Back to Journals » International Journal of Nanomedicine » Volume 15
Dental Materials Incorporated with Nanometals and Their Effect on the Bacterial Growth of Staphylococcus aureus
Authors Rawashdeh RY, Sawafta R, Malkawi HI
Received 27 February 2020
Accepted for publication 12 May 2020
Published 18 June 2020 Volume 2020:15 Pages 4325—4331
DOI https://doi.org/10.2147/IJN.S251573
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Rabeah Yousef Rawashdeh,1 Reyad Sawafta,2 Hanan I Malkawi1
1Department of Biological Sciences, Faculty of Science, Yarmouk University, Irbid, Jordan; 2QuarTek Corporation, Asheboro, NC 27203, USA
Correspondence: Rabeah Yousef Rawashdeh
Yarmouk University, Shafiq Irshidat Street, Irbid 21163, Jordan
Tel +962-2-721-1111
Email [email protected]
Purpose: The purpose of this study was to investigate the effect of different commercially used dental materials (RelyX Luting Plus and Dyract Extra) mixed with either a metallic ionic solution or a colloidal suspension of metallic nanoparticles. Both the solution and the suspension contained a mixture of silver, copper, and lithium ions.
Methods: The metal/ion-incorporated dental materials were prepared into disk-shaped samples and tested against the growth of Staphylococcus aureus. The susceptibility of bacteria against the antibacterial dental disks was tested using two methods: counting the colony-forming units per milliliter and disk diffusion (Kirby–Bauer). The incorporated materials (Dyract and Rely cement) were tested for ion release using flame atomic absorption spectroscopy.
Results: Assessment showed efficient antibacterial activity of metal ion–incorporated Rely luting cement, exhibited by the formation of inhibition zones larger than those formed by the standard antibiotic, as well as a reduction in bacterial number of sevenfold after incubation for 24 hours. Dyract material incorporated with nanoparticles showed no significant clear zones and had no inhibiting effect on bacterial colony numbers after incubation for 24 hours. The release of silver, copper, and lithium metal ions depended on the type of both dental material and the incorporated nanoagents. The metal ion–incorporated Rely Plus cement released the highest levels of metal ions, which was attributed to its antibacterial efficiency.
Conclusion: Rely Plus cement incorporated with the nanoparticle suspension demonstrated high antibacterial potency, due to the release of the highest concentrations of silver, copper, and lithium metal ions. This work is the first direct comparative study of dental materials with different forms of nanomixtures (metallic nanoparticles and soluble metallic ions) and their antibacterial effects after incubation with bacterial culture for 24 hours.
Keywords: RelyX Luting Plus, Dyract Extra, metallic ionic solution, suspension of metallic nanoparticles, antibacterial activity
Introduction
Research interest in nanotechnology has grown dramatically recently,due to the exponential increase in nanomaterial production and marketing.1–3 There are tremendous precursors used in the production of nanomaterials for utilization in many fields. Researchers favor natural sources in the synthesis of metallic nanoparticles, mainly silver nanoparticles.4–7 Metallic nanoparticles are made either of a single element or a combination of elements, eg, nanoparticles can be made solely of silver or a combination of silver and copper elements. Silver nanoparticles can be shaped differently, eg, spheres or rods. On the basis of their dimensionality, nanomaterials are classified into different groups, such as dendrimers, nanoparticles, nanofilms, and nanotubes. This further adds to the diversity of nanoscale materials.8
Various approaches and techniques have been developed for the synthesis of nanomaterials to make them one of the most applicable and widely used materials in science. For example, silver nanoparticles are used as biocides and antioxidants4–7,9 and utilized either alone or integrated into other structures/substances. Additionally, silver nanoparticles have biomedical and pharmaceutical applications, such as cancer treatment and medical imaging,10,11 not to mention other uses in engineering, cosmetics, and agriculture.12–14 One important branch in nanotechnology is nanodentistry, which has evolved as an extension of application of nanomaterials in dentistry. The insertion of dental materials with antibacterial activity inside the oral cavity provides a good strategy for prevention of the development of dental diseases.15–17
This study introduces an original methodology for assessing the biocidal activity of a nanoparticle suspension versus free ions obtained from their counterpart nanoparticles. Antibacterial activity results were compared between nanocolloids incorporated into dental materials versus a nanosolution incorporated into dental materials. A critical point for controlling the activity of nanomaterials is their mode of action.18 For metallic nanoparticles, ion release is an important and determining factor in their biological activity. Trend and duration of ion release of nanocontaining restorative materials vary according to several chemical and physical parameters, though this study did not assess the time necessary to complete the release of ions. The aim of this work was to test the antibacterial properties of two commercial dental materials (RelyX Luting Plus and Dyract Extra) following treatment with nanomixtures. The ionic solution contained metallic ions of silver, copper, and lithium while the colloidal suspension of nanoparticles contained counterpart ions in addition to the silver, copper, and lithium nanoparticle. The hypothesis was that this combination of metals is unique and serves as an excellent biocidal agent for dental application.
Methods
Preparation of Antibacterial Dental Materials
Two dental materials were used in this study: RelyX Luting Plus (RL; 3M ESPE) and Dyract Extra (Dentsply). Each of the two types of dental material was incorporated with either metallic ionic solutions or colloidal suspensions of nanoparticles (Figure 1) and then formed into disks (Metallic and ionic mixtures were kindly provided by QuarTek). The ionic solution was derived originally from a colloidal suspension of nanoparticles contains insoluble particles and free ions. Heavy particles were settled in a test tube, and the supernatant liquid containing the ions was transferred to a new tube to be used as ionic solution. Both nanomixtures (the solution and the suspension) were made of the same metals: silver, copper, and lithium. RelyX Luting Plus is a self-cured resin, while Dyract Extra was solidified using a dental ultraviolet light–curing lamp following the manufacturer’s instructions. The two nanomixtures (the solution and the suspension) were applied at a concentration of 100 µg/mL to each dental material disk. Disks were made to be fairly close in shape and size for each type of dental material.
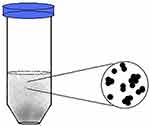 |
Figure 1 Representative drawing of the colloidal suspensions of nanoparticles used in the study. The average size of nanoparticles was 100 nm. |
Inhibitory Effect of Incorporated Dental Materials Against Growth of Staphylococcus aureus
Staphylococcus aureus bacterial cultures in liquid broth and on solid agar were subjected to treatment with incorporated-material disks. Two methods were used to evaluate antibacterial activity: disk incubation into inoculated broth and disk diffusion (Kirby–Bauer).
Disk Incubation in Inoculated Broth
S. aureus culture was prepared by inoculation in a tryptic soy broth (Difco BBL) and incubation overnight at 37°C, and then a dilution of this bacterial culture was induced to reach a bacterial count of 3.5×103 CFU/mL and subjected to treatment with incorporated-material disks. Each disk was dipped in 1 mL bacterial broth and incubated at 37°C, then CFU/mL was recorded after 2 and 24 hours of incubation with disks (Figure 2). Serial dilution was done for each sample, and from each dilution a certain volume was cultivated on tryptic soy agar media (Difco) and incubated for 24–48 hours, in order to estimate the CFU/mL of the sample.
Disk-Diffusion Method (Kirby–Bauer)
On an agar plate, Müller–Hinton agar media (HiMedia Laboratories), were inoculated overnight (log phase) with bacterial broth (S. aureus), by dipping a sterile loop swab into bacterial broth and then streaking the loop over the entire surface of the agar plate. Plates were allowed to dry for 5–15 minutes at room temperature. Disks of the different dental materials were applied and gently pressed down onto the agar plate. The antibiotic gentamicin was used as the positive control and incorporated into disk materials at a concentration of 100 µg/mL. Plates were incubated at 37°C for 24 hours. The diameter (mm) of the zone of inhibition was recorded for each disk material.
Atomic Absorption for Measuring Ion Release
Furnace atomic absorption spectrometry (PerkinElmer 272) was used to measure release of ions from dental-material disks incorporated with different forms of nanometals. Standard silver, copper and lithium solutions were prepared at concentrations of 0.5, 1, 3, 5, 10 and 20 µg/L (all standard solutions diluted in 1% HNO3). The incorporated dental–material disks were incubated in tryptic soy broth, and the ions released (Ag, Cu, and Li) measured after 2 and 24 hours of incubation. Absorption of Ag, Cu, and Li was measured at wavelengths of 328.1, 328, and 670 nm, respectively.
Results
S. aureus growth had reduced after 2 and 24 hours of incubation with the incorporated RelyX Luting Plus cement in comparison to the control (Table 1; Figure 3, A and B). After 24 hours’ incubation with Rely Plus disks mixed with the metallic ionic solution, the bacterial count had reduced sevenfold compared to the negative control (Table 1, Figure 3B) whereas for the NP suspensions the reduction was threefold. Dyract Extra–incorporated disks resulted in no inhibition of bacterial growth after their incubation in inoculated broth. Similarly to the results obtained from disk incubation into bacterial broth, the Bauer–Kirby test results for disks of RelyX Luting Plus, and not Dyract Extra, showed antibacterial activity, with the formation of larger inhibition zones (Figure 4). Inhibition-zone diameter varied between the two types of dental material incorporated with different metallic mixtures. Dyractincorporated with ionic solution showed a slight suppression in bacterial growth, illustrated by the formation of smaller inhibition zones than those formed by the antibiotic disk, and the zone of inhibition measured for the gentamicin disk was largest, with a diameterof 23 mm, for Dyract. Rely Luting Plus disks incorporated with the antibacterial mixtures showed a zone of inhibition of 15 mm, larger than the zone of inhibition formed by the control (11 mm) and the material-only disk (without metallic incorporation — 10 mm), which was expected, since the material itself contained fluoride, which affects bacterial growth; however, for disks mixed with nanometals, the zone of inhibition was larger. Dental disks incorporated with the ionic solution showed greater ion release than disks incorporated with nanoparticlesuspensions (Table 2). Moreover, Rely Plus cement incorporated with the metallic ionic solution) showed the highest silver, copper, and lithium concentrations. However, the two mixtures of the suspension and solution incorporated into Rely Plus showed similar antibacterial activity using the Bauer–Kirby method.
 |
Table 1 Average values of CFU/mL of S. aureus After 2 and 24 hours‘ Incubation with Rely Plus Dental Material Mixed with Either Ionic or Nanostructured Metals |
 |
Table 2 Concentrations (µg/mL) of Ag, Cu, and Li Released from Dental Materials After 2 Hours and 24 Hours, Calculated Based on Absorption Values Obtained by Atomic Absorption Spectrometry |
Discussion
The dental materials used in this study were compomer materials and a glass ionomer, which are used as either filling material or cement for dental prosthesis. These are well-developed dental materials that have many optimized properties, such as ease of handling, low viscosity, good flow, and low polymerization.19–21 The current study aimed to add a further enhancement property to these materials with the incorporation of antibacterial nanomixtures made of silver, copper, and lithium.
Nanomixtures incorporated into RelyX Luting Plus exhibited noticeable antibacterial activity after 2 hours’ incubation (Figure 3A), regardless of the kind of nanomixture used, with bacteria number reduced twofold. However, after 24 hours, disks with metallic ions resulted in a sevenfold reduction in bacterial colony counts, while disks with metallic particles resulted in a threefold reduction (Figure 3B). Similar results have been reported in literature for the bactericidal effect of dental materials with silver nanoparticles on S. aureus colony counts.9,22–24
Diskdiffusion is commonly used for evaluating the antibacterial activity of metallic nanoparticles. This study showed high susceptibility of bacteria to Rely materials with either form of nanomixture after 24 hours’ incubation. This result is in agreement with previous studies that used agar disk-diffusion to demonstrate the susceptibility of S. aureus to metallic nanoparticles.4–7 However, the inhibitory extent of silver nanoparticles depends to a greater degree on the synthesis approach used, and thus the diameter of the inhibition zone against S. aureus exhibits a variety of concentrations.4–7 Furthermore, instead of paper disks, dental porcelain incorporated with one kind of metallic nanoparticle was evaluated by Hashem et al25 using the disk diffusion, and the authors found that the growth of S. aureus was inhibited effectively. This is similar to the results of our work.
Furnace atomic absorption spectrometry was used in this study, and revealed that Rely Plus cement mixed with the metallic ionic solution had the highest concentrations of silver, copper, and lithium. Rely Plus cement used in a previous study demonstrated similar results, silver having the greatest diffusion.26 The size factor plays to the advantages of ion release, and thus contributes to higher antibacterial activity for Rely cement with ionic forms of metals, not that with metallic particles. Previous studies have demonstrated that smaller silver nanoparticles showmore ion release than larger particles.27,28 However, it is noteworthy that the focus of the current study was to distinguish between free ions and solid particles and their ability to exert antibacterial effects.
Possessing antibacterial properties, the self-cured material — Rely Plus cement combined with nanometallic mixtures — resulted in a marked decrease in bacterial viability compared to the control after incubation in the broth for 2 and 24 hours, while the incorporated Dyract material, which was ultraviolet light–cured, did not result in bacterial reduction, even after incubation in the broth for 24 hours. The slow release of ions from Dyract material reduces its antibacterial potency.29 In addition to the type of dental polymer, the method and extent of polymerization is another factor that accounts for ion leakage from dental material, and thus affects the functionality of antibacterial contents.26,30,31 Based on these results, the high antibacterial activity of the nanoincorporated dental material is attributed to high ion leakage.27
Conclusion
This study showed that incorporated cement material exhibited good antibacterial activity, while light-curing of dental resin retarded the diffusion of nanoparticles. The antibacterial activity of the incorporated dental material is related to the released ions. Higher ion release causes an increase in antibacterial activity.The highest concentrations of Ag, Cu, and Li were obtained from cement material mixed with the ionic solution. Since the ions were smaller, they showed more efficient antibacterial activity. The low antibacterial activity of Dyract compomer–incorporated materials can be related to the low release of ions: incorporated Dyract-material disks induced no or little toxicity to S. aureus compared to the incorporated cement (Rely Plus) disks, which exerted greater toxicity on bacteria. Rely Plus is a self-cured material, while Dyract compomer is a light-cured material, and thus it can be concluded that the method used for material polymerization may have limited ion release from the incorporated nanoparticles.
Disclosure
RS is affiliated with QuarTek Corporation. The authors report no other conflicts of interest in this work.
References
1. Inshakova E, Inshakov O. World market for nanomaterials: structure and trends. In: MATEC Web of Conferences. Volgograd State Technical University.Vol. 129. 2017:02013. doi: 10.1051/matecconf/201712902013
2. ASD Reports. Nanotechnology market - global opportunity analysis and industry forecast, 2018–2025. Publisher: Allied Market Research; 2019. Report code: ASDR-490893.
3. Janković NZ, Plata DL. Engineered nanomaterials in the context of global element cycles. Environ Sci Nano. 2019;6:2697–2711. doi:10.1039/C9EN00322C
4. Hamelian M, Zangeneh M, Shahmohammadi A, Varmira K, Veisi H. Pistacia atlantica leaf extract mediated synthesis of silver nanoparticles and their antioxidant, cytotoxicity, and antibacterial effects under in vitro condition. Appl Organo Metal Chem. 2020;34:e5278. doi:10.1002/aoc.5278
5. Hemmati S, Rashtiani A, Zangeneh MM, Mohammadi P, Zangeneh A, Veisi H. Green synthesis and characterization of silver nanoparticles using Fritillaria flower extract and their antibacterial activity against some human pathogens. Polyhedron. 2019;158:8–14. doi:10.1016/j.poly.2018.10.049
6. Zangeneh MM, Joshani Z, Zangeneh A, Miri E. Green synthesis of silver nanoparticles using aqueous extract of Stachys lavandulifolia flower, and their cytotoxicity, antioxidant, antibacterial and cutaneous wound healing properties. Appl Organo Metal Chem. 2019a;33:e5016.
7. Zangeneh MM. Green synthesis and chemical characterization of silver nanoparticles from aqueous extract of Falcaria vulgaris leaves and assessment of their cytotoxicity and antioxidant, antibacterial, antifungal and cutaneous wound healing properties. Appl Organo Metal Chem. 2019b;33:e4963.
8. Madkour LH. Classification of nanostructured materials. In: Nanoelectronic Materials. Adv. Struct. Mater. Cham: Springer; 2019:116.
9. Tanase C, Berta L, Coman NA, et al. Antibacterial and antioxidant potential of silver nanoparticles biosynthesized using the spruce bark extract. Nanomaterials. 2019;9(11):1–13. doi:10.3390/nano9111541
10. Mathur P, Jha S, Ramteke S, Jain NK. Pharmaceutical aspects of silver nanoparticles. Artif Cells Nanomed Biotechnol. 2018;46(1):115–126. doi:10.1080/21691401.2017.1414825
11. Chen M, Yu X, Huo Q, et al. Biomedical potentialities of silver nanoparticles for clinical multiple drug-resistant acinetobacter baumannii. J Nanomater. 2019;2019(3754018):7. doi:10.1155/2019/3754018
12. Alavi SV, Dehpour AA. Evaluation of the nanosilver colloidal solution in comparison with the registered fungicide to control greenhouse cucumber downy mildew disease in the north of Iran. Acta Hortic. 2010;877:1643–1646. doi:10.17660/ActaHortic.2010.877.226
13. Kokura S, Handa O, Takagi T, Ishikawa T, Naito Y, Yoshikawa T. Silver nanoparticles as a safe preservative for use in cosmetics. Nanomedicine: NBM. 2010;6(4):570–574. doi:10.1016/j.nano.2009.12.002
14. Yu J, Zhou X. Synthesis of dendritic silver nanoparticles and their applications as SERS substrates. Adv Mater Sci Eng. 2013;2013:1–4. doi:10.1155/2013/519294
15. Kasraei S, Sami L, Hendi S, Alikhani MY, Rezaei-Soufi L, Khamverdi Z. Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutans and Lactobacillus. Restor Dent Endod. 2014;39(2):109–114. doi:10.5395/rde.2014.39.2.109
16. Farahani A, Beyrami A, Piri H, Naghizadeh A, Imani H, Farahani MH. Evaluation of antibacterial properties of resin composites containing silver nanoparticles on streptococcus mutans. JDOH. 2018;5:1–6.
17. Song W, Ge S. Application of antimicrobial nanoparticles in dentistry. Molecules. 2019;24(6):1033.
18. Shaikh S, Nazam N, Rizvi S, et al. Mechanistic insights into the antimicrobial actions of metallic nanoparticles and their implications for multidrug resistance. Int J Mol Sci. 2019;20(10):2468. doi:10.3390/ijms20102468
19. Silva RA, Coutinho M, Cardozo PI, Silva LA, Zorzatto JR. Conventional dual-cure versus self-adhesive resin cements in dentin bond integrity. J Appl Oral Sci. 2011;19(4):355–362. doi:10.1590/S1678-77572011005000010
20. Pameijer CH. A review of luting agents. Int J Dent. 2012;2012:752861. doi:10.1155/2012/752861
21. Baroudi K, Mahmoud S. Improving composite resin performance through decreasing its viscosity by different methods. Open Dent J. 2015;9:235–242. doi:10.2174/1874210601509010235
22. Zhao L, Wang H, Huo K, et al. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials. 2011;32(24):5706–5716. doi:10.1016/j.biomaterials.2011.04.040
23. Nam K-Y. In vitro antimicrobial effect of the tissue conditioner containing silver nanoparticles. J Adv Prosthodont. 2011;3:20–24. doi:10.4047/jap.2011.3.1.20
24. Dianat O, Ataie M. Gutta-percha coated with nanosilver particles. Invention Registered. 2008;56019.
25. Hashem M, Cherif, Mohsen A, Abu-Eittah MR. The antibacterial and antifungal effect of silver nanoparticles and silver hydroxyapatite nanoparticles on dental ceramic. Egypt Dent J. 2019;61(2):1–7.
26. Sokołowski K, Szynkowska MI, Pawlaczyk A, Łukomska-Szymańska M, Sokołowski J. The impact of nanosilver addition on element ions release form light-cured dental composite and compomer into 0.9% NaCl. Acta Biochim Pol. 2014;61:317–323. doi:10.18388/abp.2014_1902
27. Lok C, Ho C, Chen R, et al. Silver nanoparticles: partial oxidation and antibacterial activities. J Biol Inorg Chem. 2007;12:527–534. doi:10.1007/s00775-007-0208-z
28. Peulen TO, Wilkinson KJ. Diffusion of nanoparticles in a biofilm. Environ Sci Technol. 2011;45(8):3367–3373. doi:10.1021/es103450g
29. Marczuk-Kolada G, Luczaj-Cepowicz E, Waszkiel D, Szarmach I, Jakoniuk P, Mystkowska J. Fluoride release and antibacterial properties of the polyacid-modified composite Dyract AP. Fluoride. 2009;42:147–151.
30. Kozai K, Suzuki J, Okada M, Nagasaka N. In vitro study of antibacterial and antiadhesive activities of fluoride-containing light-cured fissure sealants and a glass ionomer liner/base against oral bacteria. ASDC J Dent Child. 2000;67:117–122.
31. Sultan MA. The effect of light curing intensity on fluoride release from composite resin. Al–Rafidain Dent J. 2009;9(2):232–237. doi:10.33899/rden.2009.9102
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.