Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 12
Delayed hypersensitivity reaction to hyaluronic acid dermal filler following influenza-like illness
Authors Turkmani MG , De Boulle K, Philipp-Dormston WG
Received 13 December 2018
Accepted for publication 15 March 2019
Published 29 April 2019 Volume 2019:12 Pages 277—283
DOI https://doi.org/10.2147/CCID.S198081
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Mohammed G Turkmani,1 Koenraad De Boulle,2 Wolfgang G Philipp-Dormston3
1Derma Clinic, Riyadh, Saudi Arabia; 2Aalst Dermatology Clinic, Aalst, Belgium; 3Cologne Dermatology, Cologne, Germany
Background: Delayed reactions after facial hyaluronic acid injection are relatively rare complications. Their cause may be infectious or immune-mediated in origin, and their outbreak can be triggered, for example, by an influenza-like illness.
Objective: To describe potential adverse event of influenza like illness following dermal filler injection.
Methods: We report fourteen unusual cases of delayed hypersensitivity reaction to several brands of hyaluronic acid dermal filler following influenza like illness.
Results: Increasing evidence implicates influenza infection in the pathogenesis of late onset filler reaction.
Conclusion: Although there is a low risk of late onset adverse reaction with hyaluronic acid fillers, injecting physicians must be aware of the possible filler reaction following the influenza infection.
Keywords: fillers, hyaluronic acid, reaction, hypersensitivity, complications, influenza, infection
Introduction
Fillers have become an important treatment for patients who seek noninvasive rejuvenation. The American Society of Plastic Surgeons (ASPS) reported that approximately 2.7 million dermal filler procedures were performed by plastic surgeons and other board-certified physicians in 2017. Moreover, the American Society for Dermatologic Surgery ASDS reported that dermatologic surgeons performed 1.64 million dermal filler injections in the same year (over 10% more than 2016 and doubled since 2012).1,2 As the field of soft tissue augmentation has become increasingly popular, reports of adverse events have also risen.
Post filler injection complications vary and can be categorized based on their timing in relation to the filler injection as early events (occurring up to several days post treatment) or delayed events (occurring weeks to years post treatment).3
Delayed hypersensitivity reactions are characterized by induration, erythema, and edema and are mediated by T lymphocytes rather than antibodies.4 They typically occur 48–72 hrs after injection but may be seen as late as several weeks post injection and can persist for months.5
Herein we describe fourteen cases of delayed hypersensitivity reaction to hyaluronic acid (HA) dermal filler following influenza-like illness from two dermatology centers in Saudi Arabia and Belgium.
Methods
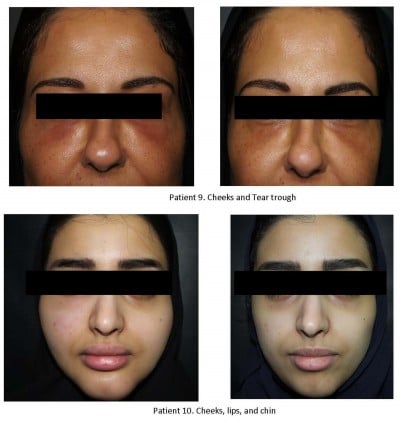
A total of fourteen females, 22–65 years old, were presented to our clinics between September 2016 and September 2018 complaining of localized redness and firm, painful swelling on their faces at the sites of previously injected fillers (Figure 1). In all cases, the reactions had started 3–5 days after patients had experienced influenza-like illness (fever, headache, sore throat, cough, and fatigue).
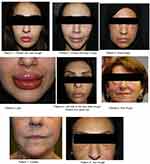 | Figure 1 Sites of reaction. |
All patients described their history of multiple injections (between 2–6) over the last four years prior to onset of their symptoms in different sites of their face. Duration of the last filler injection varied from 2–10 months before experiencing the reaction (Table 1).
 | Table 1 The injected fillers, number of injections, and time to onset |
The injections were performed by dermatologists or plastic surgeons using needles or cannula in different clinics. Patients’ files depicted no history of allergies or autoimmune disease and none of patients were injected with permanent fillers. All of the patients were unaware of the amounts of the injected fillers but fully aware of the number of injections on their face, and the filler brands which were Juvederm (Allergan Inc. Pringy, France), Teosyal (Teoxane S.A., Geneva, Switzerland), Belotero (Anteis S.A, Geneva, Switzerland), Surgiderm (Allergan Inc.) Restylane (Q-med AB Uppsala, Sweden) and Inamed (Nasdaq:IMDC) (Table 2). The most common sites of the reactions were the cheeks (n=9), tear trough (n=8), and lips (n=3).
 | Table 2 Number of injections in each anatomical site of each kind of filler |
Patients were treated with oral prednisolone 20–30 mg or Methyl prednisolone 16–24 mg daily for 5 days, followed by tempering of the dose for another 5 days. After a 2 week follow up, there was a complete resolution of the symptoms in 10 patients following the oral steroid therapy. The remaining 4 patients still presented minimal swelling that was treated with hyaluronidase one month after the onset of symptoms (Figure 2). Patients were followed up two months following the complete resolution of their symptoms and experienced another influenza-like infection or a recurrence of symptoms at the sites of filler injections.
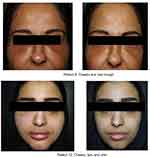 | Figure 2 Patient 1 and patient 2: sites of reaction before (left) and two weeks after (right) treatment. |
Each individual patient from all the clinics provided written informed consent for the case details and accompanying images to be published.
Institutional approval from all clinics was not required to publish the case details.
Discussion
Delayed hypersensitivity may manifest from weeks to months after an uneventful HA filler injection. Hence, it is impossible to predict the probability of occurrence. Several published reports, however, have attempted to understand the etiology in relation to HA soft tissue fillers.6–9 It has been suggested that biological/patient factors (eg, previous skin or systemic conditions such as infections and trauma),10 injection technique (eg, filler volume, repeat treatments, intramuscular implantation), and the different properties of HA fillers may explain the etiology.
Type IV hypersensitivity following HA implantation and influenza infection seems to be the most probable explanation for our observed late-onset events. This rare systemic response may be initiated by T-lymphocytes and mediated by CD4+ cells. The memory of macrophages and a trigger, such as an infection or a drug-drug interaction, could explain the unpredictability of foreign body granuloma, which may occur even many years after the biodegradation of the implant.11 Macrophages are known to be memory cells even when they move away from their site of action once degradation of their target is complete.12 Still, the exact etiology of delayed hypersensitivity in relation to HA fillers and the influenza virus infection remains not completely understood.6,13
HA molecules in all fillers are the same polysaccharide molecules that compose a major part of our skin. Therefore, HA molecule itself is not usually considered an immunogen. As suggested by Bitterman-Deutsch et al, however, in some conditions, glycosaminoglycans, such as HA, could directly trigger a specific immune response without the primary phase of inflammation, like a “superantigen”.14 Other components that are added to stabilize HA molecules in soft tissue fillers (eg, crosslinkers, conserving) may also be immunogenic.
Beleznay et al have suggested a mechanism involving the release of pro-inflammatory low molecular weight HA fragments during an accelerated breakdown of HA gels triggered by a systemic inflammatory response to an unknown antigen.7 The definition of low molecular weight HA, however, is not well defined in clinical research. Furthermore, whether low molecular weight HA fragments cause a pro-inflammatory response is open to debate in scientific literature. Therefore, due to lack of a clear understanding of the exact etiology of delayed hypersensitivity reactions in some filler patients, it is difficult to conclude that specific filler technologies are more prone to induce these reactions in comparison with others.15
The majority of patients here developed reactions at all the previously injected sites at the same time (except patient 7 who developed the reaction only in one side of the face) regardless of filler type, number of injections, or the injected volumes. Interestingly, two patients (patient #1 and #3) did not react at the previously injected sites (nasolabial fold and nose, respectively). This is likely due to the fact that the injections were done more than two years from the time of the hypersensitivity reaction, and so most of the injected fillers can be expected to have been degraded and removed by then.
An immunologic interaction between a concomitant amino penicillin treatment and an Ebstein–Barr viral infection (ie, sore throat) is known to induce maculopapular exanthems.16 The exact mechanism behind the interaction is unclear and it is not yet well explained whether a true allergic drug reaction, infectious-dependent eruption or transient loss of drug tolerance due to the infection, is responsible for the symptoms.17 The use of other medications such as antibiotics, antipyretic and non-steroidal anti-inflammatory drugs are commonly seen in daily practice for treatment of acute respiratory tract infections. HA, like viral infection, is known to activate in vitro T-lymphocytes via CD44.18 Therefore, HA could be considered as a risk factor in the development of hypersensitivity reactions when any medication is introduced.
The differential diagnosis of late onset nodules include infection (including biofilm), foreign body granulomatous reaction, and immune-mediated delayed hypersensitivity reactions. Exact diagnosis may be difficult since delayed filler reactions can present in many ways. Biofilms can present as erythematous, tender nodules and papules.19 They can also present as inert, asymptomatic granulomas that may even subside on their own, especially in the case of hyaluronic acid.19
Delayed complications of the soft tissue fillers are particularly difficult to diagnose and treat due to the time lapse from the procedure.20 Type IV hypersensitivity reactions are unresponsive to antihistamines.21 Steroids are required to alleviate the inflammatory signs. Hyaluronidase may be injected to remove the allergen in some cases.22,23
Delayed hypersensitivity reaction to hyaluronic acid following infection is relatively rare. Homsy et al24 reported multiple inflammatory collections on the face following sore throat in two patients. One case of cold sore and flu-like illness has been reported by Dr. Bhojani-Lynch,25 and one more case described a patient who developed a granulomatous lip 8 months after silicone injections and 1 week after a flu-like syndrome.24 Here, we present 10 rare cases of late onset hypersensitivity to hyaluronic acid injection following influenza-like illness.
The main limitation of this article is the absence of a histological analysis. Biopsies were not performed due to the patients’ desire to have minimally invasive resolutions for their symptoms as quickly as possible.
An immunologic interaction between dermal fillers and influenza infection may be the cause of the delayed hypersensitivity. The exact mechanism is unclear and not yet well understood; it is unknown whether the hypersensitivity is due to a true allergic filler reaction or to an influenza infection. To verify this hypothesis, an allergic work-up can be performed with the same HA that was used in each patient. It is, however, difficult to make definite conclusions from this test, as even if the results are negative, a hypersensitivity reaction cannot be excluded due to the limited sensitivity of these tests.26–28 Commercially available HA – based fillers have a wide variety of properties including the degree of cross-linking and gel concentration that have an extensive effect on their expected clinical outcome. Understanding these characteristics may be important in deciphering the etiology of delayed hypersensitivity reactions. It should be note that the actual causes are dependent not only on the characteristics of the HA fillers, but also on the response of the biological host. Other possible causes of delayed hypersensitivity in our patients may be injections of large volumes of dermal filler in many facial sites and repeated treatments. Many questions still remain; for example: Why all the patients injected with HA fillers do not display a hypersensitivity response when having influenza or influenza- like illness? Why patient number 7 was presented with the symptoms only on the left side of the face although she was injected with the same filler equally in both sides? What will happen if these patients get another flu attack? Are these patients proper candidates for receiving HA-based fillers again? We believe that more studies should be conducted to explain the mechanism of this reaction.
Disclosure
Wolfgang G Philipp-Dormston reports being a clinical trial investigator, scientific advisor, and/or speaker for Allergan, Galderma, and Merz, and no other conflicts of interest or payments for this publication. The other authors report no conflicts of interest in this work.
References
1.
2.
3. Lowe NJ, Maxwell CA, Patnaik R. Adverse reactions to dermal fillers: review. Dermatol Surg. 2005;31(11 Pt 2):1616–1625.
4. De Boulle K. Management of complications after implantations of fillers. J Cosmet Dermatol. 2004;3(1):2–15. doi:10.1111/j.1473-2130.2004.00058.x
5. Arron ST, Neuhaus IM. Persistent delayed-type hypersensitivity reaction to injectable non-animal-stabilized hyaluronic acid. J Cosmet Dermatol. 2007;6:167–171. doi:10.1111/j.1473-2165.2007.00331.x
6. Rongioletti F. Complications granulomateuses des techniques de comblement. Ann Dermatol Venereol. 2008;135:59–65. doi:10.1016/S0151-9638(08)70213-8
7. Beleznay K, Carruthers JD, Carruthers A, Mummert ME, Humphrey S. Delayed-onset nodules secondary to a smooth cohesive 20 mg/mL hyaluronic acid filler: cause and management. Dermatol Surg. 2015;41:929–939. doi:10.1097/DSS.0000000000000418
8. André P. Evaluation of the safety of a non-animal stabilized hyaluronic acid (NASHA – Q-Medical, Sweden) in European countries: a retrospective study from 1997 to 2001. J Eur Acad Dermatol Venereol. 2004;18:422–425.
9. Lee JM, Kim YJ. Foreign body granulomas after the use of dermal fillers: pathophysiology, clinical appearance, histologic features, and treatment. Arch Plast Surg. 2015;42:232–239. doi:10.5999/aps.2015.42.5.596
10. De Boulle K, Heydenrych I. Patient factors influencing dermal filler complications: prevention, assessment, and treatment. Clin Cosmet Investig Dermatol. 2015;15(8):205–214. doi:10.2147/CCID.S80446
11. Moscona RR, Bergman R, Friedman-Birnbaum R. An unusual late reaction to Zyderm I injections: A challenge for treatment. Plast Reconstr Surg. 1993;92:331. doi:10.1097/00006534-199308000-00021
12. Williams CT, Williams WJ. Granulomatous inflammation: a review. J Clin Pathol. 1983;36:723.
13. Alijotas-Reig J, Fernández-Figueras MT, Puig L. Late-onset inflammatory adverse reactions related to soft tissue filler injections. Clin Rev Allergy Immunol. 2013;45:97–108. doi:10.1007/s12016-012-8348-5
14. Bitterman-Deutsch O, Kogan L, Nasser F Delayed immune mediated adverse effects to hyaluronic acid fillers: report of five cases and review of the literature. Dermatol Report. 2015;7:5851. doi:10.4081/dr.2015.5851
15. Cyphert JM, Trempus CS, Garantziotis S. Size matters: molecular weight specificity of Hyaluronan effects in cell biology. Int J Cell Biol. 2015;2015:563818. doi:10.1155/2015/563818
16. Gonzalez-Delgado P, Blanes M, Soriano V, Montoro D, Loeda C, Niveiro E. Erythema multiforme to amoxicillin with concurrent infection by Epstein-Barr virus. Allergol Immunopathol. 2006;34:76–78. doi:10.1157/13086752
17. Onodi-Nagy K, Kinyo A, Meszes A, et al. Amoxicillin rash in patients with infectious mononucleosis: evidence of true drug sensitization. Allergy Asthma Clin Immunol. 2015;11:1. doi:10.1186/s13223-015-0099-4
18. Jordan AR, Racine RR, Hennig MJ, Lokeshwar VB. The role of CD44 in disease pathophysiology and targeted treatment. Front Immunol. 2015;6:182. doi:10.3389/fimmu.2015.00182
19. Wagner RD, Fakhro A, Cox JA, Izaddoost SA. Etiology, prevention, and management of infectious complications of dermal fillers. Semin Plast Surg. 2016;30:83–86. doi:10.1055/s-0036-1580734
20. Tseng CHW, Chen AM, Chang JYF. Hyaluronic acid injectioninduced delayed-onset foreign body granuloma. J Dental Sci. 2015;10:341–343. doi:10.1016/j.jds.2015.03.008
21. Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Plast Surg Nurs. 2015;35:13–32. doi:10.1097/PSN.0000000000000087
22. Signorini M, Liew S, Sundaram H, et al.;
23. Alijotas-Reig J, Fernández-Figueras MT, Puig L. Pseudocystic encapsulation: a late noninflammatory complication of hyaluronic acid filler injections. Dermatol Surg. 2013;39:1726–1728. doi:10.1111/dsu.12316
24. Homsy A, Rüegg EM, Jandus P, Pittet-Cuénod B, Modarressi A. Immunoligical reaction after facial hyaluronic acid injection. Case Reports Plast Surg Hand Surg. 2017;4(1):68–72.
25. Bhojani-Lynch T. Late-onset inflammatory response to hyal-uronic acid fillers. Dermal Plast Reconstruc Surg. 2017;5(12):e1532.
26. Wolkenstein P, Chosidow O, Flechet ML, et al. Patch testing in severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Derm. 1996;35:234–236. doi:10.1111/j.1600-0536.1996.tb02364.x
27. Britschgi M, Steiner UC, Schmid S, et al. T-cell involvement in drug-induced acute generalized exanthematous pustulosis. J Clin Invest. 2001;107:1433–1441. doi:10.1172/JCI10501
28. Padial MA, Alvarez-Ferreira J, Tapia B, et al. Acute generalized exanthematous pustulosis associated with pseudoephedrine. Br J Dermatol. 2004;150:139. doi:10.1111/j.1365-2133.2004.05717.x
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
