Back to Journals » OncoTargets and Therapy » Volume 13
Derlin-1 Promotes the Progression of Human Hepatocellular Carcinoma via the Activation of AKT Pathway
Authors Fan J, Tian L , Huang S , Zhang J , Zhao B
Received 11 July 2019
Accepted for publication 16 May 2020
Published 11 June 2020 Volume 2020:13 Pages 5407—5417
DOI https://doi.org/10.2147/OTT.S222895
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Federico Perche
Jiye Fan,1,2 Liying Tian,2 Shuhong Huang,3 Jing Zhang,2 Baohua Zhao1
1Life Science of College, Hebei Normal University, Shijiazhuang, Hebei 050024, People’s Republic of China; 2Department of Pharmacy, Hebei Chemical and Pharmaceutical College, Shijiazhuang, Hebei 050026, People’s Republic of China; 3Department of Neurobiology, Shandong Provincial Key Laboratory of Mental Disorders, School of Basic Medical Science, Shandong University, Jinan, Shandong 250012, People’s Republic of China
Correspondence: Baohua Zhao
Life Science of College, Hebei Normal University, South Second Ring East Road 20, Shijiazhuang, Hebei 050024, People’s Republic of China
Tel/Fax +86 311 8078 9712
Email [email protected]
Introduction: Hepatocellular carcinoma (HCC) is the third leading cause of cancer death worldwide. In the present research, we explored a new oncogene, derlin-1 (DERL1), and studied its role and mechanism in human HCC.
Methods: We assessed the expression and prognosis value of DERL1 in human HCC by using GEPIA dataset analysis and immunohistochemistry. To elucidate the specific function of DERL1, we suppressed its expression in two HCC cell lines, HuH7 and Hep3B, and overexpressed DERL1 in Hep3B cells. Cell proliferation and migration was detected by CCK8 and transwell assays. Cell flow cytometry was used to evaluate cell apoptosis.
Results: Our results demonstrated that DERL1 was highly expressed in HCC samples (n = 369) than in normal samples (n = 160). Similar results were obtained in 60 clinical samples that we collected from the local hospital. The high expression rate of DERL1 reached 78.3% (47/60). DERL1 overexpression samples were concentrated in patients with tumor diameters > 5cm or lymph node metastases. Thus, we speculated that DERL1 operated as a tumor promotor in HCC, and its expression might be proposed as a predictor for tumor metastasis of human HCC. Interference of DERL1 markedly blocked cell proliferation and migration, and induced the apoptosis of HCC cells in vitro. Phosphorylation of Akt was significantly inhibited in cells transfected with DERL1 siRNA compared to their control cells in HuH7 and Hep3B cell lines. The opposite result was observed in the DERL1 overexpression cells.
Conclusion: Our findings prove that DERL1 promotes tumor progression via AKT pathway and provide a new potential target for the clinical treatment and diagnosis of human HCC.
Keywords: derlin-1, hepatocellular carcinoma, proliferation, apoptosis
Introduction
Primary liver cancer (PLC) is the sixth most common tumor and is the third leading cause of cancer death in the world. Every year, about 10,000 people worldwide suffer from this disease and thus die, of which over 90% are patients with hepatocellular carcinoma (HCC). The high incidence of liver diseases, such as hepatitis and cirrhosis, is also a hidden danger for the deterioration of HCC. In many cases, the surgical resection rate of HCC is only 20% to 30%; 5-year survival rate is 30% to 50%; the recurrence and metastasis rates are more than 60%.1–3 The low resection and high recurrence rates of surgical operation force people to further study the molecular mechanism of the pathogenesis, metastasis and invasion of HCC, so as to develop more effective early diagnosis and treatment methods.4,5
In the present research, we explored a new oncogene, derlin-1 (DERL1), and studied its role and mechanism in human HCC. The protein encoded by DERL1 is an endoplasmic reticulum (ER)-associated protein, which participate in ER-associated degradation response and unfolded protein response (UPR).6–8 ER Stress and UPR can be observed in various tumors, which play important roles in tumorigenesis, and are closely related to tumor cell survival, tumor angiogenesis and apoptosis.9–12 In the process of ER Stress, the expression level of DERL1 is up-regulated, and it can inhibit the apoptosis induced by ER Stress.13,14 Studies have confirmed that DERL1 protein can penetrate from ER to the cell membrane in tumor cells, and its high expression was observed in pancreatic cancer, liver cancer, breast cancer, colon cancer and esophageal cancer.15–21 DERL1 is overexpressed in breast cancer tissues and is associated with lymph node metastases in breast cancer.
In this research, we investigate the role of DERL1 in proliferation, migration and apoptosis of human HCC cells. The purpose of the present study was to determine the functionary mechanism of DERL1 in vitro, and explore its potential as a new molecular marker and therapeutic target in human HCC.
Materials and Methods
Immunohistochemistry (IHC) and Histological Analysis
The HCC samples were fixed in 4%polyformaldehyde and followed by being embedded in paraffin wax. Sections were cut a thickness of 4 μm. Antigen retrieval was done by heating in a microwave for 12 min. Slides were blocked with goat blocking serum for 40 min and then were incubated with DERL1 antibody (Abcam, USA) at room temperature for 1 hour. The antibody binding was visualized using DAB Kit. Sections were then stained with hematoxylin. The result was observed by a microscope and all image processing was done using ImageJ.
Cells and Media
Human HCC cell lines, HuH7 and Hep3B, were purchased from the Type Culture Collection of the Chinese Academy of Sciences. HuH7 and Hep3B were cultured in Dulbecco’s modified Eagle’s medium (DMEM) medium containing 10% fetal bovine serum (FBS). Cells reached approximately 70% confluency were transfected with DERL1-specific siRNA (KD), control siRNA (CON), DERL1 overexpression plasmid (OE) or empty plasmid (C-OE) by Lipofectamine 6000 according to manufacturer’s guidelines.
Real-Time Fluorescence Quantitative PCR (qPCR)
Total RNA was acquired from HuH7 and Hep3B after transfected with DERL1 or control siRNA for 48 hours and reverse transcribed with Reverse Transcription Reaction Kit (CWBIO, China) following the manufacturer’s instructions. DERL1 primers used for Real-time fluorescence quantitative PCR were as follows: For, 5ʹ - CACCTCAGTTTTTGTACCGCTG-3ʹ; Rev, 5ʹ- TCACTGGTCTCCAAGTCGAAAG −3ʹ. Cycling conditions were as follows: initial denaturation at 95°C for 30 seconds; 95°C for 5 seconds, 60°C for 30 seconds, 40 cycles. qPCR was performed on an H-4800 Real-Time PCR System following the manufacturer’s instructions. The related mRNA level was normalized to GAPDH. Three repeats were performed in qPCR assay.
Western Blot Analysis
For immunoblot analysis, total lysates of HuH7 and Hep3B cells were collected using RIPA buffer after transfected for 48 hours. Proteins were separated on 10% SDS-PAGE by electrophoresis followed by transfer to a PVDF membrane. Proteins were blocked with 5% blocking liquid and probed with the following primary antibodies (1:1000): DERL1 (Abcam, UK), Active Caspase3 (ProteinTech Group, USA), Bcl2 (ProteinTech Group, USA), Bax (ProteinTech Group, USA), AKT (Abcam, UK), p-AKT (Abcam, UK), cyclin D1 (ProteinTech Group, USA), p70 (Abcam, UK) and GAPDH (Abcam, UK). The immunoblot analysis was subsequently performed with ECL chemiluminescence. The intensity of proteins was quantified using Image J software. Western blot analysis was performed in triplicate.
Cell Proliferation Assay
The proliferation of HuH7 and Hep3B cells was detected using Cell Counting Kit-8 (CCK-8).
Cells were transfected as described above followed by transfer into a 96-well plate at concentration of 3000 cells/well. Each experiment was performed in triplicate. The well was incubated with 10 μL CCK-8 solutions for 2 hours. Then, OD values were quantified on a microplate reader.
Migration Assay
Cell migration ability was estimated via transwell assay by using transwell chamber (Millipore, USA) according to the manual. Cells transfected with siRNA were seeded on a chamber at a density of 1×105 cells/well and incubated for 48 hours. The chamber was fixed with 4% polyformaldehyde for 20 min, and then stained with 0.1% crystal violet for 10 min. The migration cell numbers were counted under a microscope in five random fields. Migrated cells were analyzed from triplicate determinations.
Apoptosis Detection
Cell flow cytometry was used to evaluate cell apoptosis by fluorescent labeling of propidium iodide (PI) and Annexin v-FITC on cell surface. The cells were seeded and transfected as described above. After transfected for 36 hours, cells were starved for 24 h and then stained with PI and Annexin v-FITC. Apoptosis were analyzed using flow cytometry and Flowjo software from triplicate determinations.
Statistical Analyses
All results in this study were expressed as mean ± standard deviation (SD). Data were evaluated using GraphPad Prism 7 (GraphPad software, Inc., USA) and SPSS v17.0 (SPSS Inc., USA). Statistical difference between CON and KD group were determined using the T-test to determine which groups differed. Differences with P value less than 0.05 were considered statistically significant.
Results
DERL1 Is Overexpressed in Human HCC
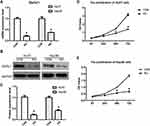
With the development of sequencing technology, it is possible to explore the molecular mechanism of cancer through extensive cooperation by using large-scale sequencing-based genomic analysis. In 2017, Tang et al developed an interactive web server GEPIA, which could analyze the RNA sequencing expression data from the TCGA and the GTEx projects using a standard processing pipeline.22 Here, we assessed DERL1 expression level and prognosis value in human HCC using GEPIA. As shown in Figure 1A, a boxplot was plotted for human liver hepatocellular carcinoma (LIHC) patients, which were divided into DERL1 high expression group and low expression group. From these results, DERL1 level in tumor (red box) was observed significantly higher than that in normal tissues (grey box) (P<0.05). In order to determine if there is a correlation between DERL1 expression and prognosis of patients with HCC, overall survival and disease-free survival curves were plotted. As shown in Figure 1B and C, there was no significant correlation between derl1 expression and prognosis of HCC patients (P>0.05).
DERL1 Overexpression Predicts Tumor Metastasis and Increased Diameter
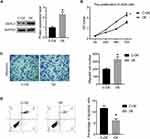
In order to further confirm DERL1 overexpressed in HCC, we collected tumor and adjacent normal tissues of 60 cases patients with HCC. All tissues were fixed and made into paraffin sections. Immunohistochemistry was performed to investigate DERL1 expression and its correlation with clinicopathologic features. Indeed, upregulation of DERL1 protein level was observed in HCC tumor tissues in comparison to adjacent normal tissues as shown in Figure 2A. DERL1 expressions were analyzed in each tissue using the methods described in materials and methods. The results showed that there were 47 cases of DERL1 high expression and 13 cases of low expression in 60 patients. High expression rate reached 78.3% (47/60). The relationship between DERL1 level and clinicopathologic feature was analyzed by χ2 test (Figure 2B). There is no significant difference in DERL1 expression among patients of different ages and sexes. Interestingly, DERL1 level was found to be higher in HCC patients with tumor diameter >5cm than that in patients with tumor diameter ≤ 5cm (P=0.0234), and also higher in patients with lymph-node metastasis than that in patients without lymph-node metastasis (P=0.0474), suggesting that DERL1 overexpression predicts tumor metastasis and increased diameter in human HCC (Figure 2B).
DERL1 Knockdown Inhibited Cell Proliferation and Migration
Although bioinformatics analysis and tissue staining suggests the upregulation of DERL1 in human HCC, it cannot determine whether DERL1 contributes to the occurrence and development of HCC. In order to confirm the regulatory role of DERL1 on oncology, DERL1 siRNA was transfected into human HCC cell lines, HuH7 and Hep3B, to build DERL1 knockdown cell lines (KD group) with control siRNA as the negative control (CON group). qPCR and Western blot were performed to detect DERL1 expression on mRNA and protein level, respectively. The data from Figure 3A–C confirmed that DERL1 expression was significantly suppressed in KD cells, which were used for subsequent functional experiments. The effect of DERL1 on proliferation was investigated by using CCK8 assay. As shown in Figure 3D and E, OD values of KD group after transfected with DERL1 siRNA for 48 h significantly decreased in comparison to CON group in HuH7 and Hep3B cells, suggesting a cell proliferation inhibition effect of DERL1 deletion.
Transwell assay was performed to detect cell migration ability of HuH7 and Hep3B cells as shown in Figure 4. The migration cell numbers of KD cells were 240±20, reduced significantly compared with that of CON cells, 50±10 in HuH7. Similar results were obtained in other human HCC cell line, Hep3B. The migration cell numbers of KD cells were 270±15, reduced significantly compared with that of CON cells, 110±23 in Hep3B. These results proved that suppression of DERL1 could prevent the migration of human HCC cells in vitro.
DERL1 Knockdown Induced Cell Apoptosis
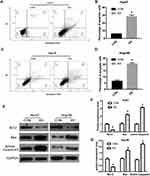
To evaluate the effect of DERL1 on cell apoptosis, DERL1 siRNA were transfected into HuH7 and Hep3B cells. Thirty-six hours later, cells were starved for 12 hours, and then harvested and stained with PI and AnnexinV FITC. The results of flow cytometry are shown in Figure 5A–D. Based on these results, apoptotic percentage of HuH7 and Hep3B cells transfected with DERL1 siRNA increased significantly compared with HuH7 and Hep3B CON cells, respectively, suggesting that DERL1 knockdown could promote the apoptosis of human HCC cells. The above changes of cell apoptosis actually should be caused by the expression changes of apoptotic factors. Hence, we next detected the expressions of apoptosis-related protein and found that Active Caspase3 and Bax, two important apoptotic factors, significantly increased in KD cells in comparison to the CON cells. On the contrary, the apoptosis inhibitory factor, Bcl-2 sharply reduced in KD cells compared with their CON cells in HuH7 and Hep3B cell line (Figure 5E–G). The above findings suggest that DERL1 knockdown induced cell apoptosis in human HCC cell lines.
Overexpression of DERL1 Promoted the Growth and Migration in HCC Cells
To further confirm the effect of DERL1 on HCC cells, we constructed the DERL1 overexpression plasmid and transfected it into Hep3B cells with an empty plasmid as the control (C-OE). As shown in Figure 6A, the protein level of DERL1 was significantly increased after the transfection of overexpression plasmid. Further, CCK8, transwell and flow cytometry were performed to detect the biological behavior of Hep3B cells. As shown in Figure 6B, OD values of OE group significantly increased in comparison to C-OE group in Hep3B cells. The migration cell numbers of OE cells were 330±35, increased significantly compared with that of C-OE cells, 200±15 in HuH7 (Figure 6C). These results proved that overexpression of DERL1 could promote the proliferation and migration of human HCC cells in vitro. Based on the results of flow cytometry, apoptotic percentage of HuH7 cells transfected with DERL1 overexpression plasmid decreased significantly compared with C-OE cells, suggesting a cell apoptosis inhibition effect of DERL1 overexpression (Figure 6D).
DERL1 Regulated the Activity of Akt Signaling Pathway
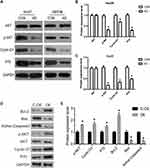
To investigate the molecular and cellular mechanisms of DERL1 promoted tumor progression, the key genes in the tumor-related pathway were detected by Western blot. As shown in Figure 7A–C, phosphorylation of Akt significantly decreased in cells transfected with DERL1 siRNA compared to their control cells in HuH7 and Hep3B cell lines. The opposite result was observed in the DERL1 overexpression cells (Figure 7D and E). As key proteins of cell proliferation, P70 and cyclin D1 were also observed to reduce in cells transfected with DERL1 siRNA in HuH7 and Hep3B cell lines. These results demonstrated that DERL1 knockdown promoted the proliferation by inhibiting the activity of Akt signaling pathway in human HCC cells.
Discussion
DERL family consists of three members, DERL1, DERL2 and DERL3, are located in the ER membrane, mainly involved in the degradation of misfolded protein.23 The human DERL1 gene is located on the 8q24.13 and encodes a protein consisting of 251 amino acids.24 As an ER-associated protein involved in ER stress, the important role of DERL1 in tumors has been gradually recognized in recent years. DERL1 expression is upregulated in various malignant tumors, and several publications have reported its important role in tumor. In a related study, DERL1 is overexpressed in human breast carcinoma and protects cancer, and the expression of DERL1 was associated with the grade and axillary lymph node metastasis of human breast carcinoma patients.18 A recent research demonstrates that DERL1 is overexpressed and promotes malignant phenotype via PI3K/AKT/and ERK pathway in bladder cancer.19 In non-small cell lung cancer (NSCLC), DERL1 promotes tumor cell invasion through EGFR/ERK-mediated upregulation of MMP-2 and MMP-9.21 The overexpression of DERL1 is also observed in human colorectal cancer and cervical cancer.
However, its expression and function in human HCC remains unknown. In the present study, we assessed the expression and prognosis value of DERL1 in human HCC by using TCGA and GTEx dataset analysis. Our results demonstrated that DERL1 was abnormal high expression in HCC samples (n = 369) than in normal samples (n = 160). Similar results were obtained in 60 cases clinical samples that we collected from the local hospital. The high expression rate of DERL1 reached 78.3% (47/60). Interestingly, patients with a tumor diameter >5cm have a high rate of DERL1 overexpression, as well as patients with lymph-node metastasis. Thus, we speculated that DERL1 operated as a tumor promotor in HCC, and its expression was posed as a predictor for tumor metastasis of human HCC.
To elucidate the specific function of DERL1, we suppressed its expression in two human HCC cell lines, HuH7 and Hep3B. Interference of DERL1 markedly blocked cell proliferation and migration, and induced the apoptosis of HCC cells in vitro. After overexpression of derl1, the proliferation and migration ability of cells increased significantly, while the number of apoptosis decreased significantly. In brief, our findings highlight the positive role of DERL1 in tumorigenesis of human HCC. Studies have shown that DERL1 affects tumor growth through Akt and ERK pathways in breast cancer and NSCLC.21,25 Therefore, we detected the expression and activity of key proteins in Akt and ERK pathways in cell groups. Phosphorylation of Akt was significantly inhibited in cells transfected with DERL1 siRNA compared to their control cells in HuH7 and Hep3B cell lines, while there is only a little trend (Hep3B) or no trend (HuH7) in phosphorylation of ERK (data not shown). Thus, we predicted that DERL1 knockdown promoted the proliferation by inhibiting the activity of Akt signaling pathway in human HCC cells. This result may be due to the organizational differences or limitations of in vitro experiments, suggesting that the mechanism by which DERL1 regulates cell activity still needs further study in more HCC cell lines and animal model.
In conclusion, the present study demonstrates that DERL1 is upregulated in HCC, and the first to report that DERL1 may be critical in the development and progression of HCC, which indicates that DERL1 may be a potential predictor of the progression of HCC. Furthermore, DERL1 contributes to the biological behavior regulation by targeting the Akt signaling pathway in HCC. Our findings expand the understanding of DERL1 functions in promoting solid tumor growth, and provide experimental and theoretical basis for further clinical application.
Data Sharing Statement
The data of this study are available. Readers can access the data from the manuscript or access them by contacting with the corresponding author.
Ethics and Consent
This research has been carried out in accordance with the World Medical Association Declaration of Helsinki, and that all subjects provided written informed consent. The study was done after agreement from the ethics committee of Hebei Normal University and with the patients’ informed consent.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Frcpc BAK, Reddy KR, Luigi Bolondi MD. Hepatocellular Carcinoma; CRC Press, 2009.
2. She WH, Chok KS. Strategies to increase the resectability of hepatocellular carcinoma. World J Hepatol. 2015;7:2147. doi:10.4254/wjh.v7.i18.2147
3. Lee JG, Kang CM, Park JS, et al. The actual five-year survival rate of hepatocellular carcinoma patients after curative resection. Yonsei Med J. 2006;47(1):105–112. doi:10.3349/ymj.2006.47.1.105
4. Qin G, Dang M, Gao H, Wang H, Luo F, Chen R. Deciphering the protein-protein interaction network regulating hepatocellular carcinoma metastasis. Biochim Biophys Acta. 2017;1865:1114–1122.
5. Cui H, Zhang Y, Zhang Q, Chen W, Zhao H, Liang J. A comprehensive genome-wide analysis of long noncoding RNA expression profile in hepatocellular carcinoma. Cancer Med. 2017;6(12):2932. doi:10.1002/cam4.1180
6. Eshraghi A, Dixon SD, Tamilselvam B, et al. Cytolethal distending toxins require components of the ER-associated degradation pathway for host cell entry. PLoS Pathog. 2014;10:e1004295. doi:10.1371/journal.ppat.1004295
7. Brambilla PG, Molinari M. Five questions (with their answers) on ER-associated degradation. Traffic. 2016;17(4):341. doi:10.1111/tra.12373
8. Chen CY, Malchus NS, Hehn B, et al. Signal peptide peptidase functions in ERAD to cleave the unfolded protein response regulator XBP1u. EMBO J. 2014;33:2492. doi:10.15252/embj.201488208
9. Verfaillie T, Garg AD, Agostinis P. Targeting ER stress induced apoptosis and inflammation in cancer. Cancer Lett. 2013;332(2):249. doi:10.1016/j.canlet.2010.07.016
10. Kania E, Pająk B, Orzechowski A. Calcium homeostasis and ER stress in control of autophagy in cancer cells. Biomed Res Int. 2015;2015:352794. doi:10.1155/2015/352794
11. Liu Y, Gong W, Yang ZY, et al. Quercetin induces protective autophagy and apoptosis through ER stress via the p-STAT3/Bcl-2 axis in ovarian cancer. Apoptosis. 2017;22(4):544–557. doi:10.1007/s10495-016-1334-2
12. Tsai YC, Weissman AM. The unfolded protein response, degradation from the endoplasmic reticulum, and cancer. Genes Cancer. 2010;1(7):764. doi:10.1177/1947601910383011
13. Nishitoh H, Kadowaki H, Nagai A, et al. ALS-linked mutant SOD1 induces ER stress- and ASK1-dependent motor neuron death by targeting Derlin-1. Genes Dev. 2008;22(11):1451. doi:10.1101/gad.1640108
14. Liang CJ, Chang YC, Chang HC, et al. Derlin-1 regulates mutant VCP-linked pathogenesis and endoplasmic reticulum stress-induced apoptosis. PLoS Genet. 2014;10:e1004675. doi:10.1371/journal.pgen.1004675
15. Ran Y, Hu H, Hu D, et al. Derlin-1 is overexpressed on the tumor cell surface and enables antibody-mediated tumor targeting therapy. Clin Cancer Res. 2008;14(20):6538–6545. doi:10.1158/1078-0432.CCR-08-0476
16. Xu L, Wang ZH, Xu D, et al. Expression of Derlin-1 and its effect on expression of autophagy marker genes under endoplasmic reticulum stress in lung cancer cells. Cancer Cell Int. 2014;14:50. doi:10.1186/1475-2867-14-50
17. Tan X, He X, Jiang Z, et al. Derlin-1 is overexpressed in human colon cancer and promotes cancer cell proliferation. Mol Cell Biochem. 2015;408(1–2):205–213. doi:10.1007/s11010-015-2496-x
18. Wang J, Hua H, Ran Y, et al. Derlin-1 is overexpressed in human breast carcinoma and protects cancer cells from endoplasmic reticulum stress-induced apoptosis. Breast Cancer Res. 2008;10(1):R7. doi:10.1186/bcr1849
19. Dong Q, Lin F, Yue Z, Tan S, Wang E. Derlin-1 overexpression confers poor prognosis in muscle invasive bladder cancer and contributes to chemoresistance and invasion through PI3K/AKT and ERK/MMP signaling. Oncotarget. 2017;8:17059. doi:10.18632/oncotarget.15001
20. Wu Z, Wang C, Zhang Z, et al. High expression of Derlin-1 is associated with the malignancy of bladder cancer in a Chinese Han population. PLoS One. 2016;11(12):e0168351. doi:10.1371/journal.pone.0168351
21. Dong QZ, Wang Y, Tang ZP, et al. Derlin-1 is overexpressed in non-small cell lung cancer and promotes cancer cell invasion via EGFR-ERK-mediated up-regulation of MMP-2 and MMP-9. Am J Pathol. 2013;182:954–964. doi:10.1016/j.ajpath.2012.11.019
22. Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–W102. doi:10.1093/nar/gkx247
23. Huang CH, Hsiao HT, Chu YR, Ye Y, Chen X. Derlin2 protein facilitates HRD1-mediated retro-translocation of sonic hedgehog at the endoplasmic reticulum. J Biol Chem. 2013;288:25330. doi:10.1074/jbc.M113.455212
24. Buerger E. The ubiquitin-domain protein HERP forms a complex with components of the endoplasmic reticulum associated degradation pathway. J Mol Biol. 2005;354:1021–1027. doi:10.1016/j.jmb.2005.10.020
25. Wang J, Hua H, Ran Y, Zhang H, Liu W. Derlin-1 is overexpressed in human breast carcinoma and protects cancer cells from endoplasmic reticulum stress-induced apoptosis. Breast Cancer Res. 2008;10:R7.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.