Back to Journals » Therapeutics and Clinical Risk Management » Volume 14
Descending necrotizing mediastinitis in a healthy young adult
Authors Ochi N , Wakabayashi T, Urakami A, Yamatsuji T , Ikemoto N, Nagasaki Y, Nakagawa N , Honda Y, Nakanishi H , Yamane H , Monobe Y, Akisada T, Katayama H , Naomoto Y , Takigawa N
Received 6 June 2018
Accepted for publication 8 September 2018
Published 17 October 2018 Volume 2018:14 Pages 2013—2017
DOI https://doi.org/10.2147/TCRM.S176520
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Nobuaki Ochi,1 Tokio Wakabayashi,2 Atsushi Urakami,3 Tomoki Yamatsuji,3 Naoto Ikemoto,4 Yasunari Nagasaki,1 Nozomu Nakagawa,1 Yoshihiro Honda,1 Hidekazu Nakanishi,1 Hiromichi Yamane,1 Yasumasa Monobe,5 Takeshi Akisada,2 Hiroshi Katayama,4 Yoshio Naomoto,3 Nagio Takigawa1
1Department of General Internal Medicine 4, Kawasaki Medical School, Okayama, Japan; 2Department of Otolaryngology, Kawasaki Medical School, Okayama, Japan; 3Department of General Surgery, Kawasaki Medical School, Okayama, Japan; 4Department of Anesthesiology and Intensive Care Medicine, Kawasaki Medical School, Okayama, Japan; 5Department of Pathology, Kawasaki Medical School, Okayama, Japan
Abstract: A 26-year-old man with right lower mandibular and chest pain, fever, and respiratory distress was urgently transported to our hospital. CT images revealed gas collection and an abscess from the neck to the mediastinum with bilateral pleural effusion. Descending necrotizing mediastinitis (DNM) induced by an odontogenic infection of a right mandibular molar abscess was diagnosed. The cervical and mediastinal areas were drained, extensive debridement was performed, necrotic tissue was excised, and broad-spectrum antibiotics were administered immediately. Prompt diagnosis and intensive care were necessary for managing the DNM, and the patient was discharged with no comorbidities.
Keywords: descending necrotizing mediastinitis, odontogenic infection, healthy young adult
Introduction
Descending necrotizing mediastinitis (DNM) is one of the most lethal forms of mediastinitis, and most commonly caused by odontogenic infections or deep cervical infections.1 The infection spreads to the mediastinum via the cervical fascial plane. The mortality rate of DNM is 25%–40% in the literature.2 Delay in the diagnosis, a treatment approach involving appropriate antibiotics, drainage of the mediastinum, or debridement leads to the high mortality rate in this life-threatening condition. Here, we report a healthy young adult with DNM who recovered from this dreadful condition due to prompt diagnosis and intensive care.
Case report
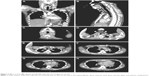
A 26-year-old man with progressive chest pain and respiratory distress was transported as an emergency case to our hospital from the local airport. He had just returned to Japan from traveling in southeastern Asia for 1 week. He had no relevant past medical history and was not on medication. He had developed pain in the right lower mandibular area over the past week. The pain had progressed from the neck to the chest; he developed a high fever during his journey and could not eat or drink. In the emergency room, peripheral oxygen saturation was 95% with receiving oxygen at 15 L/min via an oxygen mask and a reservoir bag. A chest X-ray showed a slightly widened mediastinum with pleural effusion (Figure 1A). An ECG revealed widespread ST-elevation (Figure 1B). Physical examination revealed a body mass index of 23.0 kg/m2 (height 168.0 cm; weight 65.0 kg), blood pressure of 121/68 mmHg, average heart rate of 110/min, respiratory rate of 40 breaths/min, body temperature of 37.9°C, and tenderness and redness in the anterior and bilateral sides of the neck. White blood cell count was 14,510/μL, neutrophils were 11,320/μL, red blood cell count was 5.07×106/μL, hemoglobin was 14.7 g/dL, and the platelet count was 196,000/μL. C-reactive protein level was elevated at 54.52 mg/dL (ref. 0.0–0.14 mg/dL). Blood urea nitrogen and creatinine levels were 45 mg/dL (ref. 8–20 mg/dL) and 1.06 mg/dL (ref. 0.65–1.07 mg/dL), respectively. Lactate dehydrogenase was elevated to 510 U/L (ref. 124–222 U/L). Creatine kinase and creatine kinase-MB were 269 (ref. 59–248 U/L) and 11.6 (ref. 0.0–12.0 U/L), respectively. The fasting blood glucose and HbA1c levels were normal. Although these findings indicated the possibility of acute pericarditis, an ECG did not show any findings of pericarditis or myocardial dysfunction. Contrast-enhanced CT images revealed that a large volume of gas had collected in the neck and upper and anterior mediastinum (Figure 2A–F). In addition, an abscess had formed in the retropharyngeal, bilateral submandibular, upper paratracheal, and mediastinal spaces with pleural effusion and atelectasis in the right lower basal lobe (Figure 2B–H). Thus, DNM induced by odontogenic infection with a right mandibular second or third molar abscess was highly suspected.
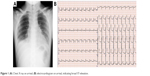  | Figure 1 (A) Chest X-ray on arrival; (B) electrocardiogram on arrival, indicating broad ST-elevation. |
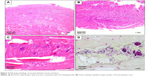
A multidisciplinary team, including physicians, otolaryngologists, surgeons, and intensivists commenced treatment within 2 hours of arrival. He received intravenous 600 mg clindamycin bid and 4.5 g tazobactam/piperacillin hydrate qid. A tracheostomy, bilateral neck drainage, neck fasciotomy, and debridement via a cervical incision were performed 5 hours after arrival. The blood culture on admission was negative. Gram-positive cocci were found in sputum and pus, and Streptococcus pyogenes, Streptococcus constellatus, Fusobacterium necrophorum, and Peptostreptococcus sp. were found later in pus culture. Continuous bilateral thoracic drainage began due to the development of a bilateral pyothorax. Subsequently, the transcervical mediastinal and right mediastinal areas were drained via thoracotomy. His condition improved steadily, and he was discharged without any comorbidities on hospital day 63 after admission. Pathological findings from the excised skeletal muscle and fascia indicated acute inflammation with necrosis (Figure 3A–C), consistent with necrotizing fasciitis, and a large number of Gram-positive cocci were observed (Figure 3D). Thus, the radiological diagnosis of DNM was confirmed histologically and bacteriologically.
Ethics approval and consent to participate
Authors’ institution does not require ethical approval for publication of a single case report. Written informed consent for publication of clinical details and images was obtained from the patient.
Discussion
We experienced a patient with severe DNM. His diagnosis was promptly provided and he was successfully treated with intensive care. Acute mediastinitis is a serious infection involving the connective tissue, which fills the interpleural spaces. The early concept of DNM was described by Pearse in 1938.3 In that study, 110 patients with suppurative mediastinitis from a descending cervical infection and 21 cases secondary to oropharyngeal infection were reported, and the mortality rate was 55%.3 The modern concept of DNM was established by Estrera et al.1 They defined the diagnostic criteria as: clinical manifestations of severe infection, establishment of a relationship between an oropharyngeal or cervical infection and subsequent mediastinitis, demonstration of radiographic features characteristic of DNM, and documentation of a necrotizing mediastinal infection at the time of operative debridement or necropsy.
The most common primary oropharyngeal infection is an odontogenic infection with a mandibular second or third molar abscess.4 Delay in the diagnosis is one of the main causes for the high mortality in patients with DNM. If a physician suspects DNM from a medical interview, a radiographic evaluation should be performed as soon as possible. Chest X-ray findings of DNM, including widening of the retrovisceral space with or without air-fluid, anterior displacement of the trachea, mediastinal emphysema, and widening of the superior mediastinal shadow are observed in only 25% of patients.5 Although a chest X-ray was insufficient for an early diagnosis of DNM in the present case, the CT images clearly indicated DNM.2,5
Mediastinitis and pericarditis often produce similar clinical symptoms and ECG findings, such as widespread ST-segment elevation.6–8 Cho et al reported a patient with DNM, who manifested a low-grade fever, sore throat, cough, and progressive chest pain, with diffuse ST-segment elevation mimicking pericarditis.9 The chest X-ray showed a widened mediastinum and thickening of the bronchial wall. In the present case, the ECG findings and clinical symptoms, including chest pain, shortness of breath, and high fever were consistent with pericarditis. However, the patient originally suffered from sustained and progressive lower mandibular pain while traveling and high fever with neck swelling and tenderness, indicating the existence of a suppurative deep neck infection.
Because the majority of deep neck infections are polymicrobial-mixed aerobic and anaerobic pathogens, broad-spectrum antibiotic therapy is desirable. In 270 cases of deep neck space infection, the major causative organisms were S. pyogenes (30.37%), Streptococcus viridans (12.96%), and Staphylococcus aureus (22.97%). Anaerobic pathogens were also detected in 16.3% (Bacteroides in 8.52% and Peptostreptococcus in 7.78%).11 Therefore, broad-spectrum antibiotics should be started, followed by modification based on the culture and drug sensitivity test results. Latent risk factors for deep neck infections, including DNM, are compromising situations, such as diabetes mellitus, heavy smoker, alcoholic, and immunodeficient status or receiving immunosuppressive therapies.10–13 In the present case, the patient had none of these risk factors and he had an odontogenic infection with overt symptoms. Ridder et al reported only 13.3% (6/45) patients presented with DNM had no preexisting comorbidity based on their retrospective single institutional study.14 Although it is possible that delays in dental or medical checks while traveling might affect DNM development, such a diagnosis should be considered even in patients without immunosuppressive conditions or disease.
Of course, the optimal surgical approach, including mediastinal drainage, surgical extensive debridement, and excision of necrotic tissue and the appropriate medical management are indispensable for recovery from this life-threatening disease. Several surgical approaches to treat DNM have been described in the literature; however, the optimal surgical strategy or standard surgical approach to DNM remains controversial.15 Although transcervical drainage is usually effective in patients with acute mediastinitis due to cervical esophageal perforation, it is insufficient to salvage patients with DNM in many cases.4
Palma et al reported some factors associated with intensive care unit (ICU) mortality of patients with DNM based on the prospective analysis of 34 cases.16 High age, high simplified acute physiology score (SAPS) II score, severe DNM type, and longer ICU stay were significantly associated with high mortality. According to their criteria, this patient was fortunately classified into the better outcome group. However, they also emphasized prompt admission to the ICU in order to manage general condition, as early and aggressive surgical approach are key factors in reducing mortality. Because of the development of treatment approach to DNM, actual mortality rate of DNM has improved recently compared with the past reports (11.1%–17.5%).14,17,18
In conclusion, a delayed diagnosis and inadequate surgical approach put patients at high risk of death from DNM. If a patient is diagnosed with DNM, a multidisciplinary team, including physicians, otolaryngologists, surgeons, and intensivists should promptly, and in a coordinated fashion, treat the patient.
Disclosure
The authors report no conflicts of interest in this work.
References
Estrera AS, Landay MJ, Grisham JM, Sinn DP, Platt MR. Descending necrotizing mediastinitis. Surg Gynecol Obstet. 1983;157(6):545–552. | ||
Freeman RK, Vallières E, Verrier ED, Karmy-Jones R, Wood DE. Descending necrotizing mediastinitis: an analysis of the effects of serial surgical debridement on patient mortality. J Thorac Cardiovasc Surg. 2000;119(2):260–267. | ||
Pearse HE. Mediastinitis following cervical suppuration. Ann Surg. 1938;108(4):588–611. | ||
Wheatley MJ, Stirling MC, Kirsh MM, Gago O, Orringer MB. Descending necrotizing mediastinitis: transcervical drainage is not enough. Ann Thorac Surg. 1990;49(5):780–784. | ||
Marty-Ané CH, Berthet JP, Alric P, Pegis JD, Rouvière P, Mary H. Management of descending necrotizing mediastinitis: an aggressive treatment for an aggressive disease. Ann Thorac Surg. 1999;68(1):212–217. | ||
Catarino PA, Westaby S. Postcardiac surgery mediastinitis mimicking acute inferior myocardial infarction. J Card Surg. 2000;15(5):309–312. | ||
Wang K, Asinger RW, Marriott HJ. ST-segment elevation in conditions other than acute myocardial infarction. N Engl J Med. 2003;349(22):2128–2135. | ||
Lange RA, Hillis LD. Clinical practice. Acute pericarditis. N Engl J Med. 2004;351(21):2195–2202. | ||
Cho YS, Choi JH. Descending necrotizing mediastinitis with diffuse ST elevation mimicking pericarditis: a case report. J Emerg Med. 2014;47(4):408–411. | ||
Gujrathi AB, Ambulgekar V, Kathait P. Deep neck space infection – a retrospective study of 270 cases at tertiary care center. World J Otorhinolaryngol Head Neck Surg. 2016;2(4):208–213. | ||
Mihos P, Potaris K, Gakidis I, Papadakis D, Rallis G. Management of descending necrotizing mediastinitis. J Oral Maxillofac Surg. 2004;62(8):966–972. | ||
Huang TT, Liu TC, Chen PR, Tseng FY, Yeh TH, Chen YS. Deep neck infection: analysis of 185 cases. Head Neck. 2004;26(10):854–860. | ||
Ohsaki A, Shirasaki F, Hirooka N. Chest Necrotizing Fasciitis with Mediastinitis. Intern Med. 2017;56(13):1755–1756. | ||
Ridder GJ, Maier W, Kinzer S, Teszler CB, Boedeker CC, Pfeiffer J. Descending necrotizing mediastinitis: contemporary trends in etiology, diagnosis, management, and outcome. Ann Surg. 2010;251(3):528–534. | ||
Singhal P, Kejriwal N, Lin Z, Tsutsui R, Ullal R. Optimal surgical management of descending necrotising mediastinitis: our experience and review of literature. Heart Lung Circ. 2008;17(2):124–128. | ||
Palma DM, Giuliano S, Cracchiolo AN, et al. Clinical features and outcome of patients with descending necrotizing mediastinitis: prospective analysis of 34 cases. Infection. 2016;44(1):77–84. | ||
Makeieff M, Gresillon N, Berthet JP, et al. Management of descending necrotizing mediastinitis. Laryngoscope. 2004;114(4):772–775. | ||
Suehara AB, Gonçalves AJ, Alcadipani FA, Kavabata NK, Menezes MB. Deep neck infection: analysis of 80 cases. Braz J Otorhinolaryngol. 2008;74(2):253–259. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.