Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Decreased Serum Dickkopf-1 Levels After Hypoglycemic Therapy in Patients with Type 2 Diabetes Mellitus
Authors Gao F, Li C, Peng J, Lu W, Zhu W, Zhou J , Lu J, Ma X
Received 1 June 2022
Accepted for publication 23 August 2022
Published 5 September 2022 Volume 2022:15 Pages 2725—2732
DOI https://doi.org/10.2147/DMSO.S376988
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Gian Paolo Fadini
Fei Gao,* Cheng Li,* Jiahui Peng, Wei Lu, Wei Zhu, Jian Zhou, Jingyi Lu, Xiaojing Ma
Department of Endocrinology and Metabolism, Shanghai Clinical Center for Diabetes, Shanghai Diabetes Institute, Shanghai Key Laboratory of Diabetes Mellitus, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jingyi Lu; Xiaojing Ma, Department of Endocrinology and Metabolism, Shanghai Clinical Center for Diabetes, Shanghai Diabetes Institute, Shanghai Key Laboratory of Diabetes Mellitus, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, 600 Yishan Road, Shanghai, 200233, People’s Republic of China, Tel +86-21-64369181, Fax +86-21-64368031, Email [email protected]; [email protected]
Aim: Dickkopf-1 (DKK-1) is a major inhibitor of Wingless protein signaling pathway, which is involved in glucose metabolism and cardiovascular diseases. The aim of the study was to investigate the changes of serum DKK-1 levels after hypoglycemic treatments and the relationship between DKK-1 and clinical parameters.
Materials and Methods: The study was a sub-study of a previously published clinical trial (the effect of Acarbose on glycemic variability in patients with type 2 diabetes mellitus using premixed insulin compared to metformin). All subjects underwent anthropometric and biochemical assessments at baseline and endpoint. Serum DKK-1 levels of 92 subjects were measured at baseline and after 12-week hypoglycemic treatment.
Results: DKK-1 levels were significantly decreased after hypoglycemic treatment for 12 weeks (P < 0.001). ΔDKK-1 levels were not correlated with improvement of metabolic parameters (all P > 0.05) but were negatively correlated with baseline DKK-1 levels (r = − 0.263, P = 0.011). Spearman correlation showed that baseline DKK-1 levels were positively related to baseline total cholesterol (r = 0.226, P = 0.030) and low-density lipoprotein cholesterol (LDL-C) (r = 0.277, P = 0.007). Compared with the higher baseline DKK-1 group (≥ 3700 pg/mL), subjects in the lower baseline DKK-1 group (< 3700 pg/mL) had significantly lower baseline glycated hemoglobin A1c levels (P = 0.008) and LDL-C levels (P = 0.048). Systolic and diastolic pressure were decreased more significantly in the lower baseline DKK-1 group than that in the higher baseline DKK-1 group (both P < 0.05).
Conclusion: Serum DKK-1 levels were decreased after hypoglycemic treatments. Patients with lower baseline DKK-1 levels were featured by more favorable cardiometabolic factors.
Keywords: cardiovascular risk factor, dickkopf-1, hypoglycemic treatment, type 2 diabetes mellitus
Introduction
Hyperglycemia is an important risk factor of vascular endothelial dysfunction, leading to macrovascular complications including cardiovascular diseases, which are the main cause of death in patients with diabetes mellitus.1 Wingless protein (Wnt) signaling pathway is involved in endothelial function, vascular smooth muscle cell proliferation, inflammation and glucose metabolism, all closely associated with vascular diseases.2–5 Dickkopf-1 (DKK-1), a member of the Dickkopf family, is a major inhibitor of Wnt signaling pathway.6,7 A study in Caucasian populations found that circulating DKK-1 levels are higher in type 2 diabetes mellitus (T2DM) with cardiovascular disease (CVD) compared with those without CVD and are associated with abnormal carotid intima-media thickness.8 Otherwise, platelet activation has been revealed to play a vital role in the development of atherosclerosis.9,10 A cross-sectional study has shown that circulating DKK-1 levels are elevated in patients with T2DM and are significantly lower in T2DM receiving aspirin treatment, which suggests that DKK-1 is associated with endothelial dysfunction and platelet activation.11 Therefore, DKK-1 might be involved in the development of diabetes and its macrovascular complications, the underlying mechanism of which still need further investigation.
DKK-1 is a secreted protein consisting of two cysteine-rich domains and a signal peptide sequence12,13 and is widely expressed in many cells and tissues, including osteoblasts, osteocytes, skin, endothelium, prostate and placenta.14 Previous studies have revealed that DKK-1 is regulated by a variety of regulatory molecules, such as p53, β-catenin, progesterone and Msh homeobox 1.15,16 Given the close association with these molecules, DKK-1 and its regulation could be therapeutic targets for diseases.
To our knowledge, there are few studies focusing on changes in DKK-1 levels after hypoglycemic intervention in patients with T2DM. In our previously published study, recruited subjects were treated with acarbose or metformin in addition to insulin. The results demonstrated that both metformin and acarbose combined with insulin effectively reduced blood glucose, while acarbose combined with insulin also reduced glycemic variability.17 Song et al18 also found that both acarbose and metformin treatment could improve glycemic and weight control in patients with newly diagnosed T2DM, while acarbose could reduce urinary albumin/creatinine ratio compared to metformin. Acarbose inhibits the activity of alpha-glucosidase in the epithelial cells of the upper brush margin of the small intestine.19 Metformin reduces hepatic glucose production and peripheral insulin resistance and thereby lowers blood glucose.20 In the current study, we conducted a post-hoc analysis of this previous study in patients with T2DM, to explore the changes of serum DKK-1 levels after hypoglycemic treatment and the relationship between DKK-1 levels and clinical parameters.
Materials and Methods
Study Population
The present study is a sub-study of a previously published clinical trial (the effect of acarbose on glycemic variability in patients with type 2 diabetes mellitus using premixed insulin compared to metformin [AIM]).17 Briefly, it was a 12-week, open-label, randomized controlled clinical trial. The previous study was registered at www.ClinicalTrials.gov with clinical trial registration number NCT02438397. Subjects were recruited from the outpatient clinic at the Department of Endocrinology of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital from 2016 to 2018. Patients with premixed insulin treatment were enrolled. The inclusion criteria were as follows: (1) diagnosis of T2DM; (2) daily dose of premixed insulin injection higher than 20 IU/d and lower than 1 IU/(kg·day); (3) age from 30 to 70 years; (4) body mass index (BMI) between 18.5 and 35.0 kg/m2; (5) 7.0% ≤ glycated hemoglobin A1c (HbA1c) ≤ 10.0%. The subjects who met the screening requirements were randomly divided into two groups (metformin group or acarbose group). The dose of acarbose was 100 mg three times a day at three meals, and the dose of metformin was 500 mg three times a day after three meals. During the 12-week observation period, premixed insulin dose was adjusted by the same experienced endocrinologist and the subjects maintained their previous lifestyle during the 12-week hypoglycemic intervention.
To explore the relationship between DKK-1 changes and hypoglycemic treatment, patients taking aspirin were excluded in the present study, as DKK-1 levels were lower in T2DM receiving aspirin treatment.11 Patients whose serum samples were not available for DKK-1 testing were also excluded. Finally, a total of 46 patients in the metformin group and 46 in the acarbose group were included in this subgroup.
Informed consent was obtained from all the subjects. The study was approved by the Ethics Committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital and was in accordance with the principle of the 1964 Helsinki Declaration.
Anthropometric and Biochemical Measurements
As described previously,17 all subjects underwent anthropometric and biochemical assessments at baseline and after treatment (at 12-week). BMI was calculated as weight in kilograms divided by the square of the height in meters (kg/m2). Standardized meal tests were performed at baseline and the endpoint. Fasting blood samples were collected after an 8-h overnight fast. After that, the patient received standardized meal tests.17 Postprandial blood was collected 2 hours later. Fasting plasma glucose (FPG), 2-h postprandial plasma glucose (PG2h), HbA1c and serum lipid profiles were assayed as previously reported.17 Fasting serum DKK-1 levels were measured at the baseline and endpoint by quantitative sandwich enzyme-linked immunosorbent assays (R&D Systems, Inc. Minneapolis, USA).
Statistical Analyses
The distribution of the variables was tested by Kolmogorov–Smirnov tests. Variables with an approximately normal distribution were presented as means ± standard error (SE), while those with a skewed distribution were shown as median (interquartile range [IQR]). Continuous variables were compared between the two groups by Student’s t-test or analysis of covariance (ANCOVA) adjusting for baseline levels. Within-group differences in the parameters before and after treatment were analyzed by the paired t-test or Wilcoxon test. Skew-distributed variables were tested by the rank sum test. Comparison of categorical variables between groups was performed by the chi-square test. The differences in clinical parameters between 12-week and baseline were shown as Δ. Spearman correlation and partial correlation were used to analyze the correlation between DKK-1 levels and other clinical parameters. The receiver operating characteristic (ROC) curve was generated to analyze the optimal cut-off point of baseline DKK-1 levels for further analysis. The optimal cut-off point was confirmed based on the Youden index. Statistical analyses were performed using SPSS software version 22.0 (SPSS, Inc., Chicago, IL). A P value of <0.05 (two-tailed) was considered statistically significant.
Results
Changes of DKK-1 Levels After Hypoglycemic Treatment
A total of 92 patients were finally included (acarbose [ACA] group, n = 46; metformin [MET] group, n = 46) in the present study. Baseline DKK-1 levels did not differ between the two intervention groups (MET vs ACA, 3865.61 ± 117.30 pg/mL vs 3602.74 ± 122.60 pg/mL, P = 0.125). Serum DKK-1 levels were significantly decreased in both groups after treatment (MET: P = 0.012; ACA: P = 0.010). However, there was no difference in ΔDKK-1 between the two groups (MET vs ACA, −163.90 ± 62.87 pg/mL vs −171.47 ± 63.31 pg/mL, P = 0.933).
Since there were no differences in both baseline DKK-1 levels and post-treatment ΔDKK-1 levels between the MET and ACA groups, we carried out a further analysis in the total population (n = 92). The characteristics of all patients before and after treatment are shown in Table 1. After hypoglycemic treatment, FPG, PG2h, HbA1c, systolic blood pressure (SBP) and BMI were significantly reduced (all P < 0.05). Serum DKK-1 levels were significantly decreased after a 12-week hypoglycemic treatment (baseline vs endpoint, 3734.18 ± 85.49 vs 3566.49 ± 85.32, P < 0.001, Table 1).
 |
Table 1 Clinical Characteristics of the Participants at the Baseline and Endpoint |
The Relationship Between ΔDKK-1 Levels and Clinical Parameters
However, ΔDKK-1 levels were not correlated with gender, age, diabetes duration, and the improvement of metabolism, including changes of BMI, blood pressure, lipid profiles, plasma glucose and HbA1c (all P > 0.05). Spearman correlation revealed that serum ΔDKK-1 levels were negatively correlated with baseline DKK-1 levels (r = −0.263, P = 0.011). After adjusting for gender, age, diabetes duration, baseline BMI, blood pressure, lipid profiles and HbA1c, multiple stepwise regression analysis revealed that baseline DKK-1 levels were independently related to ΔDKK-1 levels (standardized β = −0.342, P = 0.001).
Since baseline DKK-1 levels were closely related to ΔDKK-1 levels, we further conducted the ROC analysis. The results showed that the optimal cut-off point of baseline DKK-1 levels for assessing the reduction of DKK-1 levels was 3689.27 pg/mL with the sensitivity of 61.29%, specificity of 66.67%, and area under the curve of 0.638 (95% confidence interval: 0.514–0.763) (Figure 1).
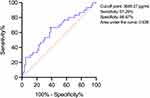 |
Figure 1 Receiver operating characteristic curve for baseline DKK-1 levels. Abbreviation: DKK-1, dickkopf-1. |
The Relationship Between Baseline DKK-1 Levels and Clinical Parameters
Spearman correlation analysis showed that baseline DKK-1 levels were not correlated with gender, age, or diabetes duration (all P > 0.05). Baseline DKK-1 levels were positively correlated with baseline total cholesterol (TC) (r = 0.226, P = 0.030) (Figure 2A) and low-density lipoprotein cholesterol (LDL-C) (r = 0.277, P = 0.007) (Figure 2B). Furthermore, baseline DKK-1 levels were positively correlated with ΔSBP (r = 0.250, P = 0.016) (Figure 2C) and ΔDBP (r = 0.240, P = 0.021) (Figure 2D). After adjusting for baseline SBP and DBP, baseline DKK-1 levels were still positively correlated with ΔSBP (r = 0.327, P = 0.002) and ΔDBP (r = 0.325, P = 0.002).
Then, subjects were divided into the lower baseline DKK-1 group (DKK-1 <3700 pg/mL, n = 44) and higher baseline DKK-1 group (DKK-1 ≥3700 pg/mL, n = 48). The clinical characteristics of the two groups stratified by baseline DKK-1 levels are shown in Table 2.
 |
Table 2 Clinical Characteristics of the Participants at the Baseline and Endpoint Divided by Baseline DKK-1 Levels |
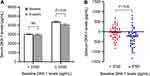
There was no significant difference in DKK-1 levels between baseline and endpoint in the lower baseline DKK-1 group (baseline vs endpoint, 3026.49 ± 65.76 pg/mL vs 2977.40 ± 81.39 pg/mL, P = 0.346) (Figure 3A). However, in the higher baseline DKK-1 group, DKK-1 levels were decreased after treatment (baseline vs endpoint, 4382.89 ± 69.09 pg/mL vs 4106.49 ± 92.12 pg/mL, P < 0.001) (Figure 3A). ΔDKK-1 levels were larger in the higher baseline DKK-1 group as compared with the lower baseline DKK-1 group (−276.39 ± 67.51 pg/mL vs −49.09 ± 51.48 pg/mL, P = 0.010) (Figure 3B).
Compared with the higher baseline DKK-1 group, subjects in the lower baseline DKK-1 group had significantly lower baseline HbA1c levels (P = 0.008) and LDL-C levels (P = 0.048). BMI, SBP and DBP were decreased significantly in the lower DKK-1 group (all P < 0.05, Table 2) but not in the higher baseline DKK-1 group (all P > 0.05, Table 2) after treatment. The reduction of SBP and DBP (both P < 0.05, Table 2), but not BMI (P > 0.05, Table 2), were significantly greater in the lower baseline DKK-1 group than that in the higher baseline DKK-1 group. After treatment, FPG, PG2h and HbA1c were significantly improved in both groups (all P < 0.01, Table 2). Covariance analysis showed that ΔHbA1c did not differ between the two groups after adjustment of baseline HbA1c (P > 0.05).
Discussion
In the present study, the serum DKK-1 levels were significantly reduced after hypoglycemic treatment. Patients with lower baseline DKK-1 levels were featured by more favorable baseline blood glucose levels and lipid profiles and had more pronounced improvement in blood pressure after treatment.
We found that a 12-week treatment with premixed insulin with acarbose or metformin was associated with a significant decrease in serum DKK-1 levels. Previous studies showed that DKK-1 might be related to diabetes mellitus, as several cross-sectional studies have revealed that plasma DKK-1 levels were markedly higher in patients with diabetes mellitus than those in healthy subjects.11,21,22 A clinical study showed that DKK-1 levels were significantly decreased after acarbose or rosiglitazone intervention.23 Ghardashi-Afousi et al24 found out that 12 weeks of high-intensity interval training could reduce both fasting glucose and DKK-1 levels. Thus, it is implied that DKK-1 levels could be reduced after hypoglycemic intervention. However, the results in the present study showed that ΔDKK-1 levels were not correlated with the improvement of metabolic indicators. Thus, the underlying mechanisms between the reduction of DKK-1 levels and hypoglycemic treatments need further investigation.
To be noted, the present study revealed that subjects with lower baseline DKK-1 levels (<3700 pg/mL) had lower baseline HbA1c, TC and LDL-C levels, moreover, subjects with lower baseline DKK-1 levels had more significant improvement of blood pressure compared with subjects with higher baseline DKK-1 levels (≥3700 pg/mL), while the glycemic improvement was similar between these two groups. Since lipid profiles and blood pressure are important risk factors of cardiovascular disease,25,26 our results implied that patients with lower baseline DKK-1 levels might have a lower risk of cardiovascular disease. Previous studies showed that DKK-1 levels are closely related to atherosclerotic diseases such as myocardial infarction or ischemic cerebrovascular disease.24,27,28 Ghardashi-Afousi et al24 found that carotid intima-media thickness, a validated surrogate marker of atherosclerosis, was positively associated with DKK-1. Ueland et al27 have demonstrated by experiments that increasing DKK-1 levels in carotid plaque may enhance the interactions between platelets and endothelial cells, which may play a role in the progression of atherosclerotic lesion. Our results provided more information on the relationship between DKK-1 and serum lipids, but the cause-and-effect linkage between DKK-1 and serum lipids still needs in vitro and in vivo experiments to clarify. In addition, we found a negative relationship between the changes of DKK-1 levels and baseline DKK-1 levels. Even though the reduction of DKK-1 was greater in higher baseline DKK-1 group, the subjects did not show better improvement in cardiometabolic parameters. As we did not evaluate the events of cardiovascular disease in the present study, the relationship between DKK-1, diabetes mellitus, and its complications and comorbidities needs to be further illuminated in future studies.
There are some limitations in this study. First of all, the present study is a sub-study of AIM study with a relatively small sample size. Second, the present study is a single-center study, limiting the generalizability of our findings. Third, the short period of intervention (12 weeks) could only demonstrate a short-term effect of hypoglycemic treatment on the levels of DKK-1 and cardiometabolic risk factors. Fourth, even though the subjects were asked to maintain their previous lifestyle, daily nutrition intake was not measured in the study. The effect of nutrition and calorie intake on DKK-1 levels needs further investigation. Moreover, there was no control group without treatment, so the relationship between hypoglycemic drugs, HbA1c, and DKK-1 levels needs to be further studied by designing placebo-controlled trials in the future.
Conclusions
In conclusion, DKK-1 levels were significantly decreased after hypoglycemic treatment. Patients with lower baseline DKK-1 levels might have better cardiometabolic state and improvement of metabolic indicators after treatment.
Abbreviations
BMI, body mass index; DBP, diastolic pressure; DKK-1, Dickkopf-1; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PG2h, 2-hour postprandial plasma glucose; SBP, systolic pressure; TC, total cholesterol; TG, triglycerides.
Data Sharing Statement
The data that support the findings of this study are available upon reasonable request from the corresponding authors (Jingyi Lu, [email protected]; Xiaojing Ma, [email protected]).
Ethical Approval and Informed Consent
The study was approved by the Ethics Committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital and was in accordance with the principle of the 1964 Helsinki Declaration. Informed consent was obtained from all individual participants included in the study.
Acknowledgments
We would like to thank all the involved clinicians, nurses, and technicians for their contribution to the study. We are grateful to all the participants for their dedication in data collection and laboratory measurements.
Funding
This work was funded by the Shanghai Municipal Commission of Health and Family planning General Program (201840232).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Park KS, Kwak S, Cho YM, et al. Vildagliptin reduces plasma stromal cell-derived factor-1α in patients with type 2 diabetes compared with glimepiride. J Diabetes Investig. 2017;8(2):218–226. doi:10.1111/jdi.12572
2. Zimmerman ZF, Moon RT, Chien AJ. Targeting WNT pathways in disease. Cold Spring Harb Perspect Biol. 2012;4(11):a008086. doi:10.1101/cshperspect.a008086
3. Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi:10.1016/j.cell.2012.05.012
4. Foulquier S, Daskalopoulos EP, Lluri G, Hermans KCM, Deb A, Blankesteijn WM. WNT signaling in cardiac and vascular disease. Pharmacol Rev. 2018;70(1):68–141. doi:10.1124/pr.117.013896
5. Jin T. The WNT signalling pathway and diabetes mellitus. Diabetologia. 2008;51(10):1771–1780. doi:10.1007/s00125-008-1084-y
6. Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169(6):985–999. doi:10.1016/j.cell.2017.05.016
7. Huang Y, Liu L, Liu A. Dickkopf-1: current knowledge and related diseases. Life Sci. 2018;209:249–254. doi:10.1016/j.lfs.2018.08.019
8. Garcia-Martín A, Reyes-Garcia R, García-Fontana B, et al. Relationship of dickkopf1 (DKK1) with cardiovascular disease and bone metabolism in caucasian type 2 diabetes mellitus. PLoS One. 2014;9(11):e111703. doi:10.1371/journal.pone.0111703
9. Davì G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357(24):2482–2494. doi:10.1056/NEJMra071014
10. Kaur R, Kaur M, Singh J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: molecular insights and therapeutic strategies. Cardiovasc Diabetol. 2018;17(1):121. doi:10.1186/s12933-018-0763-3
11. Lattanzio S, Santilli F, Liani R, et al. Circulating dickkopf-1 in diabetes mellitus: association with platelet activation and effects of improved metabolic control and low-dose aspirin. J Am Heart Assoc. 2014;3(4):e001000. doi:10.1161/JAHA.114.001000
12. Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391(6665):357–362. doi:10.1038/34848
13. Ma Y, Zhang X, Wang M, et al. The serum level of dickkopf-1 in patients with rheumatoid arthritis: a systematic review and meta-analysis. Int Immunopharmacol. 2018;59:227–232. doi:10.1016/j.intimp.2018.04.019
14. Ke HZ, Richards WG, Li X, Ominsky MS. Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocr Rev. 2012;33(5):747–783. doi:10.1210/er.2011-1060
15. Wang J, Shou J, Chen X. Dickkopf-1, an inhibitor of the Wnt signaling pathway, is induced by p53. Oncogene. 2000;19(14):1843–1848. doi:10.1038/sj.onc.1203503
16. Li J, Gong W, Li X, et al. Recent progress of wnt pathway inhibitor dickkopf-1 in liver cancer. J Nanosci Nanotechnol. 2018;18(8):5192–5206. doi:10.1166/jnn.2018.14636
17. Gao F, Ma X, Peng J, et al. The effect of acarbose on glycemic variability in patients with type 2 diabetes mellitus using premixed insulin compared to metformin (AIM): an open-label randomized trial. Diabetes Technol Ther. 2020;22(4):256–264. doi:10.1089/dia.2019.0290
18. Song L, Kong X, Yang Z, et al. Acarbose reduces low-grade albuminuria compared to metformin in Chinese patients with newly diagnosed type 2 diabetes. Diabetes Metab Syndr Obes. 2021;14:4451–4458. doi:10.2147/DMSO.S325683
19. Tibaldi J. Importance of postprandial glucose levels as a target for glycemic control in type 2 diabetes. South Med J. 2009;102(1):60–66. doi:10.1097/SMJ.0b013e318188898e
20. Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60(9):1577–1585. doi:10.1007/s00125-017-4342-z
21. Gaudio A, Privitera F, Pulvirenti I, Canzonieri E, Rapisarda R, Fiore CE. The relationship between inhibitors of the Wnt signalling pathway (sclerostin and dickkopf-1) and carotid intima-media thickness in postmenopausal women with type 2 diabetes mellitus. Diab Vasc Dis Res. 2014;11(1):48–52. doi:10.1177/1479164113510923
22. Kurban S, Selver Eklioglu B, Selver MB. Investigation of the relationship between serum sclerostin and dickkopf-1 protein levels with bone turnover in children and adolescents with type-1 diabetes mellitus. J Pediatr Endocrinol Metab. 2022;35(5):673–679. doi:10.1515/jpem-2022-0001
23. Santilli F, Liani R, Di Fulvio P, et al. Increased circulating resistin is associated with insulin resistance, oxidative stress and platelet activation in type 2 diabetes mellitus. Thromb Haemost. 2016;116(6):1089–1099. doi:10.1160/TH16-06-0471
24. Ghardashi-Afousi A, Davoodi M, Hesamabadi BK, et al. Improved carotid intima-media thickness-induced high-intensity interval training associated with decreased serum levels of Dkk-1 and sclerostin in type 2 diabetes. J Diabetes Complications. 2020;34(1):107469. doi:10.1016/j.jdiacomp.2019.107469
25. Soppert J, Lehrke M, Marx N, Jankowski J, Noels H. Lipoproteins and lipids in cardiovascular disease: from mechanistic insights to therapeutic targeting. Adv Drug Deliv Rev. 2020;159:4–33. doi:10.1016/j.addr.2020.07.019
26. Stevens SL, Wood S, Koshiaris C, et al. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ. 2016;354:i4098. doi:10.1136/bmj.i4098
27. Ueland T, Otterdal K, Lekva T, et al. Dickkopf-1 enhances inflammatory interaction between platelets and endothelial cells and shows increased expression in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29(8):1228–1234. doi:10.1161/ATVBAHA.109.189761
28. Seifert-Held T, Pekar T, Gattringer T, et al. Circulating dickkopf-1 in acute ischemic stroke and clinically stable cerebrovascular disease. Atherosclerosis. 2011;218(1):233–237. doi:10.1016/j.atherosclerosis.2011.05.015
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.