Back to Journals » Cancer Management and Research » Volume 12
Depth of Response and Early Tumor Shrinkage for Predicting Clinical Outcomes in HER2-Positive Metastatic Breast Cancer Treated with Trastuzumab
Authors Che YQ , Zhang Y , Ou KP, Wang D , Shen D , Liu HY, Luo Y
Received 11 July 2020
Accepted for publication 14 August 2020
Published 16 September 2020 Volume 2020:12 Pages 8527—8534
DOI https://doi.org/10.2147/CMAR.S269067
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Yi-Qun Che,1 Yue Zhang,1 Kai-Ping Ou,2 Di Wang,3 Di Shen,1 Hui-Ying Liu,4 Yang Luo5
1Department of Clinical Laboratory, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, People’s Republic of China; 2Department of Medical Oncology, Beijing Chaoyang District Sanhuan Cancer Hospital, Beijing 100122, People’s Republic of China; 3State Key Laboratory of Molecular Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, People’s Republic of China; 4Department of Clinical Laboratory, Jinzhou Central Hospital, Jinzhou 121000, Liaoning Province, People’s Republic of China; 5Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, People’s Republic of China
Correspondence: Yang Luo
Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. No.17 Panjiayuan Nanli, Chaoyang District, Beijing 100021, People’s Republic of China
Tel/Fax + 86-10-87788820
Email [email protected]
Background: To evaluate whether the depth of response (DepRe) and early tumor shrinkage (ETS) are predictive factors of clinical outcomes in HER2-positive metastatic breast cancer (mBC) patients treated with trastuzumab.
Methods: We performed a retrospective study of 100 HER2-positive mBC patients who received trastuzumab combined with chemotherapy as first-line treatment. ETS and DepRe were calculated. We employed Youden’s index to determine the optimal cutoff value of ETS and DepRe for predicting progression-free survival (PFS) and overall survival (OS). We used Kaplan–Meier analysis, Log-rank tests, and Cox proportional hazards regression models to evaluate the impacts of ETS and DepRe on clinical outcomes.
Results: The optimal cutoff values were 30% for ETS and 40% for DepRe; ETS and DepRe were observed in 51.0% (51/100) and in 56.0% (56/100) of patients, respectively. Both ETS≥ 30% and DepRe≥ 40% were significant tumor-size metrics for predicting PFS (ETS: median 1.43 vs 0.69 years, hazard ratio [HR] = 0.384; 95% confidence interval [CI]: 0.245 to 0.601; P=0.000030; DepRe: median 1.43 vs 0.59 years, HR = 0.390; 95% CI: 0.250 to 0.609; P=0.0000034), but only DepRe≥ 40% was a significant predictor for OS (median 4.02 vs 3.07 years, HR = 0.484; 95% CI: 0.255 to 0.919; P = 0.027). Multivariate analyses also identified DepRe as an independent prognostic factor for PFS (HR = 0.52; 95% CI: 0.29 to 0.93; P = 0.028) and OS (HR=0.37; 95% CI:0.15 to 0.90; P = 0.029).
Conclusion: ETS≥ 30% and DepRe≥ 40% were significant predictors of better clinical outcomes in mBC patients treated with first-line trastuzumab-based chemotherapy. Further validation in prospective trials with larger patient populations is needed.
Keywords: depth of response, early tumor shrinkage, metastatic breast cancer, trastuzumab, survival
Introduction
Breast cancer – the major cause of cancer death in females – remains a significant global health problem. An estimated 268.6 thousand new cases and 41.8 thousand deaths are predicted to occur in the United States alone in 2019.1 The human epidermal growth factor receptor 2 (HER2) is overexpressed in approximately 20% of breast cancers, and is specifically associated with more aggressive tumor behavior and poor prognosis.2 Currently, pertuzumab- and trastuzumab-based first-line treatments represent the standard approach for HER2-positive metastatic breast cancer (mBC) patients.3 In fact, in China, trastuzumab-based regimens are still widely used as the standard approach in first-line treatment because of economic factors. Treatment with trastuzumab has dramatically improved the prognosis of HER2-positive breast cancer patients in adjuvant setting and advanced stage.4,5 However, not every individual in this subgroup benefits to the same extent. Indeed, approximately 50% of patients do not exhibit an objective response with trastuzumab plus chemotherapy, and the majority of patients whose breast cancer initially respond to treatment eventually experience progression towards metastasis.6 Clinically applicable surrogate markers for survival have not been properly evaluated in HER2-positive mBC patients treated with trastuzumab.
It has long been recognized that patients with responding tumors have a better outcome than those with no response; patients with continued tumor growth have worse outcomes. In the absence of molecular predictors of response, early on-treatment changes have been investigated to identify those patients who benefit from continued therapy and can serve as decision-making tools for clinicians. Tumor response, as defined by Response Evaluation Criteria in Solid Tumors (RECIST), is a widely recognized endpoint in both clinical trials and in daily clinical routine. However, RECIST does not consider depth, timing, or duration of response. Achieving early and sustained tumor shrinkage is a vital treatment goal in patients with mBC. Two other shrinkage-related endpoints, depth of response (DepRe) and, early tumor shrinkage (ETS) could provide essential information in addition to that provided by RECIST.7–11 DepRe quantifies the maximum tumor shrinkage achieved during treatment, whereas, ETS can provide an early assessment of sensitivity to treatment. DepRe and ETS are reported as hallmarks of cetuximab or bevacizumab activity, featuring maximal and rapid effects on tumor size leading to favorable long-term progression-free survival (PFS) and overall survival (OS) in various cancer types and regimens, mainly colorectal cancer.12–14 Analysis of DepRe or ETS as predictive factors in association with long-term outcomes has never been performed for mBC, specifically within one treatment cohort.
The purpose of this study was to explore the values of ETS and DepRe as predictive factors in mBC patients treated with first-line trastuzumab-based chemotherapy. We also defined discriminatory ETS and DepRe cutoff values for dichotomizing patient populations to aid clinical decision-making processes.
Methods
Patients
A single-institution retrospective analysis was performed at the Cancer Hospital, Chinese Academy of Medical Sciences. The inclusion criteria were as follows: 1) pathologically confirmed BC and HER2-amplified according to a 3+ score on immunohistochemistry, and/or had a HER2/CEP17 ratio of ≥2.0 by fluorescence in situ hybridization; 2) clinically diagnosed metastasis; 3) trastuzumab combined chemotherapy regimens as first-line treatment; 4) Karnofsky Performance Score (KPS) greater than 70; 5) normal hepatic and renal function; 6) complete follow-up data. The exclusion criteria were as follows: 1) without trastuzumab maintenance treatment; 2) concurrent malignancies. Between January 2011 and December 2018, one hundred HER2-positive mBC patients who meet the inclusion and exclusion criteria above were included in this analysis. Chemotherapeutic regimens were different and the selection of regimens were depended on the patients’ physical status and discretion of the treating physician. The most commonly used chemotherapeutic agents were docetaxel-based regimens (51.0%), paclitaxel-based regimens (27.0%), vinorelbine-based regimens (17.0%), gemcitabine-based regimens (4.0%), and capecitabine (1.0%). All patients underwent diagnostic biopsy and/or surgery of a primary or metastatic breast tumor before treatment. Previously validated prognostic factors, including age, hormone receptor status, liver metastasis or not, carcinoembryonic antigen (CEA), absolute lymphocyte count (ALC), alkaline phosphatase (ALP) and lactate dehydrogenase (LDH) levels, were retrieved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Our project has been approved by the Ethics Committee of Cancer Hospital, Chinese Academy of Medical Sciences. All patients provided written informed consent. Clinical information of each patient was obtained from medical records.
Assessments
Radiological tumor responses were performed by computed tomography at 6-week intervals during the treatment of chemotherapy, then 3-month intervals in the phase of trastuzumab maintenance. RECIST version 1.1 was used to classify tumor responses as: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). In accordance with RECIST 1.1, we considered the following criteria for the identification of target lesions: all metastatic lesions must be measured with the longest diameter >10 mm and lymph node metastases must be at least 15 mm in the short axis; no more than 5 metastases were considered for each patient, with a maximum of 2 per organ; the sum of the longest diameters of the target lesions was used for comparison and statistical analysis. ETS in this study was defined as the relative change in the sum of the longest diameters of the target lesions at week 6 compared to baseline. DepRe was calculated as the relative change in the sum of the longest diameters of the target lesions at their smallest attained sizes compared to baseline. ETS or DepRe had positive values for tumor reduction, negative values for tumor growth, and zero for no change.
Statistical Analysis
PFS was calculated as the interval between the first trastuzumab administration and the first documentation of disease progression or death; OS was defined as the time from first trastuzumab administration to death by any cause. Patients free from tumor progression or alive were censored. For comparing categorical variables between two groups, the chi-squared test or Fisher’s exact test was applied. Survival curves were constructed using the Kaplan–Meier method and compared by the Log-rank test. Youden’s index was used to establish the optimal threshold point at which to distinguish the patient population based on DepRe or ETS in order to predict PFS or OS, Univariable and multivariable analyses were analyzed using Cox proportional hazards multiple regression model. Statistical analyses were performed using SPSS 22.0 and GraphPad PRISM 7.0 software. All statistical tests were two-sided, and were considered statistically significant with a P value less than 0.05.
Results
Patients
A total of 100 HER2-positive mBC patients who received first-line trastuzumab-based treatment were included. Patients received trastuzumab combined chemotherapy, with a median follow-up duration of 1.94 years (interquartile range, 1.17 to 3.32 years). Baseline characteristics of the patients are represented in Table 1.
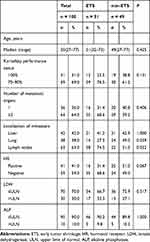 |
Table 1 Baseline Characteristics |
Identification of Cutoff Values
The clinical utility of continuous numerical variables often involves dichotomization of the patient population into two parts around a cutoff threshold which could accurately distinguish the long-term outcomes between groups. Receiver Operating Characteristics (ROC) curve was used to generate a testing the ETS or DepRe and Youden’s index were calculated. In this analysis, optimal cutoff values of 24.5% (PFS) and 29.0% (OS) for ETS and 34.5% (PFS) and 42.0% (OS) for DepRe were determined, respectively (Table 2). Cutoff values of 30% for ETS and 40% for DepRe were defined for further analyses. According to cutoff values, patients were divided into subgroups for ETS (≥30%: n = 51) or non-ETS (<30%: n = 49); DepRe (≥40%: n = 56) or non-DepRe (<40%: n = 44).
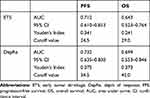 |
Table 2 Performance Indices of ETS and DepRe on Long-Term Outcomes |
RECIST Best Response in Relation to ETS versus Non-ETS
ETS was defined as a decrease in tumor size of ≥30% at the 6-week time-point. Baseline characteristics were generally similar between patients with ETS versus non-ETS. However, the baseline incidence of lung metastasis was significantly lower (27.5% vs 49.0%, P = 0.039) and lymph nodes metastasis was significantly higher (74.5% vs 51.0%, P = 0.022) in ETS patients than in non-ETS patients (Table 1). Patients with ETS≥ 30% achieved a CR in 15.7% of cases and a PR in 84.3% of cases, reflecting high sensitivity towards trastuzumab-based treatment. Additionally, the non-ETS cohort was subdivided into “minor shrinkage” (1–29%), “tumor progression” (no change or any increase), and “new metastatic lesions” in an attempt to distinguish non-ETS patients further. In the cohort having “minor shrinkage,” best overall response rates of 3.4% for CR (one patient) and 37.9% for PR (11 patients) were achieved. However, only half of patients classified as having “tumor progression” reached SD during further continuation of trastuzumab-based treatment, and none of them kept SD for more than 6 months. As expected, all patients who developed new metastatic lesions resulted in having PD according to RECIST (Table 3). Patients responding with “minor shrinkage” (n = 29) had a median PFS of 1.09 years, while patients with “tumor progression” (n = 17) progressed after 0.35 years. Patients who developed “new metastatic lesions” during chemotherapy (n = 3) had a PFS of only 0.11 years.
 |
Table 3 Achievement of Best Overall Response According to ETS and Non-ETS Patients |
Impact of DepRe and ETS on Clinical Outcomes
As shown in Table 4, PFS and OS were significantly prolonged in patients with DepRe ≥40% (P = 0.0000018 for PFS; P = 0.024 for OS), but only PFS, not OS, was significantly longer for patients in the ≥30% ETS group (P = 0.000015 for PFS; P = 0.224 for OS). Kaplan–Meier curves for PFS and OS in relation to DepRe and ETS were also constructed and each was categorized by their respective cutoff values (Figure 1). To further validate the potential prognostic factors, the multivariate Cox regression model was subsequently applied. Other potential confounding prognostic factors (LDH and ALC) at baseline were all taken into account, and it was found that both LDH ≤ upper limit of normal (ULN) (vs >ULN) and DepRe≥40% (vs.<40%) were significant predictors for both PFS and OS (Table 5), independent of other factors.
 |
Table 4 Univariate Analysis for the Prognostic Factors of PFS and OS of Trastuzumab Therapy in HER2-Positive mBC (n = 94) |
 |
Table 5 Multivariate Analysis of PFS and OS (n = 100) |
Discussion
Selecting patients who are likely to benefit from targeted anticancer agents is of paramount importance. The molecular basis underpinning HER2 dependency of tumors is complex, and it is unlikely that positive molecular predictors will soon become available.2,15 Early on-treatment changes of tumor lesions may help identify particular patients in whom the continuation of therapy is worthwhile. In a study of patients with metastatic colorectal cancer (mCRC) treated with cetuximab and chemotherapy, De Roock first reported that a decrease in tumor size by 10% at 6 weeks was associated with improved PFS and OS, and could differentiate patients with favorable outcomes within the KRAS wild-type group.16 This approach can be extended to trastuzumab because preclinical studies showed that biologic effects of trastuzumab occur very quickly and are detectable after a few days or weeks of therapy.17 From these data, we hypothesized that a rapid and substantial decrease in tumor load is a hallmark of HER2 dependency and consequently of trastuzumab sensitivity. The present cohort study demonstrates that early assessment of tumor response to trastuzumab for HER2-positive mBC predicts clinical outcome in terms of PFS but not of OS. ETS assessment can accelerate response evaluation and help clinical decision-making. Those patients who achieve ETS are clearly sensitive to trastuzumab-based treatment and should proceed with unchanged therapy. However, it is more difficult to draw conclusions for non-ETS patients, since they form a heterogeneous subgroup, where patients with minor shrinkage clearly benefit from treatment, with nearly half of patients with “minor shrinkage” achieving PR or CR at best response, and continuation with trastuzumab also remains a feasible option. By contrast, patients with early progression or development of new lesions have an unfavorable prognosis. Although most of the patients with tumor progression at 6 weeks achieved SD according to RECIST. None of them showed SD for more than 6 months, so the optimal treatment strategy remains less clear and an early switch to potentially more effective treatment could be an available choice.
DepRe, defined as the maximal tumor shrinkage achieved, has been recently studied in mCRC patients treated with cetuximab or bevacizumab.7–11 In addition, Lee and coworkers demonstrated that DepRe≥45% was associated with longer OS (median 23.5 vs 13.1 months, HR = 0.441; 95% CI: 0.203 to 0.955; P = 0.038), but not PFS (median 9.0 vs 6.3 months, HR = 0.608; 95% CI: 0.335 to 1.104; P = 0.102) in advanced gastric cancer patients receiving trastuzumab-based first-line therapy.18 We recognize that DepRe is a novel and effective measurement of treatment outcome, which could better predict patients who will survive longer. In the present study, which evaluated the predictive value of DepRe in HER2-positive mBC patients treated with trastuzumab-based therapy, we identified that the DepRe≥40% was a significant predictor for both PFS and OS. These results support the notion of using DepRe as a surrogate marker for outcomes and imply that responses to therapy might not be adequately evaluated when performed by only using RECIST; other tumor-size metrics such as ETS and DepRe could categorize patients who will respond to therapy or survive longer further. Other potential confounding factors in predicting long-term outcomes include the absolute tumor burden at baseline and host characteristics. When adjusting for the available surrogate indicators of tumor burden and host characteristics, including age, Karnofsky performance status, ALC, liver limited disease, number of involved disease sites, levels of LDH, ALP, and CEA, LDH and DepRe were effective predictors in our models, suggesting that tumor shrinkage could predict long-term outcomes irrespective of tumor burden.
Although categorization decreases the predictive value of a continuous scale (DepRe and ETS), dichotomization of populations in relation to a specific value on a continuous variable is often required for clinical decision-making. In principle, the cutoff values mainly determined on the clinical aims. By using Youden’s index, we observed the optimal cutoff values to be 30% for ETS and 40% for DepRe. ETS and DepRe can easily be obtained and makes it conveniently application.
In conclusion, our study clearly established that DepRe and ETS could be used as predictors of clinical outcomes in mBC patients treated with trastuzumab-based regimens in the first-line setting. We suggested a cutoff value of DepRe≥40% and ETS≥30% to distinguish patients with favorable clinical outcomes. Additionally, our study provided that non-ETS was a heterogeneous subgroup, that is, patients with minor shrinkage could benefit from the continuation of treatment, while patients with early tumor progression or development of new lesions could consider alternative regimens.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34.
2. Pondé N, Brandão M, El-Hachem G, Werbrouck E, Piccart M. Treatment of advanced HER2-positive breast cancer: 2018 and beyond. Cancer Treat Rev. 2018;67:10–20. doi:10.1016/j.ctrv.2018.04.016
3. Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2014;372:724–734. doi:10.1056/NEJMoa1413513
4. Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2 positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32:3744–3752. doi:10.1200/JCO.2014.55.5730
5. Mendes D, Alves C, Afonso N, et al. The benefit of HER2-targeted therapies on overall survival of patients with metastatic HER2-positive breast cancer–a systematic review. Breast Cancer Res. 2015;17:140. doi:10.1186/s13058-015-0648-2
6. Jiang H, Rugo HS. Human epidermal growth factor receptor 2 positive (HER2+) metastatic breast cancer: how the latest results are improving therapeutic options. Ther Adv Med Oncol. 2015;7:321–339. doi:10.1177/1758834015599389
7. Douillard J-Y, Siena S, Peeters M, Koukakis R, Terwey J-H, Tabernero J. Impact of early tumour shrinkage and resection on outcomes in patients with wild-type RAS metastatic colorectal cancer. Eur J Cancer. 2015;51:1231–1242. doi:10.1016/j.ejca.2015.03.026
8. Cremolini C, Loupakis F, Antoniotti C, et al. Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: results from Phase III TRIBE trial by the gruppo oncologico del nord ovest. Ann Oncol off J Eur Soc Med Oncol. 2015;26:1188–1194. doi:10.1093/annonc/mdv112
9. Taieb J, Rivera F, Siena S, et al. Exploratory analyses assessing the impact of early tumour shrinkage and depth of response on survival outcomes in patients with RAS wild-type metastatic colorectal cancer receiving treatment in three randomised panitumumab trials. J Cancer Res Clin Oncol. 2018;144:321–335. doi:10.1007/s00432-017-2534-z
10. Heinemann V, Stintzing S, Modest DP, Giessen-Jung C, Michl M, Mansmann UR. Early tumour shrinkage (ETS) and depth of response (DpR) in the treatment of patients with metastatic colorectal cancer (mCRC). Eur J Cancer. 2015;51:1927–1936. doi:10.1016/j.ejca.2015.06.116
11. Piessevaux H, Buyse M, Schlichting M, et al. Use of early tumor shrinkage to predict long-term outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol. 2013;31:3764–3775. doi:10.1200/JCO.2012.42.8532
12. Wei M, Ye Q, Wang X, et al. Early tumor shrinkage served as a prognostic factor for patients with stage III non-small cell lung cancer treated with concurrent chemoradiotherapy. Medicine. 2018;97:e0632. doi:10.1097/MD.0000000000010632
13. Shirotake S, Kondo H, Okabe T, et al. Early tumor shrinkage as a predictive factor of metastatic renal cell carcinoma in molecular targeted therapy: A single institutional study. Mol Clin Oncol. 2019;10:125–131. doi:10.3892/mco.2018.1762
14. Ueda K, Suekane S, Nishihara K, et al. Early primary renal tumor response predicts clinical outcome in patients with primary unresectable renal cell carcinoma with synchronous distant metastasis receiving molecularly targeted therapies. Mol Clin Oncol. 2017;7:205–210. doi:10.3892/mco.2017.1294
15. Loibl S, Gianni L. HER2-positive breast cancer. Lancet. 2017;389:2415–2429. doi:10.1016/S0140-6736(16)32417-5
16. De Roock W, Piessevaux H, De Schutter J, et al. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol off J Eur Soc Med Oncol. 2008;19:508–515. doi:10.1093/annonc/mdm496
17. Baselga J, Carbonell X, Castañeda-Soto N-J, et al. Phase II study of efficacy, safety, and pharmacokinetics of trastuzumab monotherapy administered on a 3-weekly schedule. J Clin Oncol. 2005;23:2162–2171. doi:10.1200/JCO.2005.01.014
18. Lee C-K, Kim -S-S, Park S, et al. Depth of response is a significant predictor for long-term outcome in advanced gastric cancer patients treated with trastuzumab. Oncotarget. 2017;8:31169–31179. doi:10.18632/oncotarget.16099
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

