Back to Journals » OncoTargets and Therapy » Volume 12
Deleted in breast cancer 1 as a potential prognostic biomarker in human cancers: a pooled analysis of 2,254 patients
Authors Liu G, Wu Q, Wang Y, Xiong Q, Fu F
Received 3 October 2018
Accepted for publication 8 January 2019
Published 22 February 2019 Volume 2019:12 Pages 1563—1574
DOI https://doi.org/10.2147/OTT.S189618
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Gang Liu,* Qiaosheng Wu,* Yili Wang, Qiuyun Xiong, Feiguo Fu
Department of Breast Surgery, The Third Hospital of Nanchang City, Key Laboratory of Breast Diseases, Nanchang, Jiangxi 330009, China
*These authors contributed equally to this work
Background: Deleted in breast cancer 1 (DBC1) is believed to be involved in human cancers. However, it is still uncertain whether DBC1 expression can be regarded as a prognostic factor in patients with various cancers. This meta-analysis aimed to evaluate the relationship between high levels of DBC1 and prognosis in tumor patients.
Methods: Electronic databases were searched and 14 studies meeting the selection criteria were included. Overall survival (OS), relapse-free survival (RFS), and 95% CIs were extracted and analyzed. HRs from individual studies were pooled using fixed- or random-effects models, depending on the heterogeneity of the included studies, and publication bias analyses were also performed to increase the reliability of the results.
Results: A total of 2,254 patients with tumors from 14 published studies were included in the meta-analysis. DBC1 overexpression was associated with worse OS (univariate analysis: HR=2.94; 95% CI: [2.38–3.63]; multivariate analysis: HR=1.98, 95% CI: [1.21–3.25]) and RFS (univariate analysis: HR=2.83, 95% CI: [2.30–3.49]; multivariate analysis: HR=2.71, 95% CI: [2.07–3.53]) for various tumors. No publication bias was observed according to test of funnel plot asymmetry and Egger’s test.
Conclusion: Current evidence supports the conclusion that the upregulation of DBC1 is correlated with poor survival among tumor patients, suggesting that DBC1 represents an independent prognostic factor significantly associated with OS and RFS, and could serve as a novel therapeutic target in patients with tumors. Nevertheless, further large-scale prospective trials and well-designed studies are warranted to confirm this finding.
Keywords: deleted in breast cancer 1, breast cancer, prognosis, survival analysis, meta-analysis
Introduction
Despite some improvements in diagnosis and therapy for various human cancers, it is still the leading cause of cancer-related mortality worldwide and is, therefore, a major public health threat.1 Nowadays, surgical resection is still the optimal therapy for various human tumors. Hence, to precisely evaluate the clinical therapeutic effect and prognosis in patients with different types of cancer, reliable and effective prognostic biomarkers are urgently needed. Although multiple biomarkers involved in neoplasms of various systems have been identified, only a few have been appropriately validated for clinical applications. Therefore, resorts to find new feasible biomarkers are needed to improve clinical management of cancer patients.
Recently, deleted in breast cancer 1 (DBC1; also known as cell cycle and apoptosis regulator protein 2) has become a research hotspot in various cancers, with extensive investigation of its functions on molecular and cell levels. DBC1 is a transcriptional coactivator for nuclear protein receptors, localized on chromosome 8p21, which was initially found because of its homozygous deleted region in human breast cancer.2 The primary mechanism of DBC1 as a tumor suppressor is thought to be its negative regulation role of silent mating type information regulation 2 homolog 1 (SIRT1).3,4 Research has shown that DBC1 is involved in numerous cellular functions, including regulation of metabolism, apoptosis, and RNA splicing.5–7 Multiple studies have reported that high levels of DBC1 are associated with worse prognosis of patients with various human tumors, including gastric carcinoma,8,9 breast carcinoma,10 colorectal cancer,11 esophageal cancer,12 diffuse large B cell lymphoma,13 sarcomas,14 clear cell renal cell carcinoma,15 ovarian carcinomas,16 hepatocellular carcinoma,17,18 and osteosarcoma.19 However, the prognostic value of DBC1 overexpression in some human tumors is unclear, and no consensus has been reached. Studies have suggested that high DBC1 levels are associated with favorable outcome in patients with hepatocellular carcinoma,18 gastric cancer,20 and gallbladder carcinoma.21
Therefore, it is necessary to verify the prognostic and clinicopathological value of DBC1 expression in tumor patients. Accordingly, we performed a meta-analysis to evaluate the prognostic value of DBC1 in various human cancers. We collected and combined all eligible published articles to evaluate the prognostic value of DBC1 on the outcomes of various cancers. We hypothesized that high levels of DBC1 would be linked to worse outcomes, with the implication that combined targeted strategies should be pursued for clinical development.
Materials and methods
Data source and literature search
We conducted a systematic literature search using PubMed, EMBASE, and Web of Science, and extracted all published articles related to DBC1 expression in human cancer by July 1, 2018. The search strategy was generated by using the following keywords in various forms and combining key words related to “DBC1” and “cancer, carcinoma, tumor, neoplasm, malignancy” and “prognosis (prognosis or prognostic), survival, outcome”. Additional manual searches were performed for supplementation on this topic as well. To obtain as many records as possible, the references cited by the primarily articles were also reviewed to identify any missing articles that were not identified by the database search strategy.
Inclusion criteria
To be eligible for inclusion in this meta-analysis, a study had to meet the following criteria: 1) the expression of DBC1 in the primary tumor tissue was evaluated by immunohistochemistry (IHC) and/or tissue microarray; 2) the correlation between DBC1 expression with cancer patients’ survival (overall survival [OS] and/or relapse-free survival [RFS]) was analyzed; 3) the correlation between DBC1 and clinicopathological features of various cancers was measured; 4) all selected tumor patients were pathologically confirmed; and 5) the median follow-up period was >36 months. All candidate manuscripts were carefully read by three independent authors. To reach a consensus, disagreements on controversial results were settled by review of the original article together with a third reviewer.
Exclusion criteria
In this meta-analysis, the exclusion criteria were as follows: 1) articles published in a language other than English; 2) non-human studies; 3) duplicate reports and inappropriate study types (such as letters, conference papers, reviews, and case reports); 4) studies with sample size of no. >30 eligible cancer patients; and 5) studies with insufficient data to calculate the HR and its 95% CI, or where the Kaplan–Meier curve could not be obtained.
Data extraction and quality assessment
Three authors independently extracted relevant information from the included articles in this pooled analysis as follows: general information consisting of first author and corresponding year of publication, country, type of cancer, number of patients, age of patients (years), gender of patients, TNM staging, number of patients with DBC1 expression, survival analysis, HRs with 95% CIs, and Newcastle–Ottawa scale (NOS) scores. In order to identify high-quality studies, all articles were assessed according to the NOS. Scores on this scale range from 0 to 9; articles with a score ≥6 were considered to be methodologically adequate.22 A consensus NOS score for each item was achieved by discussion between three independent reviewers.
Statistical analysis
HRs and their 95% CIs were used to estimate the prognostic value of DBC1 expression in various tumors. The heterogeneity of each HR across the including studies was assessed by Cochran’s Q test and Higgin’s I2 test (P≤0.10 for the Q test and/or I2 ≥50% represented statistically significant heterogeneity).23,24 A random- or fixed-effects model was applied depending on the heterogeneity analysis; for the random-effects model, I2 ≥50% indicated significant heterogeneity. HR and its 95% CI were used to determine the prognostic value of DBC1 expression in various tumors. Subgroup analysis was also applied to the interpretation of identified heterogeneity. Publication bias was calculated using funnel plots with Begg’s and Egger’s tests, for which P-values <0.05 were considered to indicate a significant bias. All P-values <0.05 were regarded as statistically significant. All statistical analyses in this meta-analysis were performed using Stata 12.0 software and Review Manager version 5.3.
Results
Study selection and characteristics
For the meta-analysis, 564 relevant studies were retrieved initially. The flow diagram in Figure 1 shows the search strategy. A total of 14 studies comprising 2,254 cases were investigated in the pooled analysis. The major baseline characteristics of the 14 eligible publications are described in Tables 1 and 2. The search encompassed two countries (Korea and China) and included literature that was published between 2009 and 2017. In the included articles, 14 studies reported OS, with respect to gastric cancer (n=3), hepatocellular carcinoma (n=2), breast cancer (n=1), colorectal cancer (n=1), esophageal cancer (n=1), diffuse large B cell lymphoma (n=1), sarcomas (n=1), clear cell renal cell carcinoma (n=1), gallbladder carcinoma (n=1), ovarian carcinomas (n=1), and osteosarcoma (n=1), and nine studies presented data for RFS. As shows in Table 3, the quality assessment of 14 included studies was performed using the modified NOS. The quality scores of these studies was 9, indicating that the methodological quality in the pooled analysis was relatively high.
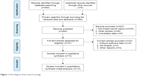  | Figure 1 Flow diagram shows search strategy. |
Meta-analysis
The impact of DBC1 overexpression on survival outcomes of various cancers is shown in Table 4. The association between high levels of DBC1 and OS is illustrated in Figure 2. The pooled analysis demonstrated that DBC1 overexpression was associated with worse survival outcomes in various tumor patients. The combined HR was 2.94 (95% CI: 2.38–3.63, P<0.00001) for OS, obtained by univariate analysis with a fixed-effects model for the 11 included studies (Figure 2A). The results showed a DBC1 overexpression combined HR of 1.98 (95% CI: 1.21–3.25, P=0.007) for OS by multivariate analysis with a random-effects model for the 13 included studies (Figure 2B). The association between DBC1 overexpression and RFS is shown in Figure 3. The pooled analysis demonstrated that high levels of DBC1 were associated with poor survival outcome in various tumor patients. A combined HR of 2.83 (95% CI: 2.30–3.49, P<0.00001) was obtained for RFS by univariate analysis with a fixed-effects model for the nine included studies (Figure 3A). The results showed a DBC1 overexpression combined HR of 2.71 (95% CI: 2.07–3.53, P<0.00001) for RFS by multivariate analysis with a fixed-effects model for the seven included studies (Figure 3B).
  | Table 4 Meta-analysis of pooled HR for various cancer patients with DBC1 overexpression |
Publication bias
As shown in Figure 4, Begg’s and Egger’s tests, as well as funnel plots, were used to estimate publication bias in the present meta-analysis. The funnel plots showed no evident asymmetry for the included studies. These findings suggested that significant publication bias did not exist in our meta-analysis.
Discussion
In past decades, much research has focused on determining the prognostic significance of potential biomarkers, in order to develop new prognostic markers to improve clinical decision-making, therapy, and patient survival. The prognostic value of DBC1 overexpression has been researched extensively in many different cancers, including gastric cancer,8,9,20 breast cancer,10 colorectal cancer,11 esophageal cancer,12 diffuse large B cell lymphoma,13 sarcomas,14 clear cell renal cell carcinoma,15 gallbladder,21 ovarian carcinomas,16 osteosarcoma,19 and hepatocellular carcinoma.17,18 Therefore, it is essential to elucidate the prognostic value of DBC1 in various human tumors. In this pooled analysis, by summarizing the results of published articles, we assessed the correlations between high levels of DBC1 expression and survival of patients with different types of cancer, with the aim of identifying a potential therapeutic target for clinical decision-making in tumor patients.
Previously published studies have indicated that DBC1 functions as a transcriptional coactivator by modulating the activities of various proteins, including SIRT1, estrogen receptor alpha, androgen receptor alpha, and retinoic acid receptor.25 Research has suggested that DBC1 is a negative regulator of SIRT1, with direct effects on the catalytic domain of SIRT1.3 DBC1-mediated downregulation of SIRT1 results in overexpression of p53 deacetylase and is involved in regulation of the p53-mediated apoptotic response.4 DBC1 was initially regarded as a tumor suppressor and, consequently, SIRT1 was assumed to be a tumor promoter. Given that previous studies have identified DBC1 as a key negative regulator of SIRT1, DBC1 may also be involved in both tumor promotion and suppression.26 A number of published articles have investigated that the involvement of DBC1 in cell proliferation, apoptosis, and histone modification, and shown that it is essential for regulating tumorigenesis.6,27,28 A previously published study showed that aberrant activation of the Wnt/b-catenin pathway by DBC1 is essential for the progression of colorectal cancer.29 Recent research showed that DBC1 could inhibit SIRT1-mediated b-catenin deacetylation, which, in turn, promotes LEF1-b-catenin complex formation.30 Huan et al have found that, in gastric cancer, DBC1 promotes anoikis resistance via the IKK-b/NF-kB signaling pathway.31 However, whether DBC1 functions as an oncogene or a tumor suppressor in human cancers remains unclear, as does the question of whether levels of DBC1 are down- or upregulated in cancers. Some researchers have shown that DBC1 is downregulated in lymphoma, soft tissue sarcomas, breast cancer, and gastric cancers.8,13,14,32 However, overexpression of DBC1 was found in other types of cancer.33–35 Therefore, it is necessary to verify the prognostic and clinicopathological value of DBC1 expression in patients with tumors.
To our knowledge, this is the first pooled analysis investigation of the association of DBC1 expression with OS and RFS in different types of human cancer. We systematically assessed the survival data of 2,254 patients with various cancers from the 14 eligible studies. This meta-analysis indicated that high levels of DBC1 are associated with unfavorable prognosis for various carcinoma patients; DBC1 overexpression was related to worse OS and RFS among different types of tumors. Therefore, further studies are imperative to clarify the underlying mechanism and role of DBC1 in pathogenesis, and its prognostic merit in different types of cancer.
Although our results are meaningful, the present pooled analysis has several pivotal limitations. The meta-analysis revealed that DBC1 overexpression was correlated with worse survival among different types of cancer patients, thereby suggesting DBC1 as a novel therapeutic target.
In a word, our pooled analysis shows the potential clinical application for DBC1 as a valuable prognostic biomarker. This meta-analysis had some limitations, as follows: 1) some included articles had relatively small sample sizes, which could result in publication bias; 2) inconsistent cutoff scores, staining procedures, and antibodies for IHC were used in detection of DBC1 expression levels in the included studies, potentially leading to the relatively high variability in this pooled analysis; 3) the included studies were mostly retrospective rather than randomized prospective trials; a better-designed prospective study with stricter quality criteria would further improve the reliability of the pooled results.
In summary, our meta-analysis indicates that DBC1 overexpression is associated with poor OS and RFS in various cancer patients; thus, it has potential as a valuable prognostic indicator and a novel therapeutic target for cancer patients. More multicenter and prospective studies are needed to clarify its clinical relevance and provide a precise molecular explanation for the abnormal expression of DBC1.
Disclosure
The authors report no conflicts of interest in this work.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global Cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. | ||
Hamaguchi M, Meth JL, von Klitzing C, et al. DBC2, a candidate for a tumor suppressor gene involved in breast cancer. Proc Natl Acad Sci U S A. 2002;99(21):13647–13652. | ||
Kim JE, Chen J, Lou Z. DBC1 is a negative regulator of SIRT1. Nature. 2008;451(7178):583–586. | ||
Zhao W, Kruse JP, Tang Y, Jung SY, Qin J, Gu W. Negative regulation of the deacetylase SIRT1 by DBC1. Nature. 2008;451(7178):587–590. | ||
Escande C, Chini CC, Nin V, et al. Deleted in breast cancer-1 regulates SIRT1 activity and contributes to high-fat diet-induced liver steatosis in mice. J Clin Invest. 2010;120(2):545–558. | ||
Kim W, Kim JE. Deleted in breast cancer 1 (DBC1) deficiency results in apoptosis of breast cancer cells through impaired responses to UV-induced DNA damage. Cancer Lett. 2013;333(2):180–186. | ||
Close P, East P, Dirac-Svejstrup AB, et al. DBIRD complex integrates alternative mRNA splicing with RNA polymerase II transcript elongation. Nature. 2012;484(7394):386–389. | ||
Cha EJ, Noh SJ, Kwon KS, et al. Expression of DBC1 and SIRT1 is associated with poor prognosis of gastric carcinoma. Clin Cancer Res. 2009;15(13):4453–4459. | ||
Bae JS, Park SH, Kim KM, et al. CK2α phosphorylates DBC1 and is involved in the progression of gastric carcinoma and predicts poor survival of gastric carcinoma patients. Int J Cancer. 2015;136(4):797–809. | ||
Lee H, Kim KR, Noh SJ, et al. Expression of DBC1 and SIRT1 is associated with poor prognosis for breast carcinoma. Hum Pathol. 2011;42(2):204–213. | ||
Zhang Y, Gu Y, Sha S, et al. DBC1 is over-expressed and associated with poor prognosis in colorectal cancer. Int J Clin Oncol. 2014;19(1):106–112. | ||
Kim SH, Kim JH, Yu EJ, Lee KW, Park CK. The overexpression of DBC1 in esophageal squamous cell carcinoma correlates with poor prognosis. Histol Histopathol. 2012;27(1):49–58. | ||
Park HS, Bae JS, Noh SJ, et al. Expression of DBC1 and androgen receptor predict poor prognosis in diffuse large B cell lymphoma. Transl Oncol. 2013;6(3):370–381. | ||
Kim JR, Moon YJ, Kwon KS, et al. Expression of SIRT1 and DBC1 is associated with poor prognosis of soft tissue sarcomas. PLoS One. 2013;8(9):e74738. | ||
Noh SJ, Kang MJ, Kim KM, et al. Acetylation status of p53 and the expression of DBC1, SIRT1, and androgen receptor are associated with survival in clear cell renal cell carcinoma patients. Pathology. 2013;45(6):574–580. | ||
Cho D, Park H, Park SH, et al. The expression of DBC1/CCAR2 is associated with poor prognosis of ovarian carcinoma. J Ovarian Res. 2015;8:2. | ||
Ha SY, Kim JH, Yang JW, Bae H, Cho HY, Park CK. Expression of DBC1 is associated with poor prognosis in hepatitis virus-related hepatocellular carcinoma. Pathol Res Pract. 2016;212(7):616–621. | ||
Li C, Liao J, Wu S, Fan J, Peng Z, Wang Z. Overexpression of DBC1, correlated with poor prognosis, is a potential therapeutic target for hepatocellular carcinoma. Biochem Biophys Res Commun. 2017;494(3–4):511–517. | ||
Wagle S, Park SH, Kim KM, et al. DBC1/CCAR2 is involved in the stabilization of androgen receptor and the progression of osteosarcoma. Sci Rep. 2015;5:13144. | ||
Kang Y, Jung WY, Lee H, Lee E, Kim A, Kim BH. Expression of SIRT1 and DBC1 in gastric adenocarcinoma. Korean J Pathol. 2012;46(6):523–531. | ||
Won KY, Cho H, Kim GY, et al. High DBC1 (CCAR2) expression in gallbladder carcinoma is associated with favorable clinicopathological factors. Int J Clin Exp Pathol. 2015;8(9):11440–11445. | ||
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. | ||
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. | ||
Dickersin K, Berlin JA. Meta-analysis: state-of-the-science. Epidemiol Rev. 1992;14:154–176. | ||
Kim JE, Sung S. Deleted in breast cancer 1 (DBC1) is a dynamically regulated protein. Neoplasma. 2010;57(4):365–368. | ||
Kim JE, Chen J, Lou Z. P30 DBC is a potential regulator of tumorigenesis. Cell Cycle. 2009;8(18):2933–2936. | ||
Anantharaman V, Aravind L. Analysis of DBC1 and its homologs suggests a potential mechanism for regulation of sirtuin domain deacetylases by NAD metabolites. Cell Cycle. 2008;7(10):1467–1472. | ||
Kuzmichev A, Margueron R, Vaquero A, et al. Composition and histone substrates of polycomb repressive group complexes change during cellular differentiation. Proc Natl Acad Sci U S A. 2005;102(6):1859–1864. | ||
Ragusa S, Cheng J, Ivanov KI, et al. PROX1 promotes metabolic adaptation and fuels outgrowth of Wnt (high) metastatic colon cancer cells. Cell Rep. 2014;8(6):1957–1973. | ||
Yu EJ, Kim SH, Kim HJ, et al. Positive regulation of β-catenin-PROX1 signaling axis by DBC1 in colon cancer progression. Oncogene. 2016;35(26):3410–3418. | ||
Huan Y, Wu D, Zhou D, Sun B, Li G. DBC1 promotes anoikis resistance of gastric cancer cells by regulating NF-κB activity. Oncol Rep. 2015;34(2):843–849. | ||
Sung JY, Kim R, Kim JE, Lee J. Balance between SIRT1 and DBC1 expression is lost in breast cancer. Cancer Sci. 2010;101(7):1738–1744. | ||
Noguchi A, Kikuchi K, Zheng H, et al. SIRT1 expression is associated with a poor prognosis, whereas DBC1 is associated with favorable outcomes in gastric cancer. Cancer Med. 2014;3(6):1553–1561. | ||
Radvanyi L, Singh-Sandhu D, Gallichan S, et al. The gene associated with trichorhinophalangeal syndrome in humans is overexpressed in breast cancer. Proc Natl Acad Sci U S A. 2005;102(31):11005–11010. | ||
Richardson AL, Wang ZC, De Nicolo A, et al. X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell. 2006;9(2):121–132. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






