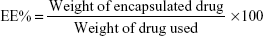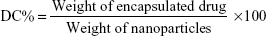Back to Journals » International Journal of Nanomedicine » Volume 10 » Issue 1
Delivery as nanoparticles reduces imatinib mesylate-induced cardiotoxicity and improves anticancer activity
Authors Marslin G , Revina AM, Khandelwal V, Balakumar K, Prakash J, Franklin G, Sheeba CJ
Received 17 October 2014
Accepted for publication 28 November 2014
Published 24 April 2015 Volume 2015:10(1) Pages 3163—3170
DOI https://doi.org/10.2147/IJN.S75962
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Carlos Rinaldi
Gregory Marslin,1 Ann Mary Revina,2,3 Vinoth Kumar Megraj Khandelwal,4 Krishnamoorthy Balakumar,5 Jose Prakash,6 Gregory Franklin,1,* Caroline J Sheeba2,3,7,*
1AgroBioPlant Group, Centre for the Research and Technology of Agro-Environment and Biological Sciences (CITAB), University of Minho, Braga, Portugal; 2Life and Health Sciences Research Institute (ICVS), School of Health Sciences, University of Minho, Braga, Portugal; 3ICVS/3B’s – PT Government Associate Laboratory, Braga/Guimarães, Portugal; 4Department of Translational Pharmacology, Consorzio Mario Negri Sud, Santa Maria Imbaro, Italy; 5Department of Pharmaceutics, PSG College of Pharmacy, Coimbatore, Tamil Nadu, India; 6Department of Pharmaceutics, Vels University, Chennai, Tamil Nadu, India; 7Regenerative Medicine Program, Department of Biomedical Sciences and Medicine, University of Algarve, Faro, Portugal
*These authors contributed equally to this work
Abstract: Clinical effectiveness of imatinib mesylate in cancer treatment is compromised by its off-target cardiotoxicity. In the present study, we have developed physically stable imatinib mesylate-loaded poly(lactide-co-glycolide) nanoparticles (INPs) that could sustainably release the drug, and studied its efficacy by in vitro anticancer and in vivo cardiotoxicity assays. MTT (methylthiazolyldiphenyl-tetrazolium bromide) assay revealed that INPs are more cytotoxic to MCF-7 breast cancer cells compared to the equivalent concentration of free imatinib mesylate. Wistar rats orally administered with 50 mg/kg INPs for 28 days showed no significant cardiotoxicity or associated changes. Whereas, increased alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase levels, and reduced white blood cell, red blood cell, and hemoglobin content were observed in the animals administered with free drug. While the histological sections from hearts of animals that received INPs did not show any significant cardiotoxic symptoms, loss of normal architecture and increased cytoplasmic vacuolization were observed in the heart sections of animals administered with free imatinib mesylate. Based on these results, we conclude that nano-encapsulation of imatinib mesylate increases its efficacy against cancer cells, with almost no cardiotoxicity.
Keywords: imatinib nanoparticles, cytostoxicity, cardiotoxicity, hematology
Introduction
Overexpression of receptor tyrosine kinase is a common mechanism associated with multiple malignancies.1 Imatinib mesylate is a tyrosine kinase inhibitor, which typically blocks the tyrosine kinase activity of BCR-Abl,2 platelet-derived growth factor receptors (PDGFRs),3 and KIT4 proteins, forming the basis of its use in the treatment of chronic myelogenous leukemia (CML) and gastrointestinal stromal tumors (GISTs). In CML, the characteristic reciprocal translocation between chromosomes results in the derivative Philadelphia chromosome, with the BCR-Abl fusion gene that produces the BCR-Abl hybrid protein, possessing constitutive tyrosine kinase activity. Imatinib mesylate is used to inhibit BCR-Abl from phosphorylating other proteins that are involved in the pathogenesis of CML.5,6 In the case of GISTs, the underlying mechanism is a gain-of-function mutation in c-kit or PDGFRα genes, causing the production of abnormal KIT and PDGFRα receptors that induce uncontrolled cell growth and cell division. Imatinib mesylate specifically antagonizes the intracellular adenosine triphosphate (ATP)-binding sites of these receptors, making it a leading drug in the treatment of GISTs.7
BCR-Abl hybrid protein only exists in cancer cells and not in healthy cells. However, KIT and PDGFR are also expressed in normal cells, where they perform crucial roles in hematopoiesis, blood vessel formation, and vasculature maintenance.8,9 PDGFRs are also known to be involved in the response of endothelial and smooth muscle cells to injury and other stressors in the cardiovascular system.10 In this context, employing a tyrosine kinase inhibitor like imatinib mesylate to treat cancer has been reported to cause unanticipated cardiotoxicity.11–14 For instance, imatinib mesylate-treated male spontaneously hypertensive rats are reported to present cardiac lesions, characterized by cytoplasmic vacuolization and myofibrillar loss, suggesting a correlation between hypertension and imatinib mesylate-induced cardiotoxicity.12 More recently, the deleterious effect of imatinib mesylate on the heart was shown to be dose-, time-, and age-dependent.14 These authors found mitochondrial impairment and cell death as the mechanisms of myocyte loss and cardiac dysfunction, which is further exacerbated in an aging population. Since the therapeutic value of imatinib mesylate is limited by such off-target cardiotoxicity, alternative strategies such as nanoformulations are necessary to overcome this problem.
Drug-encapsulated nanoparticles with sustained release properties have been attempted to minimize off-target toxicity of many cancer drugs,15–20 including imatinib mesylate.21–23 While the nano-encapsulation of imatinib mesylate improves its antitumor activity,23,24 it is also demonstrated to minimize the cytotoxicity of the drug to normal cells.21,22 Moreover, poly(lactide-co-glycolide) (PLGA) microspheres containing imatinib mesylate inhibited craniopharyngioma-mediated angiogenesis24 and intracranial xenograft glioma growth.25,26 However, there is no report on the evaluation of cardiotoxicity of imatinib mesylate-loaded nanoparticles so far. Hence, in the current investigation, we have developed and characterized imatinib mesylate-loaded PLGA nanoparticles (INPs) and have evaluated their in vitro cytotoxicity against cancer cells and cardiac toxicity in Wistar rats, in comparison with the free drug.
Materials and methods
Chemicals and reagents
Imatinib mesylate was obtained from Ranbaxy Pharmaceuticals (Delhi, India). PLGA (acid-terminated; lactide:glycolide 50:50; Mw [molecular weight] 24,000–38,000 D), polyvinyl alcohol (PVA; Mw 31,000–50,000 D), methylthiazolyldiphenyl-tetrazolium bromide (MTT), dimethylformamide (DMF), and coumarin-6 were purchased from Sigma-Aldrich Co, St Louis, MO, USA. Sodium hydroxide, phosphate buffer, potassium dihydrogen phosphate, methanol, and acetone used in the current study were acquired from Merck Millipore, Billerica, MA, USA. All reagents used in the present study were of analytical grade.
Preparation and characterization of nanoparticles
Preparation
INPs were prepared by emulsion solvent evaporation method.18 Briefly, 100 mg PLGA and imatinib mesylate (5 mg) were dissolved in 5 mL of chloroform. This solution was added drop by drop to the 20 mL aqueous phase containing 1.5% PVA and homogenized at 18,000 rpm (IKA T 25 Ultra Turrax® homogenizer). After homogenization, the nano-emulsion was stirred for 3 hours. The resulting emulsion was centrifuged at 20,000 rpm for 15 minutes to pellet down the nanoparticles. The pellet was washed three times with ultra-pure water to remove any free drug. Then, the pellet was freeze-dried and stored at 4°C until further use.
Particle size, shape, encapsulation efficiency, and drug content
Particle size distribution, mean particle size, and zeta potential of INPs were determined in a Zetasizer by dynamic light scattering and laser Doppler anemometry (Zetasizer Nano ZS; Malvern Instruments, Malvern, UK). Briefly, 500 μg of INPs was suspended in 1 mL of deionized water. An electric field of 150 mV was applied to observe the electrophoretic velocity of the particles. All measurements were made at room temperature.
The content of imatinib mesylate in INPs was measured by spectrophotometric assay. Briefly, 10 mg INPs was dissolved in 1 mL of dichloromethane and 2 mL phosphate-buffered saline (PBS). The solution was centrifuged at 10,000 rpm, and the supernatant was collected. Absorbance at 265 nm was read in a spectrophotometer (Shimadzu UV-1700) using PBS as a blank to determine the drug content from a standard graph.27 The percentage of encapsulation efficiency (EE%) and drug content (DC%) of INPs was determined using the following two formulas:
|
|
|
|
Scanning electron microscopy (SEM) characterized the INPs morphologically. Samples were prepared by dropping INP onto aluminum stubs and allowing them to air-dry. The air-dried particles were coated with gold in vacuum using a Fiscon Instrument SC 502 sputter coater and then observed under the SEM (Leica Cambridge S 360; Leica Microsystems, Wetzlar, Germany).
In vitro drug release of nanoparticles
The in vitro drug release of INPs was carried out using the previously described method with some modifications.28 Briefly, 10 mg of INPs was suspended in 2 mL PBS and transferred into a dialysis bag. The dialysis bag was then placed into a 100 mL bottle containing 50 mL PBS and was stirred at 100 rpm at 37°C. While stirring, 1 mL PBS sample was withdrawn at different time points from the bottle for 10 days. To maintain the volume, 1 mL PBS was added to the bottle after each withdrawal. Drug content of each sample was measured spectrophotometrically, as mentioned in the “Particle size, shape, encapsulation efficiency, and drug content” section.
In vitro cytotoxicity studies
Cancer cell line culture
Human breast cancer cell line MCF-7 was used in the present study. Cells were maintained in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin (Thermo Fisher Scientific, Waltham, MA, USA) at 37°C in a 5% CO2 humidified incubator.
MTT assay
The effect of imatinib mesylate in the form of free drug solution and INPs on cell proliferation was determined using MTT assay. MCF-7 cells were plated in flat-bottom 96-well plates at 5,000 cells per well. After incubation for 24 hours at 37°C, the culture medium was removed and replaced with 100 μL fresh medium containing free drug (0.148, 0.295, 0.59, and 1.18 μg), and INPs (6.56, 13.11, 26.22, and 52.44 μg); both free drug and INP additions were equivalent to final imatinib concentrations of 2.5, 5, 10, and 20 μM, respectively. Corresponding controls were maintained by adding 100 μL medium for free drug control and 100 μL medium containing 52.44 μg empty nanoparticles for INP control. After 48 hours, medium from the treatments and controls were replaced with 100 μL medium containing 50 μg MTT and were incubated for 1 hour. Then, 100 μL sodium dodecyl sulfate (SDS) solution (20% w/v, water:DMF at a 1:1 ratio, pH 4.7) was added to each well and further incubated for 24 hours to dissolve the formazan crystals formed. The absorbance was read at 570 nm in an enzyme-linked immunosorbent assay (ELISA) plate reader (Victor 1420; PerkinElmer Inc, Waltham, MA, USA). The amount of MTT that is converted to formazan after incubation with cells corresponds to the number of viable cells. The half-maximal inhibitory concentration (IC50) was determined by nonlinear regression analysis.
Cellular uptake of nanoparticles
In vitro cellular uptake of nanoparticles was studied using curcumin-loaded PLGA nanoparticles (CNPs). These nanoparticles were prepared essentially as mentioned in the “Preparation and characterization of nanoparticles” section, but using curcumin in the place of imatinib mesylate. MCF-7 cells were seeded in an 8-well ibiTreat microscopy chamber (Ibidi GmbH, Munich, Germany) and allowed to attach and grow. After 24 hours, 150 μL medium containing 100 μg/mL CNPs was added to the cells. After 90 minutes, cells were washed, and were replaced with fresh medium. To track the uptake of nanoparticles, the lysosomes of MCF-7 cells were stained with the LysoTracker® Red probe and analyzed under confocal microscope (Axiovert 135M; Zeiss International, Oberkochen, Germany).
Subacute toxicity analyses
Animals and treatments
Subacute toxicity of INPs was evaluated in Wister rats weighing about 130–150 g. This study was approved by the Animal Ethical Committee, Padmavathi College of Pharmacy, Tamil Nadu, India. Animals were divided into three groups (group 1, group 2, and group 3), each group containing six adult females. Group 1 received 2 ml PBS and was treated as a control. Group 2 received 2222 mg/kg INPs (equivalent to 50 mg/kg imatinib) suspended in PBS. Group 3 received 50 mg/kg free drug. Treatment for each group was done each day at 9 am, and was done per orally (PO) for 28 days. Throughout the study period, animals were monitored for the development of any toxicological signs and symptoms such as abnormal posture, abnormal movements, difficulties in respiration, and changes in body weight and feed intake.
Hematology and biochemistry
To know the potential side effects at the cellular level, blood biochemistry and hematological parameters were examined in treated groups of animals and compared with the control group. Blood and serum samples for hematological and biochemical studies were collected from the orbital sinus of each animal in vials rinsed with heparin and vials containing ethylenediaminetetraacetic acid (EDTA), respectively. Hematological analyses include white blood cells (WBCs), red blood cells (RBCs), and hemoglobin (Hb) content. The serum samples were analyzed for aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) to determine possible indication of myocardial damage, if any. All analyses were carried out in a smart lab batch analyzer.
Cardiotoxicity
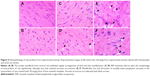
Hearts of control and treated animals were collected and observed for gross pathological and weight changes on day 29. For histopathology, heart tissue preserved in 10% formalin was embedded in paraffin using standard procedures. Sections of 4 μm thickness were obtained in a microtome and mounted on glass slides using standard techniques. After staining with hematoxylin and eosin, the slides were examined under a light microscope equipped with photography. Photomicrographs were analyzed for myocardial architecture.
Statistical analysis
Data were analyzed using GraphPad Prism 5 software (GraphPad Software, Inc, La Jolla, CA, USA). Results are expressed as the mean values ± standard deviation (SD). Statistical significance between treatments was analyzed by Student’s t-test. IC50 values were determined by nonlinear curve fitting of log concentration versus cell viability.
Results and discussion
Characterization of INPs
We have nano-encapsulated imatinib mesylate within the biodegradable, US Food and Drug Administration (FDA)-approved PLGA polymer.5 Moreover, PLGA nano-encapsulation is known to reduce the subacute toxicity, including cardiotoxicity of doxorubicin and erlotinib.17,18
Particle size distribution
Nanoparticle size is one of the most important characteristics, as it regulates the biological fate, toxicity, targeting ability, as well as drug loading, drug release, and stability of the formulation.29 Particle size distribution of the INPs is shown in Figure 1. The average diameter of INPs ranged between 250 and 300 nm, with a polydispersity index of 0.20. Zeta potential is an important characteristic for nanoparticles, as surface charge directly controls the aggregation behavior of the particles. The lowest zeta potential (−10.6 mV) indicated that the INPs were physically stable.
  | Figure 1 Histogram showing the particle size and size distribution of INPs. |
Shape of nanoparticles
A SEM image of INPs is shown in Figure 2. From the results, it is clear that the nanoparticles have a smooth spherical shape. Their smooth surface reveals the complete removal of solvent from the formulated nanoparticles, indicating their good quality.30
  | Figure 2 Scanning electron microscopic image of INPs (scale bar =2 μm). |
Encapsulation efficiency, drug content, and in vitro drug release
The encapsulation efficiency of the nanoparticles was found to be 89.94%. The drug content of the nanoparticles was 2.25% (0.0225 mg/mg INPs).
As graphically illustrated in Figure 3, the release of imatinib mesylate from the INPs occurred in a biphasic manner, with an initial burst phase followed by a diffusion-controlled slower release phase, as previously reported.25 Soon after 6 hours and 48 hours, about 40% and 60% of the encapsulated drug was released, respectively, and this initial burst can be attributed to the drug molecules adhering to the nanoparticle surface. As the hydrophilicity of PLGA nanoparticles enables an extensive water penetration to the polymeric matrix, a significant amount of drug release happens during the initial days of incubation in aqueous buffer. The initial burst was followed by a gradual rise and reached 70% on day 10, indicating a sustained release phase. Similar in vitro release kinetics of fluorescein isothiocyanate-loaded polyethylene glycol–PLGA nanoparticles, eg, approximately 40% on day 1 and a sustained release over the next 28 days has been previously demonstrated.21
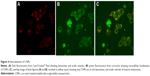
Internalization of nanoparticles by cancer cells
Fluorescent images of MCF-7 cells co-incubated with CNPs for 90 minutes and stained with LysoTracker Red taken under red filter (Figure 4A) and green filter (Figure 4B), respectively, show the localization of lysosomes and CNPs. The merged image (Figure 4C) clearly shows that the CNPs are colocalized within the lysosomes, indicating their internalization by endocytosis.
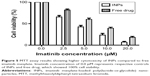
Cytotoxicity of imatinib mesylate in the form of free drug and INPs
MTT-based cell proliferation assay results of MCF-7 cells revealed that cell viability was reduced with increasing concentrations of INPs or free drug (Figure 5). However, INPs consistently showed higher viability reduction than equivalent free drug in all the concentrations tested. No cytotoxicity was observed in cells treated with just medium (free drug control) and empty nanoparticles (INP control). The IC50 value for INPs was found to be 2.6 μM at 48 hours, which was 2.9 times less than imatinib mesylate solution.
In vivo toxicity study
The results of hematological, biochemical, and heart weight analyses of rats administered with imatinib mesylate in the form of free drug (50 mg/kg) and INPs (2.222 g/kg) are summarized in Table 1.
Hematology
Although the WBC, RBC, and Hb content were reduced both in the nanodrug- and free drug-treated animals compared to the control group, the changes were not statistically significant for INPs. However, there was a significant decrease in the WBC, RBC, and Hb count of free drug-treated animals (Table 1), indicating the development of anemia and reduction of immunity in these animals.
Biochemistry
ALT, AST, and ALP are liberated into the blood after extensive tissue injury; in particular, elevated levels of AST are associated with heart muscle injury.31 Biochemical analysis of AST, ALT, and ALP levels in the serum samples did not show any significant change between control and nanodrug treated groups. Nevertheless, an increased level of these enzymes was observed in the animals administered with free drug compared to control (Table 1), indicating tissue damage, including myocardial damage.
Heart weight
Heart weight was significantly increased due to heart inflammation in free drug-treated animals over INP-treated animals and control animals. However, no significant change was noticed between INP-treated and control animals (Table 1).
Cardiac histopathology
Histopathological analysis of heart sections from INP-administered animals (Figure 6C and D) revealed no significant toxicity-related changes compared to the controls (Figure 6A and B). This is in agreement with the fact that nano-encapsulation reduces drug-induced off-target cardiotoxicity of many drugs such as doxorubicin15,20 and epirubicin.19 In addition, nano-encapsulation also improves the drug’s antitumor activity17 by making the tumor cells more sensitive to the drug.16 In this line of research, using CML stem cells, Palamà et al have reported that packaging of imatinib mesylate into a biodegradable carrier based on polyelectrolyte microcapsules increased the drug’s retention effects and antitumor activity, while improving the ex vivo purging of malignant progenitors from patient autografts.24 Both the reduction in off-target cardiotoxicity and increased antitumor activity could be attributed to the gradual release of imatinib mesylate, or reduced exposure of imatinib mesylate to the heart tissue, as it is encapsulated inside the nanoparticles.32
In the case of rats administered with free imatinib mesylate, loss of normal architecture and damage to cardiac muscle fibers were observed (Figure 6E and F), which is consistent with its previously reported semi-targeting property and heart toxicity,13,33 even at low concentrations.12 Imatinib mesylate-caused cardiotoxicity manifests cardiac mitochondrial dysfunction, ventricular dysfunction, and mild myocardial lesions.21,34
Conclusion
In spite of many reports on nanoformulations of anticancer drugs, very few studies are available on the in vivo toxicity of nanoparticles. Here, we have demonstrated that PLGA nano-encapsulation significantly reduces drug-induced cardiotoxicity of imatinib mesylate. Likewise, erlotinib-, epirubicin-, and doxorubicin-induced cardiotoxicity have also been significantly reduced by nano-encapsulation of these drugs with PLGA or polyethylene glycol–PLGA.17–19,35 Extensive studies in terms of acute and chronic toxicity, and pharmacokinetic and pharmacodynamic effects of imatinib mesylate are necessary to understand the mechanisms behind the reduced cardiac toxicity and the long-term effects of INPs.
Acknowledgments
GM and CJS were supported by the Fundação para a Ciência e a Tecnologia (FCT), Portugal, by grants SFRH/BD/72809/2010 and SFRH/BPD/89493/2012, respectively. The present work was also supported by the FCT projects PTDC/AGR-GPL/119211/2010 and UID/AGR/04033/2013. We would like to thank Dr Palanivel, Assistant Professor, Department of Pharmacology, Padmavathi College of Pharmacy, Tamil Nadu, India for help with animal studies.
Disclosure
The authors report no conflicts of interest in this work.
References
Sheeba CJ, Marslin G, Revina AM, Franklin G. Signaling pathways influencing tumor microenvironment and their exploitation for targeted drug delivery. Nanotechnol Rev. 2014;3(2):123–151. | ||
Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2(5):561–566. | ||
Carroll M, Ohno-Jones S, Tamura S, et al. CGP 57148, a tyrosine kinase inhibitor, inhibits the growth of cells expressing BCR-ABL, TEL-ABL, and TEL-PDGFR fusion proteins. Blood. 1997;90(12):4947–4952. | ||
Heinrich MC, Griffith DJ, Druker BJ, Wait CL, Ott KA, Zigler AJ. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood. 2000;96(3):925–932. | ||
Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161(2):505–522. | ||
Elias MH, Baba AA, Azlan H, et al. BCR-ABL kinase domain mutations, including 2 novel mutations in imatinib resistant Malaysian chronic myeloid leukemia patients-Frequency and clinical outcome. Leuk Res. 2014;38(4):454–459. | ||
Din OS, Woll PJ. Treatment of gastrointestinal stromal tumor: focus on imatinib mesylate. Ther Clin Risk Manag. 2008;4(1):149–162. | ||
Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22(10):1276–1312. | ||
Lennartsson J, Rönnstrand L. Stem cell factor receptor/c-Kit: from basic science to clinical implications. Physiol Rev. 2012;92(4):1619–1649. | ||
Raines EW. PDGF and cardiovascular disease. Cytokine Growth Factor Rev. 2004;15(4):237–254. | ||
Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007;7(5):332–344. | ||
Herman EH, Knapton A, Rosen E, et al. A multifaceted evaluation of imatinib-induced cardiotoxicity in the rat. Toxicol Pathol. 2011;39(7):1091–1106. | ||
Kerkelä R, Grazette L, Yacobi R, et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12(8):908–916. | ||
Maharsy W, Aries A, Mansour O, Komati H, Nemer M. Ageing is a risk factor in imatinib mesylate cardiotoxicity. Eur J Heart Fail. 2014;16(4):367–376. | ||
Chen Y, Yang W, Chang B, Hu H, Fang X, Sha X. In vivo distribution and antitumor activity of doxorubicin-loaded N-isopropylacrylamide-co-methacrylic acid coated mesoporous silica nanoparticles and safety evaluation. Eur J Pharm Biopharm. 2013;85(3 Pt A):406–412. | ||
Maksimenko A, Mougin J, Mura S, et al. Polyisoprenoyl gemcitabine conjugates self assemble as nanoparticles, useful for cancer therapy. Cancer Lett. 2013;334(2):346–353. | ||
Mao JN, Li AJ, Zhao LP, et al. PEG-PLGA nanoparticles entrapping doxorubicin reduced doxorubicin-induced cardiotoxicity in rats. Adv Mat Res. 2014;912:263–268. | ||
Marslin G, Sheeba CJ, Kalaichelvan VK, Manavalan R, Reddy PN, Franklin G. Poly(D,L-lactic-co-glycolic acid) nanoencapsulation reduces Erlotinib-induced subacute toxicity in rat. J Biomed Nanotechnol. 2009;5(5):464–471. | ||
Nasr M, Nafee N, Saad H, Kazem A. Improved antitumor activity and reduced cardiotoxicity of epirubicin using hepatocyte-targeted nanoparticles combined with tocotrienols against hepatocellular carcinoma in mice. Eur J Pharm Biopharm. 2014;88(1):216–225. | ||
Yuan A, Wu J, Song C, et al. A novel self-assembly albumin nanocarrier for reducing doxorubicin-mediated cardiotoxicity. J Pharm Sci. 2013;102(5):1626–1635. | ||
Kimura S, Egashira K, Nakano K, et al. Local delivery of imatinib mesylate (STI571)-incorporated nanoparticle ex vivo suppresses vein graft neointima formation. Circulation. 2008;118(14 Suppl):S65–S70. | ||
Mendonca LS, Moreira JN, de Lima MC, Simões S. Co-encapsulation of anti-BCR-ABL siRNA and imatinib mesylate in transferrin receptor-targeted sterically stabilized liposomes for chronic myeloid leukemia treatment. Biotechnol Bioeng. 2010;107(5):884–893. | ||
Thampi RG, Godwin SE, Harish G. Design and characterization of nanoparticulate drug delivery system of an anticancer drug: Imatinib mesylate. Indian J Res Pharm Biotech. 2014;1082–1087. | ||
Palamà IE, Leporatti S, de Luca E, et al. Imatinib-loaded polyelectrolyte microcapsules for sustained targeting of BCR-ABL+ leukemia stem cells. Nanomedicine (Lond). 2010;5(3):419–431. | ||
Karal-Yilmaz O, Ozkan A, Akgun E, et al. Controlled release of imatinib mesylate from PLGA microspheres inhibit craniopharyngioma mediated angiogenesis. J Mater Sci Mater Med. 2013;24(1):147–153. | ||
Benny O, Menon LG, Ariel G, et al. Local delivery of poly lactic-co-glycolic acid microspheres containing imatinib mesylate inhibits intracranial xenograft glioma growth. Clin Cancer Res. 2009;15(4):1222–1231. | ||
Sikharam S. Release Kinetics of Imatinib Mesylate from a Thermosensitive Polymer and its Effect on Vascular Smooth Muscle Cells [dissertation]. Salt Lake City: The University of Utah; 2011. | ||
Fan Y, Du W, He B, et al. The reduction of tumor interstitial fluid pressure by liposomal imatinib and its effect on combination therapy with liposomal doxorubicin. Biomaterials. 2013;34(9):2277–2288. | ||
Zhang XD, Wu D, Shen X, et al. Size-dependent in vivo toxicity of PEG-coated gold nanoparticles. Int J Nanomedicine. 2011;6:2071–2081. | ||
Kaleemuddin M, Srinivas P. Lyophilized oral sustained release polymeric nanoparticles of nateglinide. AAPS Pharm Sci Tech. 2013;14(1):78–85. | ||
Karmen A, Wroblewski F, and La Due JS. Transaminase activity in human blood. J Clin Invest. 1955;34(1):126–131. | ||
Kalaria DR, Sharma G, Beniwal V, Ravi Kumar MN. Design of biodegradable nanoparticles for oral delivery of doxorubicin: in vivo pharmacokinetics and toxicity studies in rats. Pharm Res. 2009;26(3):492–501. | ||
Lee SJ, Wang JY. Exploiting the promiscuity of imatinib. J Biol. 2009;8(3):30. | ||
Saad SY, Alkharfy KM, Arafah MM. Cardiotoxic effects of arsenic trioxide/imatinib mesilate combination in rats. J Pharm Pharmacol. 2006;58(4):567–573. | ||
Jain AK, Swarnakar NK, Das M, et al. Augmented anticancer efficacy of doxorubicin-loaded polymeric nanoparticles after oral administration in a breast cancer induced animal model. Mol Pharm. 2011;8(4): 1140–1151. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.