Back to Journals » Infection and Drug Resistance » Volume 13
Dendritic Cells Promote Treg Expansion but Not Th17 Generation in Response to Talaromyces marneffei Yeast Cells
Authors Tang Y, Zhang H, Xu H, Zeng W, Qiu Y, Tan C, Tang S , Zhang J
Received 25 November 2019
Accepted for publication 25 February 2020
Published 11 March 2020 Volume 2020:13 Pages 805—813
DOI https://doi.org/10.2147/IDR.S239906
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Yanping Tang,* Hui Zhang,* Haiguang Xu, Wen Zeng, Ye Qiu, Caimei Tan, Shudan Tang, Jianquan Zhang
Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianquan Zhang
Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, People’s Republic of China
Tel/ Fax +86 7715350031
Email [email protected]
Background: Dendritic cells (DCs) with both proinflammatory and tolerogenic properties have been implicated in modulation of CD4+ T cell responses in many fungal diseases. However, the role of DC in the context of Talaromyces marneffei (T. marneffei) infection has not been determined. In this study, we aimed to study the effect of the yeast form of T. marneffei yeasts on DCs, as well as the role of DCs in modulating T helper 17 (Th17) and regulatory T (Treg) cell responses to the pathogen.
Methods: Mouse bone marrow-derived DCs were stimulated with T. marneffei yeasts for 24 h. Frequencies of CD80 and CD86 expression on DCs and the levels of IL-6, IL-10 and TGF-β in the culture supernatant of yeast-stimulated DCs were detected by flow cytometry and ELISA, respectively. In co-culture experiments, CD4+ T lymphocytes of mice were isolated from the spleen using magnetic beads and co-cultured with T. marneffei yeasts, with or without DCs for 24 h. The proportions of Th17 and Treg cells in co-culture were detected by flow cytometry. The mRNA levels of RORγt and Foxp3 were detected by RT-PCR. Levels of IL-10 and TGF-β in the co-culture supernatant were detected by ELISA.
Results: The expressions of CD80 and CD86 on DCs were increased, as well as IL-6, IL-10 and TGF-β levels in the culture supernatant of T. marneffei-stimulated DCs were higher than those in DCs cultured without T. marneffei. In co-culture experiments, in the presence of DCs, T. marneffei promoted Treg expansion and Foxp3 up-regulation but limited Th17 and downregulated RORγt. Levels of IL-10 and TGF-β were higher in the co-culture containing DCs than without DCs.
Conclusion: Our findings demonstrated that the interaction between DCs and T. marneffei could promote Treg expansion but not Th17 generation. These findings provide a mechanism by which DCs may promote immune tolerance in T. marneffei infection.
Keywords: dendritic cells, Talaromyces marneffei, Th17 cells, Treg cells
Introduction
Talaromyces marneffei (T. marneffei), the only thermally dimorphic Penicillium species, is mainly endemic to Southeast Asia, including southern China.1,2 The unique thermally dimorphic fungus grows as a mycelium at 25°C and as yeast at 37°C.3 In recent years, with the rise in the prevalence of human immunodeficiency virus (HIV), the occurrence rate of opportunistic infections of T. marneffei penicilliosis has been significantly increasing.4 Previous studies have indicated that T. marneffei is the third most common agent of clinical infections among patients with acquired immune deficiency syndrome in Southeast Asia, after Mycobacterium tuberculosis and Cryptococcus neoformans, and is associated with increasing mortality rates.2,5 In addition, T. marneffei infection occurs in patients with underlying immune defects without HIV infection.6–8 Recurrent, refractory, and disseminated T. marneffei infections are probably due to immune dysfunction in hosts, but the mechanisms are not fully elucidated.8
Cell-mediated immunity is a major host defense mechanism against T. marneffei.9 In general, the clearance of fungal pathogens mainly relies on the activation of macrophages and cluster differentiation (CD)4+ T cell responses.2 T. marneffei infection can be fatal in individuals with impaired CD4+ T cell function.9 As the most powerful antigen-presenting cells, dendritic cells (DCs) function in pathogen recognition, antigen presentation, and T lymphocytes differentiation.10 There is accumulating evidence regarding the critical role of DCs in modulating the subset of CD4+ T lymphocytes in fungal disease.10–12 The role of DCs in response to pathogens is heterogeneous.10,13 Depending on the fungal pathogen and host microenvironment, DCs can develop into “inflammatory” DCs, which promote the polarization of Th1 and Th17, and the activation of M1 macrophages, or “immunomodulatory” DCs, which promote the generation of Treg cells, Th2 cells, and M2 macrophages.10 However, the role of DCs in T. marneffei infection has not yet been verified.
Th1 and Th17 cells are two major proinflammatory subsets of CD4+ T lymphocytes. Interferon(IFN)-γ producing Th1 cells contribute to macrophage activation and reactive oxygen species production which are critical for killing intracellular T. marneffei.3 While the important role of Th1 cells in host defense against T. marneffei is clearly established,14 the role of Th17 is not well-defined. There is evidence for a protective role of Th17 cells, which drive the antifungal immune response by producing chemokines to recruit neutrophils and macrophages, in addition to the regulation of antimicrobial peptides.15,16 Indeed, a prominent Th17 immune response is effective against infections with Candida,17–19 Aspergillus fumigatus,20 C. neoformans,21 and other fungal pathogens.11,22 In addition, besides the loss of IFN-γ production, impaired production of interleukin (IL)-17A by lymphocytes was also evident in patients with systemic penicilliosis harboring gain-of-phosphorylation signal transducer and activator of transcription (STAT) 1 mutations.7 Based on these findings, it is possible that the Th17 immune response protects against T. marneffei.7 In addition, forkhead box protein 3 (Foxp3+)-expressing regulatory T (Treg) cells, sharing a common precursor with Th17 cells, have emerged as another important subset of CD4+ T lymphocytes.23 Although they share a requirement for TGF-β with Th17 cells for differentiation, Tregs often shows a regulatory role in down-regulating immune responses and contribute to the maintenance self-tolerance.24,25 In most fungal models, Treg cells promote fungal dissemination and immunosuppression.26,27 However, the mechanisms by which Treg cells respond to T. marneffei are still unknown.
In this study, we investigated the in vitro effect of the yeast form of T. marneffei on the maturation and cytokines released by DCs, and explored the role of DCs in Th17 and Treg cell responses to T. marneffei. The pathogenic yeast is the known form of invasion and proliferation,2 while there is no evidence suggesting that the mycelia form of T. marneffei contribute to infection. So we used the yeasts form of T. marneffei to stimulate cells.
Materials and Methods
Animals and Strains
Male BALB/c mice (6–8 weeks old) were obtained from the Guangxi Medical University Laboratory Animal Center. Animals were kept in sterile cages under specific pathogen-free conditions with food and water. All experiments were performed according to the protocols approved by the Laboratory Animal Ethics Committee of Guangxi Medical University (Nanning, China). The T. marneffei strain (GXHCBR) used in our study was isolated from lung, liver, and spleen tissues of an infected bamboo rat in Hechi, Guangxi, China as described previously.28 The strain was previously identified by gold-standard deoxyribonucleic acid sequencing of the fungal internal transcribed spacer region.28 T. marneffei strain was plated in potato dextrose agar medium and incubated at 25°C for 10 days. Colonies were washed with sterile phosphate-buffered saline, and then yeast cells of T. marneffei were isolated by centrifugation.
Preparation of Bone Marrow-Derived DCs
After euthanasia, the thigh bones and tibias of mice were removed. The mononuclear cells of the bone marrow were isolated and collected as previously described.29 Mononuclear cells (1 × 106/mL) were seeded on 6-well plates and cultured in endotoxin free RPMI-1640 medium (Gibco, Waltham, MA, USA) containing granulocyte-macrophage colony-stimulating factor (GM-CSF; 40 ng/mL; PeproTech, London, UK) and IL-4 (10 ng/mL; PeproTech). Half of the old medium was replaced with fresh endotoxin free medium every other day. DCs were collected on the 7th day. The percentage of CD11c+ cells was determined by flow cytometry and the purity was above 70%.
DCs Co-Stimulated with T. marneffei Yeast Cells
DCs were stimulated with or without live T. marneffei yeast cells in 5% CO2 at 37°C for 24 h. The ratio of DCs to yeasts was 1:5 according to a previous study.30 The surface molecules CD80 and CD86 on DCs with or without the stimulation of yeast cells were detected by flow cytometry. The concentrations of IL-6, TGF-β and IL-10 in the culture supernatants were determined by ELISA (Cusabio, Wuhan, China) according to the manufacturer’s instructions.
Isolation of CD4+ T Lymphocytes from the Spleen
Single-cell spleen suspensions were prepared as previously described.31 In brief, the spleen tissues were cut into small pieces and grinded gently with the plunger of a 5-mL syringe until single-cell suspensions were obtained. Samples were filtered through nylon mesh to remove debris, then the spleen cell suspensions were centrifuged at 300 ×g for 10 min at 4°C. The erythrocytes in cell suspensions were eliminated by a lysis solution (Solarbio, Beijing, China). The CD4+ T cells from the spleen single-cell suspensions were isolated by negative selection using the Dynabeads™ Mouse CD4 Kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The purity of CD4+ T cells was above 90% as detected by flow cytometry.
In vitro Cultures of T. marneffei Yeast Cells, DCs, and CD4+ T Lymphocytes
Referring to the methods of co-culture described previously,29,32 the isolated splenic CD4+ T lymphocytes were cultured with live T. marneffei yeast cells with or without DCs. The ratio of CD4+ T lymphocytes to DCs to yeasts was 10: 1: 5. After co-culturing in endotoxin free RPMI-1640 medium in 5% CO2 at 37°C for 24 h, Th17 and Treg cells were detected by flow cytometry. The concentrations of IL-17A, IL-10, and TGF-β in the culture supernatant were determined by ELISA (Cusabio) according to the manufacturer’s instructions.
Flow Cytometry
To evaluate the activation of DCs after stimulation with T. marneffei, DCs were stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD11c, peridinin-chlorophyll-protein (PerCP)-Cy5.5-conjugated anti-CD80, and allophycocyanin (APC)-conjugated anti-CD86 (all purchased from BD Pharmingen, Franklin Lakes, NJ, USA) at 4°C for 30 min and fixed in 1% paraformaldehyde. For the detection of Treg cells, CD4+ T lymphocytes in the co-culture were stained with the surface markers PerCP-conjugated anti-CD4 (BD Pharmingen) and phycoerythrin (PE)-conjugated anti-CD25 (eBioscience, San Diego, CA, USA) at 4°C for 30 min. After surface marker staining, cells were fixed/permeabilized using Foxp3/Transcription Factor Staining Buffer (eBioscience) according to the manufacturer’s instructions, and stained with APC-conjugated anti-Foxp3 (eBioscience). For the detection of Th17 cells, CD4+ T lymphocytes in the co-culture were incubated with phorbol-12-myristate-13-acetate (25 ng/mL; Sigma-Aldrich, St. Louis, MO, USA), ionomycin (1 μg/mL, Sigma-Aldrich), and brefeldin A (Sigma-Aldrich) in 5% CO2 at 37°C for 4 h. The cells were stained with PerCP-conjugated anti-CD4 (BD Pharmingen) at 4°C for 30 min. Subsequently, the cells were fixed and permeabilized for 20 min at 4°C using Fixation/Permeabilization Solution (BD Pharmingen) and then stained with PE-conjugated anti–IL-17 (BD Pharmingen). The matched isotype controls used for flow cytometry were all purchased from BD Pharmingen. Flow cytometry data were acquired using the FAC Canto II system (BD Bioscience) and were analyzed using FlowJo 7.6 software (Treestar, Ashland, OR, USA).
RT-PCR
Total RNA from CD4+ T lymphocytes was isolated using TRIzol reagent (Invitrogen). The quality and quantity of total RNA were analyzed using a spectrophotometer (Nanodrop2000, Thermo Scientific, Waltham, MA, USA). RNA samples were reverse -transcribed into cDNA using a reverse transcription kit (TaKaRa, Kusatsu, China). The mRNA levels of RAR-related orphan receptor-γt (RORγt) and Foxp3 to the control β-actin in individual samples were determined by RT-PCR using SYBR Green I (SYBR®Premix Ex Taq™, TaKaRa) and the Applied Biosystems Step One Plus System (ThermoFisher Scientific). The forward and reverse primer sequences were as follows: β-actin, 5′-CATCCGTAAAGACCTCTATGCCAAC-3′ and 5′-ATGGAGCCACCGATCCACA-3′; RORγt, 5′-GCTCCATATTTGACTTTTCCCACT-3′ and 5′- GATGTTCCACTCTCCTCTTCTCTTG-3′; and Foxp3, 5′- AGTGCCTGTGTCCTCAATGGTC-3′ and 5′-AGGGCCAGCATAGGTGCAAG-3′. DNA was amplified for 40 cycles under the following conditions: denaturation at 95°C for 30 s, extension at 95°C for 5 s, and then 60°C for 30 s. mRNA levels were evaluated by the 2−ΔΔCt method. All experiments were repeated at least three times.
Statistical Analysis
Data are expressed as means ± SD. Differences between two groups were analyzed by the two-tailed Student’s t-test or non-parametric Mann–Whitney U-test. Differences between three or more groups were assessed by ANOVA followed by LSD or Tamhane’s T2 post hoc tests. The data were analyzed using SPSS 17.0 (IBM, Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
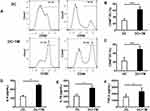
T. marneffei Induced the Activation and Secretion of IL-6, IL10 and TGF-β in DCs in vitro
To determine whether DCs react to T. marneffei yeast cells, we evaluated the positive expression of CD80 and CD86 on DCs with or without stimulation by T. marneffei for 24 h in vitro. The frequencies of CD80 and CD86 expression were markedly higher in yeast-simulated DCs than in un-stimulated DCs (62.57 ± 8.36% vs 29.24 ± 5.99% and 48.87 ± 7.17% vs 22.35 ± 7.83%, respectively; both P < 0.001, Figure 1A–C). These results confirmed that DCs could recognize and be activated by T. marneffei yeast cells. In addition, we detected significantly higher soluble IL-6, IL-10 and TGF-β levels in the supernatant of DCs stimulated with the yeast cells than in the supernatant of un-stimulated DCs cultured alone (all P < 0.05; Figure 1D–F).
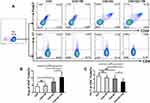
T. marneffei Promoted Treg Expansion but Not Th17 in the Presence of DCs in vitro
DCs modulate the differentiation of lymphocytes in a pathogen-specific manner.10–12 To investigate the role of DCs in the immune response of Th17 and Treg cells to T. marneffei, we co-cultured CD4+ T lymphocytes with T. marneffei with or without DCs. Surprisingly, the proportion of Treg cells was significantly higher in the co-culture of CD4+ T lymphocytes and T. marneffei with DCs than without DCs (P < 0.05, Figure 2A and B). In contrast, the proportion of Th17 cells was lower in the co-culture of CD4+ T lymphocytes and T. marneffei together with DCs compared to that without DCs (P < 0.05; Figure 2A–C). There were no differences in the proportions of Treg cells or Th17 cells between the co-culture of CD4+ T lymphocytes and T. marneffei compared with CD4+ T lymphocytes alone (P > 0.05; Figure 2B and C).
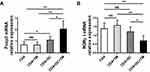
T. marneffei Up-Regulated Foxp3 and Down-Regulated RORγt Expression in CD4+ T Lymphocytes in the Presence of DCs
We further evaluated the expression levels of Foxp3 and RORγt, which are the key transcription factors in Th17 and Treg cells, respectively, in the co-culture containing CD4+ T cells, DCs and T. marneffei by qRT-PCR. Foxp3 mRNA expression was up-regulated in the co-culture consisting of CD4+ T lymphocytes, T. marneffei yeast cells and DCs, whereas RORγt mRNA expression was down-regulated compared with levels in the co-culture consisting of CD4+ T lymphocytes and T. marneffei yeast cells, but without DCs (P < 0.05; Figure 3A and B). There were no differences in the Foxp3 mRNA expression or RORγt mRNA expression between the co-culture of CD4+ T lymphocytes and T. marneffei compared with CD4+ T lymphocytes alone (P > 0.05; Figure 3A and B).
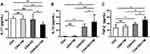
T. marneffei Induced IL-10 and TGF-β Secretion in the Co-Culture Consisting of DCs and CD4+ T Lymphocytes
The concentration of IL-17 in the culture supernatant was lower in the co-culture consisting of CD4+ T lymphocytes, T. marneffei yeast cells, and DCs than in the culture conditions lacking DCs (P < 0.05; Figure 4A), consistent with the decrease in the Th17 proportion and down-regulation of RORγt expression. Conversely, the levels of IL-10 and TGF-β were higher in supernatant of the co-culture consisting of CD4+ T lymphocytes, T. marneffei yeast cells, and DCs, than in that without DCs (P < 0.05; Figure 4B and C). There were no differences in the IL-17, IL-10 or TGF-β levels between the co-culture of CD4+ T lymphocytes and T. marneffei compared with CD4+ T lymphocytes alone (P > 0.05; Figure 4A–C).
Discussion
DCs play a critical role in modulating the inflammatory and regulatory responses to fungal infection.10,12 Mature DCs can recognize pathogens, secrete varies of cytokines, and thereby regulate the differentiation and proliferation of helper T cell subsets.12 Our results demonstrated that DCs exhibit greater surface co-stimulatory molecule expression (CD80 and CD86) and secrete higher levels of IL-6, IL-10 and TGF-β when directly stimulated with T. marneffei yeast cells. Moreover, T. marneffei yeast-stimulated DCs increased Treg cells, and limited Th17 cells together with increased IL-10 in the co-culture. These findings suggested that DCs could promote Treg expansion and limit the proinflammatory pattern of Th17 in response to pathogenic T. marneffei.
Naïve CD4+ T lymphocytes could be activated by T cell receptor signaling and co-stimulatory interactions, and differentiate into different subtypes of Th cells according to the kind of cytokines in the inflammatory milieu.33 For Th17, IL-6 plays an important role during the initial phase of differentiation by activating STAT3, which directly promotes the transcription of Th17-specific genes such as Rorc (encoding RORγt in T cells), and the synthesis of IL-17 and IL-23.23 IL-6 and low levels of TGF-β support Th17 cell development, while high levels of TGF-β support Treg differentiation.33 Interestingly, in our study, although DCs exhibited increases in the secretion of IL-6 and TGF-β, we found that Treg cells, but not Th17 cells were increased in the co-culture. Notably, the abundant secretion of IL-10 only occurred in the co-culture containing DCs, CD4+ T lymphocytes and T. marneffei yeast cells, but was not observed in the absence of DCs. Our findings indicate that DCs are likely one of the key generators of IL-10 in response to T. marneffei infection. The differential recognition of specific components on the cell wall of yeasts/spores and hyphae, such as zymosan or mannoproteins by pathogen recognition receptors, could activate the specific signaling of DCs, which is critical for the phenotype of immune responses.21,34–37 The primed DCs could secrete large amounts of IL-10, leading to impaired Th cell responses and an increased tolerogenic T cell population.21,34,35 Although we demonstrated that T. marneffei yeast cells could induce IL-10 secreted by DCs, further studies are needed to identify the specific components are recognized by DCs and promote IL-10 production in T. marneffei infection.
Treg cells may be another source of increasing IL-10 in T. marneffei infection. It has been found that the persistent secretion of IL-10 and TGF-β may establish an autocrine loop to promote Treg expansions.24,26,33 Although Treg cells could not directly recognize T. marneffei yeast cells, they could be modulated by the cytokine environment and immune checkpoints pathways mediated by DCs.10,38 DCs recognizing antigens of T. marneffei could contribute to the production and secretion of IL-10 and TGF-β, and thereby provide the cytokine environment for Treg expansions. In addition, DCs could also promote the development of Treg cells and inhibit the development of Th1 and Th17 cells via specific immunomodulatory molecules such as programmed cell death protein (PD)-1 and cytotoxic T lymphocyte-associated protein (CTLA)-4 pathways.10,38,39
Th17 and Treg cells always play opposing roles in fungal infection.12,27,40 An impaired balance of Th17/Treg is associated with disease progression, while the inhibition or depletion of Treg cells could potentially rescue Th1/Th17 immunity and improve prognosis.12,27,40 Foxp3 could inhibit RORγt and down-regulate the expression of RORγt-mediated IL-17.41 Therefore, the dominance of Treg cells could be associated with the down-regulation of RORγt expression and the suppression of Th17 cells. On the basis of previous studies,2,7,28 we predicted that increased inflammatory effector T cells (such as Th1 and Th17) and M1 macrophages activation in response to T. marneffei are beneficial for pathogen elimination. Given that Th17 is critical for host defense in fungal infections,17–19,21,22 the immune cell pattern switching to a tolerogenic phenotype may be harmful for the defense against T. marneffei. The sustained expression of IL-10 is associated with the persistence of many fungal or parasite infections.21,42,43 Conversely, blocking IL-10 could improve fungal clearance in infected mice.21,43 We speculated that the accumulation of IL-10-producing DCs and Treg cells may be one of the reasons accounting for the persistence and refractoriness of T. marneffei infection, but further evidence is needed.
This is the first study that focusd on the tolerogenic role of DCs in the modulation of Treg and Th17 cells in response to T. marneffei yeast cells. There are some limitations in this study. Firstly, we confirmed that DCs promoted the increase of Treg cells in response to T. marneffei yeast cells, but we did not study whether the additional Foxp3+ Treg cells came from expansion of pre-existing Treg cells or from de novo conversion of naïve T cells. Secondly, our study lacks of in vivo observations.
Conclusions
Taken together, we demonstrated that DCs could promote Treg cell expansions and limit Th17 cell responses after the recognition of T. marneffei yeast cells. Our findings provide direct evidence that DCs could serve a tolerogenic role in response to T. marneffei yeast cells. Accordingly, this role could induce immune tolerance and thereby may be harmful to the host defense against T. marneffei infection.
Abbreviations
HIV, human immunodeficiency virus; DC, dendritic cell; T. marneffei, Talaromyces marneffei; CD, cluster differentiation; IL, interleukin; ELISA, enzyme-linked immunosorbent assays; RT-PCR, real-time polymerase chain reaction; Foxp3, forkhead box protein 3; Th17, T Helper cell 17; Treg, regulatory T; IFN, interferon; TGF, transforming growth factor; STAT1, signal transducer and activator of transcription 1; RNA, ribonucleic acid; FITC, fluorescein isothiocyanate; PerCP, peridinin-chlorophyll-protein; APC, allophycocyanin; PE, phycoerythrin; RORγt, RAR-related orphan receptor γt; PD-1, programmed cell death protein-1; CTLA-4, cytotoxic T lymphocyte-associated protein 4; SD, standard deviation.
Ethics Approval
The protocols of the present study were approved by the Laboratory Animal Ethics Committee of Guangxi Medical University (Nanning, China). Animal ethics review followed the Guiding Opinions on the Treatment of Laboratory Animals issued by the Ministry of Science and Technology of the People’s Republic of China and the Laboratory Animal-Guideline for Ethical Review of Animal Welfare issued by the National Standard GB/T35892-2018 of the People’s Republic of China.
Acknowledgments
This study was funded by grants from the National Natural Science Foundation of China [grant number 81760010 and 81460009] and Guangxi Natural Science Foundation of China [grant number 2015GXNSFAA139189].
Author Contributions
Yanping Tang and Hui Zhang should be considered co-first authors. All authors made substantial contributions to the conception and design of the study, the acquisition, analysis, and interpretation of data, and drafting the manuscript and all authors gave final approval for publication and agree to be accountable for all aspects of the work.
Disclosure
No conflicts of interest, financial or otherwise, are declared by the authors.
References
1. Qiu Y, Tang Y, Zhang J, et al. A retrospective analysis of seven patients with acquired immunodeficiency syndrome and pharyngeal and/or laryngeal Talaromyces marneffei infection. Clin Otolaryngol. 2017;42(5):1061–1066. doi:10.1111/coa.2017.42.issue-5
2. Vanittanakom N, Cooper CR
3. Boyce KJ, Andrianopoulos A. Fungal dimorphism: the switch from hyphae to yeast is a specialized morphogenetic adaptation allowing colonization of a host. FEMS Microbiol Rev. 2015;39(6):797–811. doi:10.1093/femsre/fuv035
4. Limper AH, Adenis A, Le T, Harrison TS. Fungal infections in HIV/AIDS. Lancet Infect Dis. 2017;17(11):e334–e343. doi:10.1016/S1473-3099(17)30303-1
5. Bulterys PL, Le T, Quang VM, Nelson KE, Lloyd-smith JO. Environmental predictors and incubation period of AIDS-associated penicillium marneffei infection in Ho Chi Minh City, Vietnam. Clin Infect Dis. 2013;56(9):1273–1279. doi:10.1093/cid/cit058
6. Kawila R, Chaiwarith R, Supparatpinyo K. Clinical and laboratory characteristics of penicilliosis marneffei among patients with and without HIV infection in Northern Thailand: a retrospective study. BMC Infect Dis. 2013;13:464. doi:10.1186/1471-2334-13-464
7. Lee PP, Mao H, Yang W, et al. Penicillium marneffei infection and impaired IFN-gamma immunity in humans with autosomal-dominant gain-of-phosphorylation STAT1 mutations. J Allergy Clin Immunol. 2014;133(3):894–896.e895. doi:10.1016/j.jaci.2013.08.051
8. Chan JF, Lau SK, Yuen KY, Woo PC. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg Microbes Infect. 2016;5(3):e19. doi:10.1038/emi.2016.18
9. Kudeken N, Kawakami K, Kusano N, Saito A. Cell-mediated immunity in host resistance against infection caused by Penicillium marneffei. J Med Vet Mycol. 1996;34(6):371–378. doi:10.1080/02681219680000671
10. Roussey JA, Olszewski MA, Osterholzer JJ. Immunoregulation in fungal diseases. Microorganisms. 2016;4(4):47. doi:10.3390/microorganisms4040047
11. LeibundGut-Landmann S, Wuthrich M, Hohl TM. Immunity to fungi. Curr Opin Immunol. 2012;24(4):449–458. doi:10.1016/j.coi.2012.04.007
12. Romani L. Immunity to fungal infections. Nat Rev Immunol. 2011;11(4):275–288. doi:10.1038/nri2939
13. Iberg CA, Jones A, Hawiger D. Dendritic cells as inducers of peripheral tolerance. Trends Immunol. 2017;38(11):793–804. doi:10.1016/j.it.2017.07.007
14. Sisto F, Miluzio A, Leopardi O, Mirra M, Boelaert JR, Taramelli D. Differential cytokine pattern in the spleens and livers of BALB/c mice infected with Penicillium marneffei: protective role of gamma interferon. Infect Immun. 2003;71(1):465–473. doi:10.1128/IAI.71.1.465-473.2003
15. Richardson JP, Moyes DL. Adaptive immune responses to Candida albicans infection. Virulence. 2015;6(4):327–337. doi:10.1080/21505594.2015.1004977
16. Conti HR, Gaffen SL. IL-17-mediated immunity to the opportunistic fungal pathogen Candida albicans. J Immunol. 2015;195(3):780–788. doi:10.4049/jimmunol.1500909
17. Deng Z, Ma S, Zhou H, et al. Tyrosine phosphatase SHP-2 mediates C-type lectin receptor-induced activation of the kinase Syk and anti-fungal TH17 responses. Nat Immunol. 2015;16(6):642–652. doi:10.1038/ni.3155
18. Underhill DM, Pearlman E. Immune interactions with pathogenic and commensal fungi: a two-way street. Immunity. 2015;43(5):845–858. doi:10.1016/j.immuni.2015.10.023
19. Saijo S, Ikeda S, Yamabe K, et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32(5):681–691. doi:10.1016/j.immuni.2010.05.001
20. Schlitzer A, McGovern N, Teo P, et al. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity. 2013;38(5):970–983. doi:10.1016/j.immuni.2013.04.011
21. Murdock BJ, Teitz-Tennenbaum S, Chen GH, et al. Early or late IL-10 blockade enhances Th1 and Th17 effector responses and promotes fungal clearance in mice with cryptococcal lung infection. J Immunol. 2014;193(8):4107–4116. doi:10.4049/jimmunol.1400650
22. Wheeler ML, Limon JJ, Underhill DM. Immunity to commensal fungi: detente and disease. Annu Rev Pathol. 2017;12:359–385. doi:10.1146/annurev-pathol-052016-100342
23. Lochner M, Wang Z, Sparwasser T. The special relationship in the development and function of T helper 17 and regulatory T cells. Prog Mol Biol Transl Sci. 2015;136:99–129.
24. Lee YK, Mukasa R, Hatton RD, Weaver CT. Developmental plasticity of Th17 and Treg cells. Curr Opin Immunol. 2009;21(3):274–280. doi:10.1016/j.coi.2009.05.021
25. Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev. 2014;13(6):668–677. doi:10.1016/j.autrev.2013.12.004
26. Ferreira MC, de Oliveira RT, da Silva RM, Blotta MH, Mamoni RL. Involvement of regulatory T cells in the immunosuppression characteristic of patients with paracoccidioidomycosis. Infect Immun. 2010;78(10):4392–4401. doi:10.1128/IAI.00487-10
27. Galdino NAL, Loures FV, de Araujo EF, da Costa TA, Preite NW, Calich VLG. Depletion of regulatory T cells in ongoing paracoccidioidomycosis rescues protective Th1/Th17 immunity and prevents fatal disease outcome. Sci Rep. 2018;8(1):16544. doi:10.1038/s41598-018-35037-8
28. Dai X, Mao C, Lan X, et al. Acute Penicillium marneffei infection stimulates host M1/M2a macrophages polarization in BALB/C mice. BMC Microbiol. 2017;17(1):177. doi:10.1186/s12866-017-1086-3
29. Kuang LJ, Deng TT, Wang Q, et al. Dendritic cells induce Tc1 cell differentiation via the CD40/CD40L pathway in mice after exposure to cigarette smoke. Am J Physiol Lung Cell Mol Physiol. 2016;311(3):L581–589. doi:10.1152/ajplung.00002.2016
30. Romagnoli G, Nisini R, Chiani P, et al. The interaction of human dendritic cells with yeast and germ-tube forms of Candida albicans leads to efficient fungal processing, dendritic cell maturation, and acquisition of a Th1 response-promoting function. J Leukoc Biol. 2004;75(1):117–126. doi:10.1189/jlb.0503226
31. Liang Y, Shen Y, Kuang L, et al. Cigarette smoke exposure promotes differentiation of CD4+ T cells toward Th17 cells by CD40-CD40L costimulatory pathway in mice. Int J Chron Obstruct Pulmon Dis. 2018;13:959–968. doi:10.2147/COPD.S155754
32. Jiang Y, Zhao S, Yang X, Liu Y, Wang C. Dll4 in the DCs isolated from OVA-sensitized mice is involved in Th17 differentiation inhibition by 1, 25-dihydroxyvitamin D3 in vitro. J Asthma. 2015;52(10):989–995. doi:10.3109/02770903.2015.1056349
33. Caza T, Landas S. Functional and phenotypic plasticity of CD4+ T cell subsets. Biomed Res Int. 2015;2015:521957. doi:10.1155/2015/521957
34. Dillon S, Agrawal S, Banerjee K, et al. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J Clin Invest. 2006;116(4):916–928. doi:10.1172/JCI27203
35. Manoharan I, Hong Y, Suryawanshi A, et al. TLR2-dependent activation of beta-catenin pathway in dendritic cells induces regulatory responses and attenuates autoimmune inflammation. J Immunol. 2014;193(8):4203–4213. doi:10.4049/jimmunol.1400614
36. Rizzetto L, Kuka M, De Filippo C, et al. Differential IL-17 production and mannan recognition contribute to fungal pathogenicity and commensalism. J Immunol. 2010;184(8):4258–4268. doi:10.4049/jimmunol.0902972
37. Smeekens SP, van de Veerdonk FL, van der Meer JW, Kullberg BJ, Joosten LA, Netea MG. The Candida Th17 response is dependent on mannan- and beta-glucan-induced prostaglandin E2. Int Immunol. 2010;22(11):889–895. doi:10.1093/intimm/dxq442
38. Hasegawa H, Matsumoto T. Mechanisms of tolerance induction by dendritic cells in vivo. Front Immunol. 2018;9:350. doi:10.3389/fimmu.2018.00350
39. Sirvent S, Soria I, Cirauqui C, et al. Novel vaccines targeting dendritic cells by coupling allergoids to nonoxidized mannan enhance allergen uptake and induce functional regulatory T cells through programmed death ligand 1. J Allergy Clin Immunol. 2016;138(2):558–567.e511. doi:10.1016/j.jaci.2016.02.029
40. de Araujo EF, Feriotti C, Galdino NAL, Preite NW, Calich VLG, Loures FV. The IDO-AhR axis controls Th17/Treg immunity in a pulmonary model of fungal infection. Front Immunol. 2017;8:880. doi:10.3389/fimmu.2017.00880
41. Zhou L, Lopes JE, Chong MM, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453(7192):236–240. doi:10.1038/nature06878
42. Loevenich K, Ueffing K, Abel S, et al. DC-derived IL-10 modulates pro-inflammatory cytokine production and promotes induction of CD4+IL-10+ regulatory T cells during Plasmodium yoelii infection. Front Immunol. 2017;8:152. doi:10.3389/fimmu.2017.00152
43. Teitz-tennenbaum S, Viglianti SP, Roussey JA, Levitz SM, Olszewski MA, Osterholzer JJ. Autocrine IL-10 signaling promotes dendritic cell type-2 activation and persistence of murine cryptococcal lung infection. J Immunol. 2018;201(7):2004–2015. doi:10.4049/jimmunol.1800070
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.