction.
TUNEL Assay
The cell apoptosis was evaluated using Colorimetric TUNEL Apoptosis Assay Kit (Baiaolaibo Science and Technology Co., Ltd. Beijing, China) according to the manufacturer’s instruction. In brief, the tissue sections were blocked with H2O2 (3% in methanol) for 5 min and then labeled with TdT labeling reaction mix for 1 h at 37°C. Nuclei exhibiting DNA fragmentation were visualized with 3,3ʹ-diaminobenzidine (DAB) for 15 min and observed under a light microscope.
Western Blot
Cytoplasmic Protein Extraction Kit provided by Beyotime Biotechnology (Shanghai, China) was used to separate the nuclear-cytoplasm. The expression levels of Nrf2 in the separated nuclear or cytoplasm and other proteins in the current study were determined by Western blot. The total protein lysates of rats' kidney tissue were extracted by RIPA lysate containing 1 mmol/L PMSF. The protein concentration was assessed by the BCA method. SDA-PAGE electrophoresis (10%) was performed to separate the protein (50 μg/well). After electrophoretic separation, the protein was transferred to the cellulose nitrate membrane and then blocked in a blocking solution containing 5% skimmed milk for 1 h at room temperature. The diluted primary antibody was added according to the instructions overnight at 4°C. Primary antibodies were all purchased from Proteintech Group (Chicago, IL, USA), Abcam (Cambridge, MA, USA), Bioworld Technology (St. Louis, USA) or Cell Signaling Technology (Beverly, MA, USA) and used at the following dilutions: anti-Bax (1:1000, ab32503), anti-Bcl-2 (1:500, ab692), anti-Caspase 3 (1:500, ab13847), anti-Cleaved Caspase-3 (1:500, ab49822), anti-Caspase 9 (1:500, ab52298), anti-Cleaved-caspase 9 (1:1000, CST. #9509), anti-β-actin (1:1000, ab82270), anti-NQO1 (1:10,000, ab80588), anti-HO-1 (1:1000, ab13248), anti-γ-GCS (1:1000, #BS2926, Bioworld Technology), anti-GSR (1:500, 18257-1-AP, Proteintech Group), anti-GCLc (1:1000, ab190685), anti-Nrf2 (1:1000, ab137550), anti-Keap1 (1:1000, ab139729) and anti-GAPDH (1:2500, ab9485). Following extensive washing, horseradish peroxidase-linked goat polyclonal anti-rabbit IgG secondary antibody (1:2000, cat. no. 7074) was applied to incubate membranes for 1 h at room temperature. GAPDH and β-actin served as the loading control.
Oxidative Stress and Lipid Peroxidation Detection
The contents of peroxide malondialdehyde (MDA), myeloperoxidase (MPO), glutathione (GSH), antioxidant enzyme (SOD, GPx, CAT) and nitric oxide (NO) in lipids were detected by corresponding specific commercial kits (Shanghai Yuanye Science and Technology Co., Ltd.) according to the manufacturer’s instruction.
Immunohistochemistry (IHC) Staining
In brief, the target retrieval solution was used to immerse the tissue sections in a water bath for 30 min. H2O2 (3%) was used to block the endogenous peroxidase for 15 min, and goat serum was used to block the nonspecific bindings for 50 min. Then, primary antibodies (cleaved caspase 3, MPO and Nrf2) and secondary antibody polymer HRP were applied to stain the slides. Then, DAB and methyl green were respectively applied to stain and counterstain the slices. Images were taken with a microscope.
Statistical Analysis
Data in this study were analyzed using SPSS 22.0 software. The measurement data were expressed as mean ± SD. Student’s t-test was used for comparison between two groups, and one-way ANOVA was used for comparison among three or more groups. P-value was calculated by the Log rank test and the difference was statistically significant at P<0.05.
Results
Effects of DFE Preconditioning on the Function Parameters in Serum of I/R-Induced Renal Injury
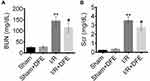
To investigate the effect of DFE on renal I/R injury, rats renal I/R injury models were constructed. After 24 h of reperfusion, blood samples (1.3 mL/sample, 8 samples in each group) were collected by cardiac puncture to determine BUN and SCr. The contents of BUN and SCr were detected using corresponding commercial kits. As shown in Figure 1A, I/R treatment induced a significant increase of BUN content in rats' renal serum compared to the sham group. DFE preconditioning obviously reduced the BUN level compared with the I/R group. Similar results were found in the assessment of SCr. The SCr contents in blood samples of the I/R group were markedly higher than that in the sham group (Figure 1B). However, DFE preconditioning could remarkably alleviate the increase of SCr induced by I/R (Figure 1B). These results showed that DFE pretreatment controlled the changes of renal function parameters caused by I/R to some extent in rat I/R injury models.
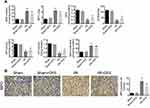
DFE Preconditioning Alleviated I/R-Induced Renal Histological Injury
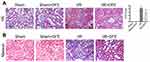
To assess rats renal histological injury degree induced by I/R, HE staining assay and ATN value were applied. Firstly, histopathological changes in the renal cortex were observed after HE staining. In sham and sham + DFE groups, the renal tissue structure showed no significant changes, and the glomerular and tubular structures remained normal (Figure 2A). The value of ATN was observed and evaluated in I/R rats after 24 h of reperfusion, the structure of the kidney extramedullary part was severely damaged and the renal tubules showed obvious vacuoles and lumen dilation (Figure 2A). In addition, the neutrophils of the I/R group infiltrated in the lumen, the epithelial cells became flat and irregular and even showed necrosis and abscission, the intercellular substance was congested and edematous, and the inflammatory cells infiltrated in the lumen (Figure 2A). Compared with the sham group, the ATN value of the I/R group increased significantly (Figure 2A). In the I/R + DFE group, the severity of pathological and outer medulla tissue damages was observed to be reduced to some extent. And, the renal tubule tissue of the I/R + DFE group was relatively intact compared to the I/R group. We also found that the renal tubules were swollen with slight water-like degeneration and balloon-like degeneration without obvious necrosis in the I/R + DFE group. Moreover, the intercellular substance was congested and edematous and the inflammatory cells infiltration was not obvious after DFE treatment. And the ATN value of the I/R + DFE group was reduced accordingly (Figure 2A). In addition, Masson staining was carried out to observe the renal fibrosis. Images from Masson staining exhibited that the blue-stained areas, that indicated renal fibrosis, in the I/R group were more than that in sham groups, and DFE treatment could reduce the blue-stained areas (Figure 2B). These results revealed that DFE preconditioning inhibited I/R-induced renal histological injury.
DFE Preconditioning Reduced I/R-Induced Renal Inflammation
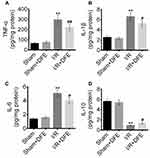
To determine the effect of DFE pretreatment on I/R-induced renal inflammation, the expression levels of inflammatory factors in renal serum (IL-1β, IL-6, TNF-α and IL-10) were detected using ELISA assay. Figure 3A–C shows that I/R treatment significantly enhanced the expression levels of anti-inflammation proteins TNF-α, IL-1β and IL-6 compared to sham groups. Also, the expressions of anti-inflammation factors in the I/R + DFE group were obviously lower than that in the I/R group. Furthermore, the pro-inflammation factor IL-10 expressions were notably increased in the I/R groups compared to sham groups (Figure 3D) as well as the expression level of IL-10 in the I/R + DFE group was obviously suppressed compared with the I/R group (Figure 3D). The above results investigated that I/R injury caused renal inflammation, and DFE could effectively repress it.
DFE Preconditioning Decreased I/R-Induced Cells Apoptosis
The number of apoptotic cells in rats renal tissues was evaluated by TUNEL assay. As shown in Figure 4A, there were only a few cells apoptosis in the sham group, but a large number of cells in I/R group. However, the number of apoptotic cells in the I/R + DFE group was significantly less than that in the I/R group. Then, the protein expressions of apoptosis-related factors containing pro-apoptosis factors (Cleaved-caspase 3, Cleaved-caspase 9 and Bax) and anti-apoptosis factor Bcl-2 were measured via Western blot. Figure 4B shows that I/R injury was accompanied by an increase in pro-apoptotic protein expression and a decrease in anti-apoptotic protein expression. It also showed that DFE preconditioning obviously inhibited the increase of pro-apoptotic protein expression and the decrease of anti-apoptotic protein expression which were induced by I/R. Cleaved-caspase 3 played an important role in cell apoptosis, it was always seen as a protein that could promote the cell apoptosis progression.24 To further verify the effect of I/R and DFE on Cleaved-caspase 3 in rats renal I/R models, IHC staining was performed to detect the expression of Cleaved-caspase 3. Compared with the I/R group, DFE treatment significantly inhibited the increase of Cleaved-caspase 3 expression caused by I/R (Figure 4C). The results of TUNEL assay, Western blot and IHC staining assay confirmed that DFE preconditioning suppressed the renal cells' apoptosis induced by I/R injury in vivo.
DFE Pretreatment Alleviated I/R-Induced Oxidative Stress and Lipid Peroxidation
To explore the effect of I/R and DFE on oxidative stress and lipid peroxidation in rats, the contents of MDA, MPO, GSH, SOD, GPx, CAT and NO in the renal cells lipid of four groups were detected by commercial kits. Briefly, the contents of peroxide-related factors that contained MDA, MPO and NO were obviously increased while the contents of antioxidant-related factors (GSH, SOD, GPx and CAT) were markedly decreased in the I/R group compared to the sham group (Figure 5A). Figure 5A also exhibited that I/R + DFE treatment significantly reduced MDA, MPO and NO contents and enhanced GSH, SOD, GPx and CAT contents when compared with I/R group. Moreover, the number of MPO-positive cells was assessed using IHC staining. Similarly, the MPO-positive cells in the I/R group were more than that in sham groups, and DFE treatment could repress the occurrence of MPO-positive cells to a certain degree (Figure 5B). The above results elucidated that I/R could result in renal oxidative stress and lipid peroxidation, and DFE inhibited the I/R-induced renal oxidative stress and lipid peroxidation in rats renal I/R models.
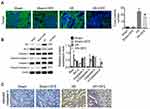
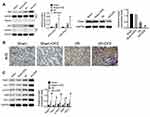
DFE Preconditioning Regulated the Nrf2-Keap1-ARE Signaling Pathway After Renal I/R Injury
To clarify the molecular mechanism of DFE acting on I/R injury, the cells' nuclear and cytoplasm of rats models kidney was separated through Nuclear and Cytoplasmic Protein Extraction Kit. And then the expression of Nrf2 in the cell cytoplasm and nuclear and the expression of Keap1 in the whole cell were detected using Western blot. Totally, Nrf2 expression in the cell cytoplasm and nuclear and Keap1 expression in the whole cell were all up-regulated in I/R groups compared with sham groups (Figure 6A). And compared with the I/R group, the I/R + DFE group significantly up-regulated the expressions of Nrf2 in the cytoplasm and nuclear and Keap1 in the whole cell (Figure 6A). Furthermore, the distribution of Nrf2 in cells nuclear and the downstream genes of Nrf2 (HO-1, NQO-1, GSR, GCLc and γ-GCS) expressions were determined by IHC staining and Western blot, respectively. Nrf2 translocation in nuclear was significantly enhanced in the I/R + DFE group compared with the I/R group (Figure 6B). Several downstream ARE genes (HO-1, NQO-1, GSR, GCLc and γ-GCS) of Nrf2 were significantly up-regulated at the protein level through enhanced Nrf2 translocation in nuclear (Figure 6C). These results illustrated that DFE regulated renal I/R injury through modulating the Nrf2-Keap1-ARE signaling pathway.
Discussion
Kidney I/R injury refers to the fact that the kidney is damaged to some extent due to the interruption or reduction of blood supply.25 After the restoration of blood supply, renal damage is aggravated rather than restored to normal. And it further leads to abnormal functions, metabolic disorders and structural damage. Renal I/R injury is the main and important cause of acute renal injury.26 Under normal physiological conditions, the kidney can self-regulate blood supply to a certain extent to maintain its stable blood perfusion.26,27 The renal cortex and medulla junction area are more sensitive to ischemia, so they are prone to damage.4 When the blood supply is restored, a large number of reactive oxygen species and inflammatory factors will accumulate, which will further aggravate the damage.28 Aseptic inflammation often occurs after renal ischemia, which is closely related to cell injury or death. The prognosis of renal I/R injury is often poor and the kidney cells are non-renewable. Therefore, it is urgent to prevent and treat I/R injury.
Inhalation anesthetics have been repeatedly reported can relieve a variety of organs and tissues sensitive to I/R injury. Riha et al29 reported that isoflurane preconditioning could relieve I/R-induced myocardial injury and arrhythmias via constructing rats myocardial I/R injury models. Ohsumi et al30 found that the early lung I/R injury could be alleviated when lungs were preconditioning or postconditioning with sevoflurane in rats lung I/R models. DFE, a commonly used inhalation anesthetic, was first synthesized by Ross Terrell in the 1960s and used clinically in the 1990s.31 The blood and tissue solubility of DFE is low, and it has the advantages of easy regulation and maintenance. And the clinical application of DFE is increasingly widespread because of its fast recovery of anesthesia.32,33 Although DFE has been reported to protect renal function and tissue after renal I/R,23 the mechanism of its action on DFE remains unclear.
Animal experimental studies have found that some methods have certain effects on the treatment of renal I/R injury.34,35 In the current study, we explored the effects of DFE on renal I/R injury and its potential mechanism by constructing rats' renal I/R injury models. The values of BUN and SCr in the kidney serum of four rat groups were detected at the beginning of this study. BUN and SCr were both commonly renal function parameters.36 The results showed that I/R injury caused an increase of BUN and SCr, and DEF preconditioning could reverse it. The ATN value was often used to assess the severity of renal injury. Therefore, the degree of renal tissue damage to four groups was assessed by HE staining, ATN value and Masson trichrome staining. Compared to sham and sham + DFE groups, significant damages were observed in the kidney extramedullary, tubular, epithelial and interstitial tissues in I/R group, and the ATN value and fibrosis of I/R group was obviously higher than that of sham groups. However, these damages, ATN value and fibrosis of rat kidney tissues in the I/R + DFE group were lighter than that in the I/R group. These results showed that DFE preconditioning could suppress the damages of kidney function caused by I/R injury.
The expressions of TNF-α, IL-1β, IL-6 and IL-10 in serum were reported to be associated with inflammation in a number of diseases, such as acute necrotizing pancreatitis,37 ankylosing spondylitis38 and neuroma.39 In the current study, the TNF-α, IL-1β, IL-6 and IL-10 expressions in kidney serum of 4 groups was measured by ELISA assay. The results showed that I/R injury enhanced pro-inflammation factors (TNF-α, IL-1β and IL-6) expressions and repressed anti-inflammation factor IL-10 expression. DFE effectively inhibited the expressions of TNF-α, IL-1β and IL-6 as well as promoted the expression of IL-10 when the kidney suffered from I/R injury. Furthermore, the cell apoptosis, the expression levels of apoptosis-related proteins (Bax, Bcl-2, Caspase 3, Cleaved-caspase 3, Caspase 9, Cleaved-caspase 9) and the expression of Cleaved-caspase 3 were determined. The above results elucidated that I/R injury increased the cell apoptosis in rats' kidney. While DFE suppressed the renal I/R injury-induced cell apoptosis. These results confirmed that DFE inhibited renal cells' inflammation and apoptosis caused by I/R injury.
Moreover, I/R injury usually resulted in oxidative stress and lipid peroxidation in kidney tissue. The contents of MDA, MPO, GSH, SOD, GPx, CAT and NO, common peroxide-related factors, in the renal lipid of four groups were assessed using corresponding commercial kits. I/R injury also resulted in an increase of the peroxide-related factors (MDA, MPO and NO) and a decrease of the antioxidant-related factors (GSH, SOD, GPx and CAT). Also, our results exhibited that I/R + DFE treatment significantly inhibited MDA, MPO and NO expressions and promoted GSH, SOD, GPx and CAT expressions when compared with the I/R group. The number of MPO-positive cells was then detected by IHC and the results showed that renal I/R injury promoted MPO cells' occurrence and DFE inhibited it. These results illustrated that DFE could alleviate the renal oxidative stress and lipid peroxidation induced by I/R injury in vivo.
Moreover, we investigated the molecular mechanism of DFE in renal I/R injury. Nrf2 is reported as a key factor in oxidative stress, which regulates the expression of antioxidant proteins and Phase II detoxifying enzymes by interacting with ARE.40 Nrf2 is located in the cytoplasm, and Nrf2 combines with Keapl to form a complex in the cytoplasm, and is rapidly degraded by ubiquitin protease, thus maintaining its low-activity physiological state.41,42 When the body is attacked by oxygen-free radicals and endogenous toxins, Nrf2 and Keapl are dissociated, and their half-life is prolonged significantly.43 Then, Nrf2 translocates into the nucleus and binds with Maf, Jun D and ATF4 to form heterodimers.43 Expression of antioxidant proteins and phase II detoxifying enzymes is induced by transcription of ARE, an antioxidant response element in heterodimers and genes.44 Up to now, more than 200 codable endogenous protective genes regulated by the Nrf2-ARE signaling pathway have been identified. They played a crucial role in enhancing tissue antioxidant capacity, protecting tissue cells from toxic damage, anti-cancer, anti-inflammation and anti-apoptosis, such as HO-1, NQO1, gamma-glutamyl cysteine synthase.45 I/R injury induces the dissociation of Nrf2 and Keap 1, and then leads to Nrf2 translocation to the nucleus, binding to ARE, and activates two antioxidant and detoxification genes, such as HO-1 and NQO1.46,47 Therefore, the up-regulation of HO-1 and NQO1 could protect the kidney from oxidative stress caused by I/R injury. In this study, we verified that DFE preconditioning attenuated renal I/R injury by activating the Nrf2-Keap1-ARE signaling pathway.
In conclusion, our study confirmed that DFE preconditioning inhibited renal functional and histological damages, inflammation, cell apoptosis, oxidative stress and lipid peroxidation induced by I/R injury in rat models through activating the Nrf2-Keap1-ARE signaling pathway. These findings might provide novel insight and evidence in the use of DFE in renal I/R injury prevent.
Disclosure
Yan Zheng and Hui Lu are co-first authors. The authors declare that they have no competing interests in this work.
References
1. Eltzschig HK, Eckle T. Ischemia and reperfusion—from mechanism to translation. Nat Med. 2011;17(11):1391. doi:10.1038/nm.2507
2. Fleming SD, Tsokos GC. Complement, natural antibodies, autoantibodies and tissue injury. Autoimmun Rev. 2006;5(2):89–92. doi:10.1016/j.autrev.2005.09.006
3. Jennings RB, Ganote CE, Reimer KA. Ischemic tissue injury. Am J Pathol. 1975;81(1):179.
4. Kurts C, Panzer U, Anders HJ, et al. The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol. 2013;13(10):738–753. doi:10.1038/nri3523
5. Mehta RL, Cerdá J, Burdmann EA, et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385(9987):2616–2643. doi:10.1016/S0140-6736(15)60126-X
6. Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380(9843):756–766. doi:10.1016/S0140-6736(11)61454-2
7. Dwyer KM, Vrazas JI, Lodge RS, et al. Treatment of acute renal failure caused by renal artery occlusion with renal artery angioplasty. Am Jo Kidney Dis. 2002;40(1):189–194. doi:10.1053/ajkd.2002.33929
8. Vaziri ND, Bai Y, Ni Z, et al. Intra-renal angiotensin II/AT1 receptor, oxidative stress, inflammation, and progressive injury in renal mass reduction. J Pharm Exp Ther. 2007;323(1):85–93. doi:10.1124/jpet.107.123638
9. Zhou S, Sun Y, Zhuang Y, et al. Effects of kallistatin on oxidative stress and inflammation on renal ischemia-reperfusion injury in mice. Curr Vasc Pharmacol. 2015;13(2). doi:10.2174/1570161113666150204142716.
10. Slofstra SH, Groot AP, Obdeijn MHP, et al. Gene expression profiling identifies C/EBPdelta as a candidate regulator of endotoxin-induced disseminated intravascular coagulation. Am J Respir Crit Care Med. 2007;176(6):602–609. doi:10.1164/rccm.200609-1250OC
11. Luh SP, Tsai CC, Shau WY, et al. Effects of gabexate mesilate (FOY) on ischemia-reperfusion-induced acute lung injury in dogs. J Surg Res. 1999;87(2):152–163. doi:10.1006/jsre.1999.5730
12. Pichlmayr I, Lehmkuhl P, Lips U. Inhalation anesthetics. Fed Proc. 1987;37(11):2501–2503.
13. Zhang Y, Dutton RC, Sonner JM. Inhaled anesthetics have hyperalgesic effects at 0.1 minimum alveolar anesthetic concentration. Anesth Analg. 2000;91(2):462–466.
14. Eger EI. Characteristics of anesthetic agents used for induction and maintenance of general anesthesia. Am J Health Syst Pharm. 2004;61 Suppl 4(20):S3–S10. doi:10.1093/ajhp/61.suppl_4.S3
15. Bodh D, Singh K, Gopinathan A, et al. Comparative evaluation of halothane and isoflurane maintenance anesthesia in water buffaloes (Bubalus bubalis). J Appl Anim Res. 2014;42(3):269–277. doi:10.1080/09712119.2013.842484
16. Péréon Y, Bernard JM, Nguyen TTS, et al. The effects of desflurane on the nervous system: from spinal cord to muscles. Anesth Analg. 1999;89(2):490–495.
17. Xia Z, Luo T. Sevoflurane or desflurane anesthesia plus postoperative propofol sedation attenuates myocardial injury after coronary surgery in elderly high-risk patients. Anesthesiology. 2004;100(4):1038–1039. doi:10.1097/00000542-200404000-00050
18. Haghighi SS, Sirintrapun SJ, Keller BP, et al. Effect of desflurane anesthesia on transcortical motor evoked potentials. J Neurosurg Anesthesiol. 1996;8(1):47–51. doi:10.1097/00008506-199601000-00011
19. Oshima Y, Sakamoto S, Yamasaki K, et al. Desflurane inhalation before ischemia increases ischemia–reperfusion-induced vascular leakage in isolated rabbit lungs. SpringerPlus. 2016;5(1):2031. doi:10.1186/s40064-016-3741-9
20. Preckel B, Schlack W, Comfere T, et al. Effects of enflurane, isoflurane, sevoflurane and desflurane on reperfusion injury after regional myocardial ischaemia in the rabbit heart in vivo. Br J Anaesth. 1998;81(6):905–912. doi:10.1093/bja/81.6.905
21. Bing Z, Xia W, Xiaoguang C, et al. Desflurane affords greater protection than halothane in the function of mitochondria against forebrain ischemia reperfusion injury in rats. Anesth Analg. 2008;106(4):1242–1249. doi:10.1213/ane.0b013e318164f2a5
22. Obal D, Dettwiler S, Favoccia C, et al. Effect of sevoflurane preconditioning on ischaemia/reperfusion injury in the rat kidney in vivo. Eur J Anaesthesiol. 2006;23(4):319–326. doi:10.1017/S0265021505002000
23. Obal D, Rascher K, Favoccia C, et al. Post-conditioning by a short administration of desflurane reduced renal reperfusion injury after differing of ischaemia times in rats. Br J Anaesth. 2006;97(6):783–791. doi:10.1093/bja/ael245
24. Duan WR, Garner DS, Williams SD, et al. Comparison of immunohistochemistry for activated caspase-3 and cleaved cytokeratin 18 with the TUNEL method for quantification of apoptosis in histological sections of PC-3 subcutaneous xenografts. J Pathol. 2003;199(2):221–228. doi:10.1002/path.1289
25. Wu H, Chen G, Wyburn KR, et al. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;82(10):2847–2859. doi:10.1172/JCI31008
26. Dobashi K, Ghosh B, Orak J, et al. Kidney ischemia-reperfusion: modulation of antioxidant defenses. Mol Cell Biochem. 2000;205(1–2):1–11. doi:10.1023/A:1007047505107
27. Park KM, Chen A, Bonventre JV. Prevention of kidney ischemia/reperfusion-induced functional injury and JNK, p38, and MAPK kinase activation by remote ischemic pretreatment. J Biol Chem. 2001;276(15):11870–11876. doi:10.1074/jbc.M007518200
28. Daemen MA, Van‘t Veer C, Denecker G, et al. Inhibition of apoptosis induced by ischemia-reperfusion prevents inflammation. J Clin Invest. 1999;104(5):541–549. doi:10.1172/JCI6974
29. Ríha H, Neckář J, Papoušek F, et al. Suppression of ischemic and reperfusion ventricular arrhythmias by inhalational anesthetic-induced preconditioning in the rat heart. Physiol Res. 2011;60(4):709. doi:10.33549/physiolres
30. Ohsumi A, Marseu K, Slinger P, et al. Sevoflurane Attenuates Ischemia-Reperfusion Injury in aRatLungTransplantation Model. The Annals of Thoracic Surgery. 2017;103(5):1578–1586. doi:10.1016/j.athoracsur.2016.10.062
31. Terrell RC. The invention and development of enflurane, isoflurane, sevoflurane, and desflurane. Anesthesiology. 2008;108(3):531–533. doi:10.1097/ALN.0b013e31816499cc
32. Patel SS, Goa KL. Desflurane. Drugs. 1995;50(4):742–767. doi:10.2165/00003495-199550040-00010
33. Fassoulaki A, Lockhart SH, Freire BA, et al. Percutaneous loss of desflurane, isoflurane, and halothane in humans. Anesthesiology. 1991;74(3):489–498. doi:10.1097/00000542-199103000-00015
34. Kelly KJ. Distant effects of experimental renal ischemia/reperfusion injury. Clin J Am Soc Nephrol. 2003;14(6):1549–1558. doi:10.1097/01.ASN.0000064946.94590.46
35. Regner KR, Zuk A, Why SKV, et al. Protective effect of 20-HETE analogues in experimental renal ischemia reperfusion injury. Kidney Int. 2009;75(5):511–517. doi:10.1038/ki.2008.600
36. Goldenberg I, Moss AJ, Mcnitt S, et al. Relation between renal function and response to cardiac resynchronization therapy in multicenter automatic defibrillator implantation trial–cardiac resynchronization therapy (MADIT-CRT). Heart Rhythm. 2010;7(12):1777–1782. doi:10.1016/j.hrthm.2010.09.005
37. Hai-Jun LI, Peng SY, Liu YB, et al. Effect of glycine on changes of TNF-α, IL-1β, IL-6, IL-8 and IL-10 in rat serum and pancreas tissue with acute necrotizing pancreatitis. Chin J Pathophysiol. 2006;22(2):239–243.
38. Gratacós J, Collado A, Filella X, et al. Serum cytokines (IL-6, TNF-α, IL-1β AND IFN-γ) in ankylosing spondylitis: a close correlation between serum IL-6 and disease activity and severity. Rheumatology. 1994;33(10):927–931. doi:10.1093/rheumatology/33.10.927
39. Khan J, Noboru N, Young A, et al. Pro and anti-inflammatory cytokine levels (TNF-α, IL-1β, IL-6 and IL-10) in rat model of neuroma. Pathophysiology. 2017;24(3):3. doi:10.1016/j.pathophys.2017.04.001
40. Zucker SN, Fink EE, Bagati A, et al. Nrf2 amplifies oxidative stress via induction of Klf9. Mol Cell. 2014;53(6):916–928. doi:10.1016/j.molcel.2014.01.033
41. Zheng S, Zhang S, Chan JY, et al. Keap1 controls postinduction repression of the Nrf2-mediated antioxidant response by escorting nuclear export of Nrf2. Mol Cell Biol. 2007;27(18):6334–6349. doi:10.1128/MCB.00630-07
42. Wruck CJ, Claussen M, Fuhrmann G, et al. Luteolin protects rat PC 12 and C6 cells against MPP + induced toxicity via an ERK dependent Keapl-Nrf2-ARE pathway. In: Neuropsychiatric Disorders An Integrative Approach. J Neural Transm Suppl. 2007;(72):57–67. doi:10.1007/978-3-211-73574-9_9.
43. Gañángómez I, Wei Y, Yang H, et al. Oncogenic functions of the transcription factor Nrf2. Free Radic Biol Med. 2013;65:750–764. doi:10.1016/j.freeradbiomed.2013.06.041
44. Li T, He S, Liu S, et al. Effects of different exercise durations on Keap1-Nrf2-ARE pathway activation in mouse skeletal muscle. Free Radic Res. 2015;49(10):1269–1274. doi:10.3109/10715762.2015.1066784
45. Hou DX, Korenori Y, Tanigawa S, et al. Dynamics of Nrf2 and Keap1 in ARE-mediated NQO1 expression by wasabi 6-(methylsulfinyl)hexyl isothiocyanate. J Agric Food Chem. 2011;59(22):11975–11982. doi:10.1021/jf2032439
46. Wang JJ, Cui P. Neohesperidin attenuates cerebral ischemia-reperfusion injury via inhibiting the apoptotic pathway and activating the Akt/Nrf2/HO-1 pathway. J Asian Nat Prod Res. 2013;15(9):1023–1037. doi:10.1080/10286020.2013.827176
47. Zeng X, Li J, Li Z. Ginsenoside Rd mitigates myocardial ischemia-reperfusion injury via Nrf2/HO-1 signaling pathway. Int J Clin Exp Med. 2015;8(8):14497–14504.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.