Back to Journals » Cancer Management and Research » Volume 13
Demographics, Changes in Treatment Patterns, and Outcomes of Bone and Soft Tissue Sarcomas in Korea—A Sarcoma-Specific, Institutional Registry-Based Analysis
Authors Jeong H, Im HS, Kim W, Lee JS, Song SY, Song JS, Cho KJ, Chung HW, Lee MH, Kim JE, Ahn JH
Received 7 September 2021
Accepted for publication 2 November 2021
Published 24 November 2021 Volume 2021:13 Pages 8795—8802
DOI https://doi.org/10.2147/CMAR.S337606
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Hyehyun Jeong,1,* Hyeon-Su Im,1,2,* Wanlim Kim,3 Jong-Seok Lee,3 Si Yeol Song,4 Joon Seon Song,5 Kyung-Ja Cho,5 Hye Won Chung,6 Min Hee Lee,6 Jeong Eun Kim,1,* Jin-Hee Ahn1,*
1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea; 2Division of Hematology and Oncology, Ulsan University Hospital, Ulsan, Republic of Korea; 3Department of Orthopedic Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea; 4Department of Radiation Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea; 5Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea; 6Department of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea
*These authors contributed equally to this work
Correspondence: Jeong Eun Kim; Jin-Hee Ahn
Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, 88, Olympic-ro 43-gil, Songpa-gu, Seoul, Republic of Korea
Tel +82-2-3010-3945
; +82-2-3010-3222
Fax +82-2-3010-6961
Email [email protected]; [email protected]
Purpose: Because of the heterogeneity of sarcomas, establishing a well-collected, sarcoma-specific database is important for sarcoma research. We analyzed the first histology-based, sarcoma-specific institutional registry in Korea, which collected 28 years of patient data according to a predefined data format.
Patients and Methods: Adult bone and soft tissue sarcoma patients who were treated from June 1989 to January 2017 were identified and analyzed, based on the ICD-O-3 codes.
Results: Among the 3420 patients included, soft tissue and bone sarcomas comprised 77.8% (n = 2661) and 22.2% (n = 759), respectively. Median age at diagnosis was 50 (range, 16– 98) in soft tissue sarcomas and 37 (range, 16– 85) in bone sarcomas. Males and females comprised 45.5% and 54.5% of soft tissue sarcomas and 52.7% and 47.3% of bone sarcomas, respectively. Among the 3407 patients with treatment data available, 90.5% of the patients with soft tissue sarcomas and 80.8% of the patients with bone sarcomas received surgery first, of which 57.8% and 71.7% did not receive any subsequent treatment, respectively. Overall, the proportion of patients who received surgery alone decreased from 85.7% to 60.5% from the pre-2000 period to the 2010– 2017 period. However, the use of adjuvant chemotherapy increased in patients with soft tissue sarcomas (from 8.0% to 17.2% in the same period), and the use of perioperative radiotherapy also increased in both groups (from 1.4% to 22.7% in soft tissue sarcomas, and 0% to 14.5% in bone sarcomas in the same period). In both soft tissue and bone sarcomas, old age (≥ 65 years) and diagnosis in the early study period were associated with poorer survival.
Conclusion: We presented a comprehensive summary of our sarcoma registry, including the demographics, changes in treatment patterns, and survival outcomes. This study will provide a framework for future studies.
Keywords: bone sarcoma, soft tissue sarcoma, sarcoma-specific registry, real-world evidence
Introduction
Sarcomas are solid tumors of mesenchymal origin and are roughly categorized into soft tissue sarcomas and bone sarcomas. They comprise more than 100 diseases, which have a variety of clinical presentations and different prognoses.1,2 Because they can occur anywhere throughout the whole body, the prevalence of sarcomas is difficult to estimate. Also, due to their wide heterogeneity, a specific subcategory of sarcomas often includes only a limited number of patients, which makes it difficult to conduct randomized controlled trials. Therefore, large-sized, population-based or institutional registries have been used in many previous sarcoma studies.3,4
Although these large-sized databases have provided important groundwork for generating clinical evidence in the management of sarcomas, their datasets have several pitfalls. For example, they might underestimate the incidence of the diseases because of inadequate coding, as sarcomas are often miscoded according to their anatomic site rather than being recorded with an adequate pathological diagnosis.5,6 Also, large-sized datasets from billing or administrative data often miss clinically relevant data.3 Therefore, establishing an accurate, sarcoma-specific registry based on histologic diagnosis and including clinically meaningful variables is important for sarcoma research. Several well collected, sarcoma-specific registries, and successful analyses using them, have been reported, mostly in Western populations.7–9 In Korea, no sarcoma-specific, registry-based analyses have been reported previously.
This study aimed to explore and present the overall demographics, changes in treatment patterns, and survival outcomes of Korean sarcoma patients who were treated in a tertiary referral center that has a multidisciplinary expert sarcoma team. Here, we analyzed the first institutional sarcoma-specific registry data in Korea. We identified patients based on histologic diagnosis and collected 28 years of patient data, according to a predefined data format.
Materials and Methods
Study Overview and the AMC Sarcoma Registry
This study is a single-center, registry-based analysis focused on bone and soft tissue sarcoma patients who were treated at Asan Medical Center (AMC), a tertiary referral center in Seoul, Republic of Korea. This study was approved by the Institutional Review Board of the AMC and conducted in accordance with the Declaration of Helsinki. The Board granted an informed consent waiver for this retrospective study due to its retrospective design and the use of de-identified data without patient contact or intervention.
The AMC sarcoma registry was first launched in 2014. Adult patients (≥16 years) who visited AMC since June 1989 and were histologically diagnosed with bone and soft tissue sarcoma of intermediate or malignant potential were identified based on International Classification of Diseases for Oncology, Third Edition (ICD-O-3) codes. The ICD-O-3 codes were curated by trained abstractors using diagnoses generated by expert pathologists. The data source for this study is a deidentified electronic health record (EHR) system (Asan BiomedicaL research Environment, ABLE), which served as an honest broker.10 The registry includes the following information: 1) patient demographics, including age at diagnosis and sex; 2) tumor characteristics, including histologic diagnosis of the tumor and its anatomical location; 3) treatment-related variables, including information on surgery, chemotherapy, and radiotherapy; and 4) survival status and dates, retrieved from Korea Central Cancer Registry data and inhospital survival data. The registry is regularly updated by dedicated data managers who add new patients, as per inclusion criteria, and update existing patient data. In this study, patients who visited AMC between June 1989 and January 2017 were included. Patients without any record of treatment or without survival outcomes or dates were excluded.
Statistical Analysis
Overall survival (OS) was defined as the time from the date of diagnosis to the date of death from any cause. Baseline characteristics of the patients were assessed using a descriptive method. Survival outcomes were estimated using the Kaplan–Meier method and compared by Log rank tests. All tests were two-sided, and a p-value of <0.05 was considered statistically significant. All statistical analyses were performed using R version 4.0.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patients
During the study period, a total of 4360 adult sarcoma patients (≥16 years) who visited AMC between 1989 and 2017 were identified. Of these, patients without any treatment history (n = 924) or survival data (n = 16) were excluded, leaving a total of 3420 patients included in the analysis, including 2661 soft tissue sarcomas and 759 bone sarcomas.
The baseline characteristics of the patients are summarized in Table 1. Median age at diagnosis was 50 (range, 16–98) in soft tissue sarcomas and 37 (16–85) in bone sarcomas. Males and females comprised 45.5% and 54.5% of soft tissue sarcomas and 52.7% and 47.3% of bone sarcomas, respectively. For soft tissue sarcomas, the most common histologic category was undifferentiated/unclassified sarcomas (22.8%, n = 607), followed by fibroblastic/myofibroblastic tumors (21.0%, n = 560), and adipocytic tumors (14.7%, n = 392). By specific histology, leiomyosarcoma was the most common tumor (13.0%, n = 346) (Figure 1A). For bone sarcomas, the most common histologic category was chondrogenic tumors (27.5%, n = 209), followed by osteogenic tumors (22.5%, n = 171), and osteoclastic giant cell-rich tumors (20.6%, n = 156). By specific histology, giant cell tumor of bone (20.6%, n = 156) was the most common tumor (Figure 1B).
 |
Table 1 Baseline Characteristics |
 |
Figure 1 Pie chart for histologic diagnosis. (A) Soft tissue sarcoma. (B) Bone sarcoma. |
Treatment Patterns
Treatment patterns were analyzed in 3407 patients whose treatment dates were available (Table 1). The initial treatment strategy included surgery in 92.9% of the patients (n = 3164); specifically, 93.2% (n = 2470) of soft tissue sarcomas and 91.8% (n = 756) of bone sarcomas. Among those, neoadjuvant/adjuvant chemotherapy or radiotherapy was administered in 28.0% (n = 743) and 25.4% (n = 192) of the patients with soft tissue and bone sarcomas, respectively. In soft tissue sarcomas, adjuvant chemotherapy was more commonly administered than neoadjuvant chemotherapy (adjuvant, 15.2% vs neoadjuvant, 1.8%) whereas in bone sarcomas, the rates of patients receiving neoadjuvant and adjuvant chemotherapy were similar (adjuvant, 10.4% vs neoadjuvant, 10.8%). Surgical margin status was available in 42% of the surgically treated patients (n = 1341). Among those, R0 resection was achieved in 71.6% (n = 793) and 86.3% (n = 202) in soft tissue and bone sarcomas, respectively; and R1 resection was achieved in 25.9% (n = 287) and 11.5% (n = 27) in soft tissue and bone sarcomas, respectively.
Among the 243 patients who did not receive surgery as initial treatment, chemotherapy alone was the most common approach in both the soft tissue sarcomas (62.4%, n = 113/181) and bone sarcomas (58.1%, n = 36/62). The median duration of first chemotherapy in non-surgically treated patients was 2.2 months (95% CI, 1.5–3.8) and 3.1 months (95% CI, 1.9–5.0) in soft tissue and bone sarcomas, respectively.
Overall, the most commonly used first-line chemotherapeutic regimens were CYVADIC (cyclophosphamide, vincristine, doxorubicin, dacarbazine; n = 174) and ID (ifosfamide, doxorubicin; n = 95) in soft sarcomas; and HD-MTX/AP (high-dose methotrexate, doxorubicin, cisplatin; n = 88) and VAC/IE (vincristine, doxorubicin, cyclophosphamide, etoposide, ifosfamide; n = 60) in bone sarcomas. The median radiation dose given was 54 Gy (interquartile range, 45–60 Gy).
Treatment flow by sequence is illustrated in Figure 2. About 90.5% and 80.8% of the soft tissue and bone sarcoma patients received upfront surgery as the first treatment method, respectively. Among those initially surgically treated patients, 57.8% of the patients with soft tissue sarcomas and 71.7% of the patients with bone sarcomas did not receive any subsequent treatment. Also, among patients who were treated with initial chemotherapy, 26.8% of soft tissue sarcomas proceeded with subsequent surgery, whereas 66.2% of bone sarcomas proceeded with subsequent surgery.
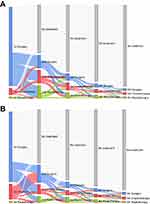 |
Figure 2 Sankey diagram showing sequential treatment flow of the study population. (A) Soft tissue sarcoma. (B) Bone sarcoma. |
Treatment Pattern by Diagnosis Time
Treatment patterns by year of diagnosis are shown in Figure 3. Across all time periods, the most common first treatment was surgery alone in both soft tissue and bone sarcomas. However, the proportion of patients who received surgery alone as initial treatment decreased over time (overall, 85.7% [n = 335] in the pre-2000 period to 60.5% [n = 1087] in the 2010–2017 period). The use of adjuvant chemotherapy and perioperative radiotherapy increased in patients with soft tissue sarcomas (8.0% [n = 23] in the pre-2000 period to 17.2% [n = 247] in the 2010–2017 period). The use of perioperative radiotherapy also increased in both groups (1.4% [n = 4] to 22.7% [n = 326] in soft tissue sarcomas, and 0% [n = 0] to 14.5% [n = 52] in bone sarcomas).
Survival Outcomes in the Overall Study Population
Figure 4 illustrates OS by patient characteristics. The median follow-up duration was 38.6 months (95% confidence interval [CI], 36.6–41.4 months). The 5-year survival rates were 78.3% (95% CI, 76.2–80.3) and 82.0% (95% CI, 78.5–85.0) in soft tissue sarcomas and bone sarcomas, respectively (Figure 4A). In terms of age at diagnosis, elderly patients (≥65 years at diagnosis) had poorer OS than other age groups both in soft tissue and bone sarcomas. In soft tissue sarcomas, the 5-year survival rate of elderly patients was 64.4% (95% CI, 57.6–70.4), whereas it was 82.3% (95% CI, 76.1–87.1%) for adolescents and young adults (AYA) (<30 years) and 80.5% (95% CI, 78.2–82.6%) for adults (30–64 years). In bone sarcomas, the 5-year survival rates of AYA, adults, and elderly patients were 81.5% (95% CI, 75.4–86.2%), 85.1% (95% CI, 80.6–88.6%), and 62.7% (95% CI, 45.7–75.7%), respectively (Figure 4B). Survival outcomes improved over time, especially in soft tissue sarcomas. In soft tissue sarcomas, the 5-year survival rates of patients diagnosed during the pre-2000, 2000–2009, and 2010–2017 periods were 60.6% (95 CI, 53.7–66.7%), 70.1% (95% CI, 66.9–73.2%), and 92.7% (89.9–94.8%), respectively. In bone sarcomas, those were 74.8% (95% CI, 64.4–82.6%), 74.8% (95% CI, 69.0–79.6), and 93.4% (95% CI, 89.0–96.1%). Similar results were observed when patients diagnosed after 2013 were excluded from the analysis to ensure a longer follow-up duration for recently diagnosed patients; five-year survival rates for patients diagnosed between 2010 and 2013 were 91.5% (95% CI, 88.3–93.8%) and 93.7% (95% CI, 88.8–96.5%) for soft tissue and bone sarcomas, respectively. Detailed survival outcomes of the patients are shown in the Supplementary Table S1.
 |
Figure 4 Overall survival by (A) origin of tumors, (B) age at diagnosis, and (C) year of diagnosis. Abbreviations: AYA, adolescents and young adults; GU/GYN, genitourinary and gynecologic. |
Discussion
In this study, we analyzed our histology-based, sarcoma-specific institutional registry, which includes a relatively large amount of patient data from 28 years of records, constructed according to a predetermined data collection plan. Here, we comprehensively presented the patient characteristics, changes in treatment patterns, and survival outcomes. To our knowledge, this is the first sarcoma-specific registry data to be reported in Korea.
We observed that among 3420 patients included in the study, surgery comprised the mainstay of treatment, with more than 90% of patients with soft tissue sarcomas and 80% of patients with bone sarcomas receiving surgery as their first treatment. This is consistent with previous studies that used Korean National Health Insurance Service (NHIS) data (82%) or American Surveillance, Epidemiology, and End Results data (88%).11,12 After the initial surgery, a majority of patients (58% of soft tissue sarcomas and 72% of bone sarcomas) did not receive any subsequent treatment. Moreover, surgery was a major treatment option across all courses of treatment, as it accounted for more than 1/3 of subsequent treatments.
Although a majority of patients received surgery alone, the proportion of patients who received perioperative radiotherapy or adjuvant chemotherapy increased over time. This can be ascribed to increased understanding of the effectiveness of adequate radiotherapy, advances in radiotherapy techniques, and (despite conflicts) the benefits of adjuvant chemotherapy for sarcoma management, in terms recurrence-free survival.13–15 These changes in treatment patterns along with the implementation of multidisciplinary team-based treatment decision during the study period may explain the improved survival outcomes in more recently diagnosed patients, consistent with previous reports of improved survival outcomes over time.16,17 However, careful interpretation is required, taking into consideration other factors, such as heterogeneity of sarcomas, data censoring issues, and conflicting survival outcomes from previous studies of perioperative, multimodal treatment. The prognosis of patients who did not receive surgery was poor, which suggests a poor prognosis in unresectable/advanced staged sarcoma.18,19 Also, elderly patients showed poor prognoses, despite treatment, consistent with previous data.20
One of the major strengths of our registry is that it includes a relatively large number of patients who were treated in a tertiary referral center that has an expert multidisciplinary team for sarcoma management, with longitudinal follow-up data for each patient. Although the incidence or prevalence of sarcoma in Korea has not been clearly demonstrated, a previous study that identified sarcoma patients using the International Classification of Diseases, 10th revision (ICD-10) reported a nationwide annual incidence of 760–1175/year.11 Therefore, despite being a single-institutional registry, we believe that our data includes a considerable number of patients.
Because of the heterogeneity of the disease, the usefulness of large-scale, population-based databases in sarcoma research has been well recognized.21 For example, datasets generated from administrative/billing data allow the inclusion of a large-sized, nationwide patient population. However, such datasets often lack clinically relevant variables.22 Also, the identification of sarcoma patients using disease codes, especially disease site-based codes, is often inaccurate, as sarcomas can arise throughout the body.6 Therefore, we identified patients based on ICD-O-3 codes generated by expert pathologists and curated by trained abstractors, as they include both site and histologic information. Another strength of our database is that dedicated data managers update the registry regularly according to a predetermined case report form, which leads to high data completeness. For survival outcomes, both inhospital deaths and out-of-hospital deaths are recorded by integrating EHR data with NHIS data. Other clinical data are derived from a deidentified EHR system (ABLE), which acts as an honest broker and protects personal data. The ABLE system is also linked to an inhospital tissue/blood bank (Bio-Resource Center), which allows researchers to integrate genomic data with clinical information.
This study is limited by its single-center nature. Similar to other electronic health record-based, real-world databases, some of the clinically relevant data such as the presence of measurable disease or response rates were not available. Also, although we have showed the feasibility of our registry-based analysis, this institutional dataset is not suitable for estimating epidemiology in the entire population, or for histologic subtype-specific survival analysis, if it is a rare disease. There are currently ongoing attempts to build prospectively collected, sarcoma-specific, multicenter cohorts.23,24 These multicenter collaborative approaches will provide further improvements in the quality and completeness of data, along with a higher level of statistical significance. Nonetheless, our registry is one of the rare sarcoma-specific databases in Asia and the first in Korea. It includes a relatively large number of patients who were accurately identified by histological diagnoses throughout a long study period. The result of this study will provide a framework for future studies, which would use the registry.
Data Sharing Statement
Data supporting the findings of this study are available from the corresponding authors, Jeong Eun Kim and Jin-Hee Ahn, upon reasonable request.
Ethical Statement
This study was approved by the Institutional Review Board of the AMC and conducted in accordance with the Declaration of Helsinki. The Board granted an informed consent waiver for this study due to its retrospective design and the use of de-identified data without patient contact or intervention.
Acknowledgments
This study was presented in part as a poster to the 2019 Connective Tissue Oncology Society (CTOS) Annual Meeting, and the 45th Annual Meeting of Korean Cancer Association/the 5th International Cancer Conference. Jeong Eun Kim and Jin-Hee Ahn are co-correspondence authors for this study. Hyehyun Jeong and Hyeon-Su Im are co-first authors for this study.
Funding
This study was supported by a grant from Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea (#2018-0570) and a grant “Elimination of Cancer Project Fund” from Asan Cancer Institute of Asan Medical Center, Seoul, Korea.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. The WHO Classification of Tumors Editorial Board. WHO Classification of Tumours Soft Tissue and Bone Tumours.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
3. Johnson AC, Ethun CG, Liu Y, et al. Studying a rare disease using multi-institutional research collaborations vs big data: where lies the truth? J Am Coll Surg. 2018;227(3):357–366 e353. doi:10.1016/j.jamcollsurg.2018.05.009
4. Lloyd S, Park HS, Decker RH, Wilson LD, Yu JB. Using the surveillance, epidemiology, and end results database to investigate rare cancers, second malignancies, and trends in epidemiology, treatment, and outcomes. Curr Probl Cancer. 2012;36(4):191–199. doi:10.1016/j.currproblcancer.2012.03.008
5. Lyu HG, Stein LA, Saadat LV, et al. Assessment of the accuracy of disease coding among patients diagnosed with sarcoma. JAMA Oncol. 2018;4(9):1293–1295. doi:10.1001/jamaoncol.2018.2979
6. Hess LM, Zhu YE, Sugihara T, Fang Y, Collins N, Nicol S. Challenges of using ICD-9-CM and ICD-10-CM codes for soft-tissue sarcoma in databases for health services research. Perspect Health Inf Manag. 2019;16:1a.
7. Brennan MF, Antonescu CR, Moraco N, Singer S. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann Surg. 2014;260(3):
8. Jorgensen PH, Lausten GS, Pedersen AB. The Danish sarcoma database. Clin Epidemiol. 2016;8:685–690. doi:10.2147/CLEP.S99495
9. Raut CP, Miceli R, Strauss DC, et al. External validation of a multi-institutional retroperitoneal sarcoma nomogram. Cancer. 2016;122(9):1417–1424. doi:10.1002/cncr.29931
10. Choi HJ, Lee MJ, Choi CM, et al. Establishing the role of honest broker: bridging the gap between protecting personal health data and clinical research efficiency. PeerJ. 2015;3:e1506. doi:10.7717/peerj.1506
11. Kim HS, Nam CM, Jang SY, et al. Characteristics and treatment patterns of patients with advanced soft tissue sarcoma in Korea. Cancer Res Treat. 2019;51(4):1380–1391. doi:10.4143/crt.2018.476
12. Gadgeel SM, Harlan LC, Zeruto CA, Osswald M, Schwartz AG. Patterns of care in a population-based sample of soft tissue sarcoma patients in the United States. Cancer. 2009;115(12):2744–2754. doi:10.1002/cncr.24307
13. Wang D, Abrams RA. Radiotherapy for soft tissue sarcoma: 50 years of change and improvement. Am Soc Clin Oncol Educ Book. 2014;244–251. doi:10.14694/EdBook_AM.2014.34.244
14. Frustaci S, Gherlinzoni F, De Paoli A, et al. Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: results of the Italian randomized cooperative trial. J Clin Oncol. 2001;19(5):1238–1247. doi:10.1200/JCO.2001.19.5.1238
15. Le Cesne A, Ouali M, Leahy MG, et al. Doxorubicin-based adjuvant chemotherapy in soft tissue sarcoma: pooled analysis of two STBSG-EORTC Phase III clinical trials. Ann Oncol. 2014;25(12):2425–2432. doi:10.1093/annonc/mdu460
16. Italiano A, Mathoulin-Pelissier S, Cesne AL, et al. Trends in survival for patients with metastatic soft-tissue sarcoma. Cancer. 2011;117(5):1049–1054. doi:10.1002/cncr.25538
17. Kollar A, Rothermundt C, Klenke F, et al. Incidence, mortality, and survival trends of soft tissue and bone sarcoma in Switzerland between 1996 and 2015. Cancer Epidemiol. 2019;63:101596. doi:10.1016/j.canep.2019.101596
18. Judson I, Verweij J, Gelderblom H, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled Phase 3 trial. Lancet Oncol. 2014;15(4):415–423. doi:10.1016/S1470-2045(14)70063-4
19. Recine F, Bongiovanni A, Riva N, et al. Update on the role of trabectedin in the treatment of intractable soft tissue sarcomas. Onco Targets Ther. 2017;10:1155–1164. doi:10.2147/OTT.S127955
20. Hoven-Gondrie ML, Bastiaannet E, Ho VK, et al. Worse survival in elderly patients with extremity soft-tissue sarcoma. Ann Surg Oncol. 2016;23(8):2577–2585. doi:10.1245/s10434-016-5158-7
21. Lyu HG, Haider AH, Landman AB, Raut CP. The opportunities and shortcomings of using big data and national databases for sarcoma research. Cancer. 2019;125(17):2926–2934. doi:10.1002/cncr.32118
22. Park HS, Lloyd S, Decker RH, Wilson LD, Yu JB. Limitations and biases of the surveillance, epidemiology, and end results database. Curr Probl Cancer. 2012;36(4):216–224. doi:10.1016/j.currproblcancer.2012.03.011
23. REtroperitoneal SArcoma Registry: an international prospective initiative. Available from: https://ClinicalTrials.gov/show/NCT03838718.
24. GISAR German Interdisciplinary Sarcoma Registry. Available from: https://ClinicalTrials.gov/show/NCT04122872.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

