Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Depression and associated factors among patients with tuberculosis in Ethiopia: a cross-sectional study
Authors Molla A , Mekuriaw B , Kerebih H
Received 11 March 2019
Accepted for publication 27 May 2019
Published 8 July 2019 Volume 2019:15 Pages 1887—1893
DOI https://doi.org/10.2147/NDT.S208361
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Alemayehu Molla,1 Birhanie Mekuriaw,1 Habtamu Kerebih2
1Department of Psychiatry, College of Medicine and Health Science, Dilla University, Dilla, Ethiopia; 2Department of Psychiatry, School of Medicine, College of Medicine and Medical Sciences, University of Gondar, Gondar, Ethiopia
Purpose: The study aimed to assess the magnitude of depression and associated factors among patients with tuberculosis (TB) visiting outpatient clinics in Ethiopia.
Patients and methods: A cross-sectional study was conducted among 415 TB patients. Study participants were selected through a systematic random sampling technique. Patient Health Questionnaire-9 (PHQ-9) was used to assess depression using face-to-face interviews. Data were analyzed using SPSS version 20. Bivariate and multivariate binary logistic analyses were done to identify associated factors with depression. P-values less than 0.05 were considered statistically significant, and the strength of the association was presented by adjusted OR (AOR) with 95% CI.
Results: The magnitude of depression was found to be 31.1% with 95% CI (26.5–35.7). Extrapulmonary TB [AOR =1.8, 95% CI (1.02, 3.24)], poor social support [AOR =3.3, 95% CI (1.8, 6.03)] and perceived TB stigma [AOR =2.0, 95% CI (1.28, 3.18)] were variables found to be statistically significantly connected with depression.
Conclusion: The magnitude of depression was high compared to the general population and some other studies. Therefore, the current study area and other settings which provide TB screening and treatment need to assess patients for depression and provide intervention, giving more emphasis to patients with risk factors.
Keywords: depressive illness, outpatient clinics, tuberculosis patients
Introduction
Tuberculosis (TB) is a chronic infectious disease caused by bacteria (Mycobacterium tuberculosis) and commonly affects the lung.1 It is a public health concern due to its high morbidity and mortality throughout the world in all population groups.2 Although all age groups are at risk to develop TB, it affects adults in their most productive years and over 95% of cases and deaths are in developing countries like Ethiopia.3 Moreover, Multidrug-resistant TB (MDR-TB) is also a major public health crisis and a health security threat currently worsening in developing countries.4 In addition to its biological deteriorative impact, TB causes social, psychological and economic problems for the individuals, families, and community as a large, and it has a bimodal relationship with depression.5,6
Mental illness comprised about 13% of the total global burden of disease and is expected to rise to 15% by the year 2020.7,8 Among the psychiatric problems, depression is the fourth leading cause of disease burden worldwide; representing 4.5% of total disability-adjusted life years. Depression is predicted to become the second leading cause of the global disease burden by the year 2020.7,9–11 The disability associated with depression in relation to chronic medical disease is high, where people are already struggling for survival and the catastrophic impact of a chronic and disabling illness.12 Furthermore, depression hinders good treatment outcome, and it can cause disability and poor quality of life.13
Some studies showed that the prevalence of depression among patients with TB is higher than in the general population. A study from South Africa showed the prevalence of depression was 32.9%.14 In Nigeria depression was reported as 27.7% and 45.5%,15,16 and in Ethiopia, 54.0% of TB patients were reported to have depression.3 Older age, level of education, social supports, female sex, perceived TB stigma and duration of illness were some factors identified as being associated with depression.3,14–17
Overall, different psychosocial problems like depression and other mild and moderate psychiatric features are very common among patients with TB. Even though some studies are available with regard to depression related to TB in Ethiopia, still little attention was given to consider depression among patients with TB. The aim of this study was to determine the magnitude of depression and associated factors among patients with TB.
Patients and methods
An institution based cross-sectional study was conducted at Saint Peter’s specialized hospital, which is a federal hospital located in Gulele subcity in Addis Ababa, Ethiopia. Patients aged 15 and above visiting an outpatient TB clinic were included in the study. Ethical clearance was obtained from the University of Gondar and Amanuel Mental Specialized Hospital Institutional Review Board (IRB). Identification or name of participants was not recorded to maintain confidentiality. Data were collected after obtaining written informed consent from participants and caregivers were required to provide consent for participants under the age of 18 years.
The sample size was determined using a single population proportion formula with a confidence interval of 95%, α=0.05, and P=50%. Proportional allocation was done for non-MDR-TB patients and MDR-TB patients using data from the registration book, and participants from both types were selected through a systematic random sampling technique. Finally, 415 (308, from non-MDR-TB and 107 from MDR-TB) patients were interviewed during data collection.
The questionnaire has four components: sociodemographic characteristics, clinically related factors, Oslo social support scale, patient health questionnaire (PHQ-9) and perceived TB stigma scale.
Outcome variables were assessed using PHQ-9 questionnaires which have 9 items, and each item has a 4-point Likert scale (0=not at all to 3=nearly every day). Individuals who score 10 and above were considered as having depression.18 The PHQ-9 has been previously used in Ethiopia in other studies.19,20 PHQ-9 is also culturally validated in the Ethiopian context with specificity and sensitivity of 67% and 86%, respectively.18
Social support was assessed by a 3-item Oslo social support scale, which ranges from 3 to 14. Oslo-3 has three categories: score of 3–8 is poor support, 9–11 moderate support and 12–14 strong support.21
TB stigma was assessed using a 12-item perceived TB stigma scale. This stigma scale consists of a 4-point Likert scale and item scores of the stigma questions were summed to construct a single stigma variable. Respondents were classified as having or not having perceived TB stigma using the mean of the stigma scale as a cutoff point.22
Data were collected by two psychiatric nurses using pre-tested face-to-face interviewer-administered questionnaires. To control the quality of data the questionnaire was designed, modified appropriately and was translated to the local language (Amharic) to be understood by all participants and translated back to English. Training was provided to data collectors and supervisors for two days on methodology, ethical concern and how to supervise the data collection process. The questionnaire was pretested and data collectors were supervised, and the filled questionnaires were checked daily.
Data were entered to Epi-data version 3.1 and exported to SPSS version 20 (IBM Coproration, Armonk, NY, USA) for further analysis. Bivariate analysis was done to see the association of each independent variable with the outcome variable. Finally, from multivariate analysis, a p-value less than 0.05 was considered statistically significant, and adjusted OR with 95% CI was used to determine association.
Results
Sociodemographic distribution of the participants
About 415 participants were included in the study, and the response rate was 98.1%. The age range of the participants was 15–78 years with a mean age of 34.56 (±12.6). Over half (222; 53.5%) were male. Among participants, 161 (38.8%) were orthodox religion followers and 134 (32.3%) were Amhara in their ethnicity. Regarding the educational status of participants, 118 (28.4%) of them had a primary level of education. The majority of respondents were from urban areas (328; 79%). The monthly income of respondents ranged from 100 to 5,000 Ethiopian birr (Table 1).
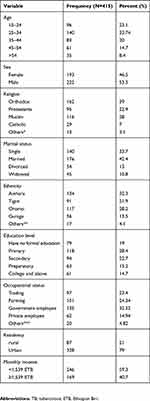 |
Table 1 Sociodemographic distributions of TB patients attending outpatient treatment at Saint Peter’s Specialized Hospital, Addis Ababa, Ethiopia, 2018 |
Clinical and psychosocial factors of the respondents
The majority of respondents had a diagnosis of pulmonary TB (344; 83%) and 257 (61.9%) of participants were in the new TB treatment category. Two-thirds of the participants were in the intensive phase of treatment (277; 65.5%). Among psychosocial factors poor social support and perceived TB stigma were reported by 177 (42.7%) and 187 (45.1%) of participants, respectively. Among study participants, 40 (9.6%) had comorbid HIV illness (Table 2).
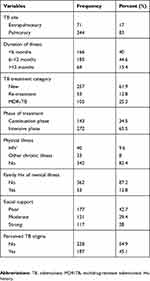 |
Table 2 Clinical and psychosocial factors of TB patients attending outpatient treatment at Saint Peter’s Specialized Hospital, Addis Ababa, Ethiopia, 2018 |
Current substance use characteristics of respondents
Thirty-one (7.5%) of respondents reported that they had used alcohol, 12 (2.9%) had smoked cigarettes and 17 (4.1%) had chewed khat in the three months before data collection (Table 3).
 |
Table 3 Current substance use characteristics of participants with tuberculosis visiting outpatient clinics at Saint Peter’s Specialized Hospital, Addis Ababa, Ethiopia, 2018 |
Prevalence depression among patients with tuberculosis
The prevalence of depression among patients with TB was 31.1% with 95% CI (26.5–35.7). Among respondents with depression, 66 (51.2%) were female and 95 (73.64%) of depressed participants had a diagnosis of pulmonary TB. Regarding the treatment category of TB, 75 (58.14%) of respondents were in the new treatment category, 41 (31.8%) were in the MDR-TB treatment category. Sixty-four (49.6%) of participants with depression had poor social support and 75 (58.12%) reported perceived stigma related to TB (see Figure S1).
Factors associated with depression among patients with tuberculosis
In bivariate binary logistic analysis variables, being female, having extrapulmonary TB, duration of TB illness >12 months, treatment category of MDR-TB, comorbid HIV illness, poor social support and perceived TB stigma were found to have P-values less than 0.25. These variables fulfilled the minimum requirements for further multivariate binary logistic regression.
From multivariate binary logistic regression variables; extrapulmonary TB 1.8 (95% CI, 1.02, 3.24) poor social support 3.3 (95% CI, 1.8, 6.03), and perceived TB stigma 2 (95% CI, 1.28, 3.18) were statistically significant with depression at P-value less than 0.05 (see Table 4).
 |
Table 4 Logistic regression analysis of factors associated with depression among patients with tuberculosis visiting outpatient clinics at Saint Peters Hospital, Addis Ababa Ethiopia, 2018 (N=415) |
Discussions
The magnitude of depression in the current study was 31.1% with 95% CI (26.5–35.7). Regarding the magnitude, the finding was similar to studies carried out in Nigeria 27.7%,15 South Africa 32.9%14 and India 35%.23
The finding of the current study was lower than studies done in India (39.5%),24 Greece (49.2%),25 Ethiopia (43.3%),17 Nigeria (45.5%, 41.9%),16,26 Pakistan (69.55%, 46.5%),4,27 and Tanzania (46.9%).28 The possible reason for the discrepancy might be variation in study design and sample size used. The study conducted in India used a consecutive sampling technique, and the Beck Depression iInventory was the tool they used to assess depression. The difference in study design might be another possible reason for the variation, as an ongoing prospective study was used in Nigeria.16 Another possible reason might be socioeconomic, cultural and sample size differences between the previous and the current studies.
However, the finding was higher than other studies conducted in India (22%),29 and UK (20.8%).30 The variation might be due to the difference in study design; the Indian study was a retrospective study from medical records, and hospital anxiety and depression (HADS depression D) was used to assess depression. A difference in study participants might be another possible reason for the variation. Participants form continuation phase of TB treatment in previous study might have improvement due to better duration of treatment contact with professional and their interaction could minimize their depressive feeling.31
Participants with poor social support were 3.3 times more likely to have depression as compared with participants with good social support. This is supported by study results from Pakistan (46.5%),27 Ethiopia (43.4%)17 and India (39.5%).24 This might be due to a lack of social support, and somatic illness (TB) may lead to increased psychological distress (mental disorders); on the other hand, good social support is vital for good disease prevention.32
Diagnosis of extrapulmonary TB was another factor associated with depression; patients with extrapulmonary TB were about 1.8 times more likely to be depressed as compared with their counterparts. This is in agreement with a study from Nigeria.16 This could be due to extrapulmonary TB having a poorer prognosis than pulmonary TB, which might lead patients to be depressed more.
The study revealed that perceived TB stigma was also associated with depression; patients with perceived TB stigma were 2 times more likely to have depression. This is in line with studies conducted in Pakistan (46.5%)27 and Ethiopia (43.3%).17 Patients with perceived TB stigma might use health services less due to low self-esteem, and social isolation, and as a result, this might predispose them to developing depression.
While there are important findings in the current study, care should be taken in interpreting the results due to the following limitations. Side effects of the anti-TB medication were not addressed. Since the entire sample was taken from a single hospital in the capital city, the findings of this study might not be generalized to other areas, especially in rural settings.
Conclusions
The magnitude of depression was high compared to the general population and some other studies. Therefore, the current study area and other settings which provide TB screening and treatment need to assess patients for depression and provide intervention, giving more emphasis to patients with risk factors. Additionally, further research using different study designs on risk factors of depression like TB medication side effects should be conducted to broaden the current findings.
Acknowledgments
The authors acknowledge the College of Medicine and Health Sciences, University of Gondar and Amanuel Specialized Mental Health Hospital for their financial and technical support for data collection. The authors are also very grateful to Saint Peter’s Specialized Hospital TB clinical staff, data collectors, and all the study participants involved in the study.
Author contributions
All authors contributed towards data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agreed to be accountable for the all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Zaman K. Tuberculosis: a global health problem. J Health Popul Nutr. 2010;28(2):111. doi:10.3329/jhpn.v28i2.4879
2. Sulehri MA, Dogar IA, Sohail H, et al. Prevalence of depression among tuberculosis patients.
3. Ambaw F, Mayston R, Hanlon C, Alem A. Burden and presentation of depression among newly diagnosed individuals with TB in primary care settings in Ethiopia. BMC Psychiatry. 2017;17(1):57. doi:10.1186/s12888-017-1489-6
4. Javaid A, Mehreen S, Khan M, Ashiq N, Ihtesham M. Depression and its associated factors with multidrug-resistant tuberculosis at baseline. J Depress Anxiety. 2017;6(253):2167–1044.1000253. doi:10.4172/2167-1044.1000253
5. Oh K, Choi H, Kim E, Kim H, Cho S. Depression and risk of tuberculosis: a nationwide population-based cohort study. Int J Tuberculosis Lung Dis. 2017;21(7):804–809. doi:10.5588/ijtld.17.0038
6. Koyanagi A, Vancampfort D, Carvalho AF, et al. Depression comorbid with tuberculosis and its impact on health status: cross-sectional analysis of community-based data from 48 low-and middle-income countries. BMC Med. 2017;15(1):209. doi:10.1186/s12916-017-0975-5
7. Reddy M. Depression: the disorder and the burden. Indian J Psychol Med. 2010;32(1):1. doi:10.4103/0253-7176.70510
8. Vigo D, Thornicroft G, Atun R. Estimating the true global burden of mental illness. Lancet Psychiatry. 2016;3(2):171–178. doi:10.1016/S2215-0366(15)00505-2
9. Chisholm D, Flisher A, Lund C, et al. Scale up services for mental disorders: a call for action. Lancet. 2007;370(9594):1241–1252.
10. Miranda JJ, Patel V. Achieving the millennium development goals: does mental health play a role? PLoS Med. 2005;2(10):e291. doi:10.1371/journal.pmed.0020291
11. Michaud CM, Murray CJ, Bloom BR. Burden of disease—implications for future research. Jama. 2001;285(5):535–539.
12. Katon W, Sullivan MD. Depression and chronic medical illness. J Clin Psychiatry. 1990;51(Suppl 6):3–11.
13. Ambaw F, Mayston R, Hanlon C, Medhin G, Alem A. Untreated depression and tuberculosis treatment outcomes, quality of life and disability, Ethiopia. Bull World Health Organ. 2018;96(4):243. doi:10.2471/BLT.17.206417
14. Peltzer K, Naidoo P, Matseke G, Louw J, Mchunu G, Tutshana B. Prevalence of psychological distress and associated factors in tuberculosis patients in public primary care clinics in South Africa. BMC Psychiatry. 2012;12(1):89. doi:10.1186/1471-244X-12-86
15. Issa BA, Yussuf AD, Kuranga SI. Depression comorbidity among patients with tuberculosis in a university teaching hospital outpatient clinic in Nigeria. Ment Health Fam Med. 2009;6(3):133.
16. Ige OM, Lasebikan VO. Prevalence of depression in tuberculosis patients in comparison with non-tuberculosis family contacts visiting the DOTS clinic in a Nigerian tertiary care hospital and its correlation with disease pattern. Ment Health Fam Med. 2011;8(4):235.
17. Duko B, Gebeyehu A, Ayano G. Prevalence and correlates of depression and anxiety among patients with tuberculosis at WolaitaSodo University Hospital and Sodo Health Center, WolaitaSodo, South Ethiopia, Cross sectional study. BMC Psychiatry. 2015;15(1):214. doi:10.1186/s12888-015-0598-3
18. Gelaye B, Williams MA, Lemma S, et al. Validity of the patient health questionnaire-9 for depression screening and diagnosis in East Africa. Psychiatry Res. 2013;210(2):653–661. doi:10.1016/j.psychres.2013.07.015
19. Bitew H, Andargie G, Tadesse A, Belete A, Fekadu W, Mekonen T. Suicidal ideation, attempt, and determining factors among HIV/AIDS patients, Ethiopia. Depress Res Treat. 2016;2016.
20. Molla A, Mengesha A, Derjaew H, Kerebih H. Suicidal ideation, attempt, and associated factors among patients with tuberculosis in Ethiopia: a cross-sectional study. Psychiatry J. 2019;2019.
21. Dalgard OS, Dowrick C, Lehtinen V, et al. Negative life events, social support and gender difference in depression. Soc Psychiatry Psychiatr Epidemiol. 2006;41(6):444–451. doi:10.1007/s00127-006-0051-5
22. Van Rie A, Sengupta S, Pungrassami P, et al. Measuring stigma associated with tuberculosis and HIV/AIDS in southern Thailand: exploratory and confirmatory factor analyses of two new scales. Trop Med Int Health. 2008;13(1):21–30. doi:10.1111/j.1365-3156.2007.01971.x
23. Kumar K, Kumar A, Chandra P, Kansal HM. A study of prevalence of depression and anxiety in patients suffering from tuberculosis. J Family Med Primary Care. 2016;5(1):150. doi:10.4103/2249-4863.184641
24. Balaji AL, Abhishekh HA, Kumar NC, Mehta RM. Depression in patients with pulmonary tuberculosis in a tertiary care general hospital. Asian J Psychiatr. 2013;6(3):251–252. doi:10.1016/j.ajp.2012.12.017
25. Moussas G, Tselebis A, Karkanias A, et al. A comparative study of anxiety and depression in patients with bronchial asthma, chronic obstructive pulmonary disease and tuberculosis in a general hospital of chest diseases. Ann Gen Psychiatry. 2008;7(1):7. doi:10.1186/1744-859X-7-7
26. Aniebue P, Okonkwo K. Prevalence of depressive symptoms amongst pulmonary tuberculosis patients at the University of Nigeria Teaching Hospital, Enugu. Int J Med Health Dev. 2006;11(2):120–124.
27. Husain MO, Dearman SP, Chaudhry IB, Rizvi N, Waheed W. The relationship between anxiety, depression and illness perception in tberculosis patients in Pakistan. Clin Pract Epidemiol Mental Health. 2008;4(1):4. doi:10.1186/1745-0179-4-4
28. Buberwa G. Prevalence of Depression among Tuberculosis Patients Attending Clinics in Temeke Municipal, Dar Es Salaam. Tanzania: Muhimbili University of Health and Allied Sciences; 2013.
29. Francis D. Prevalence of depression among patients with tuberculosis at Perundurai TB hospital, Tamilnadu–depression, a comorbidity of TB. Eur Psychiatry. 2017;41:S472. doi:10.1016/j.eurpsy.2017.01.541
30. Cleland JA, Lee AJ, Hall S. Associations of depression and anxiety with gender, age, health-related quality of life and symptoms in primary care COPD patients. Fam Pract. 2007;24(3):217–223. doi:10.1093/fampra/cmm009
31. Nezenega ZS, Tafere TE. Patient satisfaction on tuberculosis treatment service and adherence to treatment in public health facilities of Sidama zone, South Ethiopia. BMC Health Serv Res. 2013;13(1):110. doi:10.1186/1472-6963-13-438
32. Bøen H, Dalgard OS, Johansen R, Nord E. Socio-demographic, psychosocial and health characteristics of Norwegian senior centre users: a cross-sectional study. Scand J Public Health. 2010;38(5):508–517. doi:10.1177/1403494810370230
Supplementary material
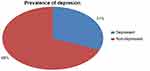 |
Figure S1 Magnitude of depression among patients with tuberculosis visiting outpatient clinics at Saint Peter’s Specialized Hospital, Addis Ababa, Ethiopia. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
