Back to Journals » OncoTargets and Therapy » Volume 14
Decitabine and Cisplatin are Synergistic to Exert Anti-Tumor Effect on Gastric Cancer via Inducing Sox2 DNA Demethylation
Authors Zhu Z, Lin S, Wu X , Xu J, Li L, Ye W, Li J, Huang Z
Received 15 September 2020
Accepted for publication 10 December 2020
Published 22 January 2021 Volume 2021:14 Pages 623—636
DOI https://doi.org/10.2147/OTT.S276168
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Gaetano Romano
Zhipeng Zhu,1 Sihao Lin,1 Xiaofang Wu,2 Jiuhua Xu,2 Lulu Li,1 Weipeng Ye,2 Jiayi Li,3 Zhengjie Huang1,2
1Department of Gastrointestinal Surgery, Xiamen Cancer Center, The First Affiliated Hospital of Xiamen University, Xiamen, Fujian 361003, People’s Republic of China; 2Department of Clinical Medicine, Fujian Medical University, Fuzhou, Fujian 350004, People’s Republic of China; 3Department of Medical Oncology, Xiamen Cancer center, The First Affiliated Hospital of Xiamen University, Xiamen, Fujian 361003, People’s Republic of China
Correspondence: Zhengjie Huang
Department of Gastrointestinal Surgery, Xiamen Cancer Center, The First Affiliated Hospital of Xiamen University, 55 Zhen Hai Road, Si Ming District, Xiamen, Fujian 361003, People’s Republic of China
Tel +86-592-2139280
Fax +86-592-2137368
Email [email protected]
Background: Cisplatin is a vital chemotherapy regimen for gastric cancer (GC), while partial response is observed (approximately 40%) because of drug resistance. Thus, it is urgent to improve drug sensitivity to improve the therapeutic effect of cisplatin on GC.
Purpose: The study was performed to explore the synergistic effect of decitabine and cisplatin in GC.
Materials and Methods: Cancer and matched adjacent tissues from patients with GC were obtained and quantitative real-time PCR (qRT-PCR), Western blot and immunohistochemistry were performed to evaluate Sox2 expression level. Methylation-specific PCR (MSP) was performed to assess the effect of 5-aza-2ʹ-deoxycytidine (5-Aza-CdR) on Sox2 promoter. Cell proliferation assay, scratch-wound migration assay and Transwell invasion ability were performed to assess the effect of 5-Aza-CdR on proliferation, migration and invasion ability. Meantime, the effect of 5-Aza-CdR was also investigated in gastric cell lines BGC-823 and nude mouse xenograft tumor model. Finally, the anti-cancer effect of decitabine, cisplatin and their combination treatment were investigated in a BGC-823 and nude mouse xenograft tumor model, Sox2 methylation level, Sox2 expression of BGC-823 and xenograft tumors were analyzed by MSP, qRT-PCR and Western blot.
Results: Sox2 expression was significantly associated with different differentiated degrees, depth of invasion (0.0011), lymph node metastasis (0.0013), and TNM stage (0.0002). Next, methylation inhibitor 5-Aza-CdR restored Sox2 expression to promote proliferation, migration and invasion in vitro and in vivo. Finally, cisplatin and decitabine was found to be synergistic to inhibit proliferation of xenograft tumors. Likewise, cisplatin and decitabine were also synergistic to induce Sox2 DNA demethylation to promote Sox2 mRNA and protein expression in BGC-823 and xenograft tumors.
Conclusion: Cisplatin and decitabine could be synergistic to induce Sox2 DNA demethylation to promote expression of the Sox2 gene, which exerted an anti-tumor effect on GC. It may suggest an insight for innovative therapeutics of GC.
Keywords: Sox2, DNA demethylation, gastric cancer, decitabine, cisplatin, anti-tumor
Introduction
GC is one the most commonly occurring gastrointestinal tumors, which has remained the second leading cause of cancer-related mortality over the past few years.1 Multiple risk factors participate in the emergence and development of GC, including environmental factors, genetic factors, and epigenetic alterations.2 Briefly, epigenetic alterations could regulate gene expression without changes in DNA sequence, which lead to genetic changes in various tumor oncogenes and suppressor genes.3 As the main mechanisms in epigenetic regulations, DNA methylation plays an important role in cell biology, gene silencing and embryonic development, and aberrant DNA methylation participates in the initiation and progression in various cancers.4
An analysis involving 15 types of cancers from 600 samples indicated that aberrant DNA methylation appears on various types of cancer-related genes with different frequencies of different cancer-related genes in different parts.5,6 Hypermethylation is a prompter of suppressor genes which can reduce genes silencing, such as CDH13, p16, MGMT, and E-cadherin. And hypermethylation status in several tumor suppressor genes may be the early driver event in GC, including E-cadherin, Runx3 (runt-related transcription factor 3 gene), CHFR, and DAPK.
It was known that Sox2 is significantly associated with differentiation, initiation, progression and malignant biological behavior in the gastrointestinal tract.7–9 Previous studies revealed that Sox2 is up-regulated in gastric cancer cells9 and gastric stem cells,10 which function as oncogene to promote the occurrence and development of GC, and Sox2 overexpression is associated with poor prognosis.11 However, more studies showed Sox2 could be considered as a tumor suppressor gene, which plays a vital role in anti-cell proliferation, anti-metastasis, and anti-apoptosis.7,12–15 Patients with positive Sox2 expression have longer overall survival than patients with negative Sox2 expression.13,16 Furthermore, Sox2 protein expression may be considered as an independent prognostic factor for survival prognosis in GC.17
Chemotherapy is still the main therapeutic regimen in polychemotherapy to treat advanced gastric cancer. With wide application of Cisplatin, Irinotecan, Taxus, 5-fluorouracil and so on, the effect of chemotherapy has been remarkably improved. However, a considerable proportion of patients cannot benefit from chemotherapy because of drug-resistance. Cisplatin is a cell cycle related non-specific cytotoxic drug, which can inhibit the DNA replication process and damage their cell membrane structure, and it is effective in malignant tumors from various systems, organs and tissue sources. Cisplatin and its derivant account for 70% of chemotherapy regimens,18 which is frequently applied in polychemotherapy regimens in GC, while partial response is observed only in approximately 40% because of drug-resistance.19 Thus it is urgent to improve drug-sensitivity to improve the therapeutic effect of chemotherapy on GC.
Recently, the association between tumor resistance and methylation as a prompter has become a hot topic. It is known that DNA methylation is a reversible process and DNA methyltransferase (DNMT) is the most important molecule to restore the function of tumor suppressor genes, including DNMT1, DNMT2, and DNMT3. DNMT1 is closely associated with cancers.20 Nowadays, decitabine is the representative drug belonging to DNMT1 inhibitor, which was firstly approved to treat myelodysplastic syndrome by FDA,21 the clinical significance for solid tumor, such as lung cancer22 and prostate cancer.23 Viet et al24 revealed decitabine could resduce cisplatin resistance in head and neck squamous cell carcinoma, which indicated DNA methylation may be considered as a biomarker of cisplatin resistance. However, few studies have reported the synergistic effect of decitabine and cisplatin in GC.
Materials and Methods
Clinical Tissue Samples, Cell Lines and Animals
Gastric cancer and matched adjacent tissues were obtained from the Department of Gastrointestinal Surgery, The First Affiliated Hospital of Xiamen University, the People's Republic of China, between September 2010 and February 2016. All samples were collected with patients’ informed consent, and all tissues were pathology confirmed. This study was approved by the Ethics Committee of the First Affiliated Hospital of Xiamen University.
The gastric cell lines BGC-823 and GES-1 were provided by the Department of Cancer Center, The First Affiliated of Xiamen University (Xiamen, People's Republic of China). All cells were cultured in RPMI-1640 medium containing 10% heat-inactivated fetal bovine serum (FBS) and maintained at 37°C in a humidified chamber containing 5% CO2.
The animals consisted of 72 BALB/c-nu/nu nude mice (4 weeks, 20–25 g) and were obtained from Xiamen University Laboratory Animal Center.
Quantitative RT-PCR (qRT-PCR)
Total RNA was extracted using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) after tissue samples and cell lines were harvested. cDNA was synthesized using ReverTra AceH qPCR RT Kit (TOYOBO) with 1 mg total RNA. The primer upstream sequence of Sox2 was 5ʹ-ATGGGTTCGGTGGTCAAGTC −3ʹ and the primer downstream sequence was 5ʹ-CCCTCCCATTTCCCTCGTTT −3ʹ. The primer upstream sequence of GAPDH was 5ʹ- GTGGACCTGACCTGCCGTCT −3ʹ and the primer downstream sequence was 5ʹ- GGAGGAGTGGGTGTCGCTGT −3ʹ. Quantitative RT-PCR was performed for 30 cycles of denaturation (at 94°C for 30 seconds), annealing (at 56°C for 30 seconds) and elongation (at 72°C for 1 minute).
Western Blot
Western blot was performed as described previously. The primary antibodies were anti-Sox2 antibody and anti-GAPDH antibody, and goat anti-mouse/rabbit double antibodies were used as secondary antibodies.
Immunohistochemical Staining
Immunohistochemistry analysis was conducted as described previously. After deparaffinizing and rehydration, immunostaining was performed at 4°C overnight with anti-Sox2 antibody and peroxidase-conjugated anti-mouse secondary antibody. Then, streptomycin antibiotic protein-peroxidase was added into incubator at 37°C for 45 minute. Next, DAB chromogenic fluid was used to perform chromogrnic reaction. Following hematoxylin dye solution for redyeing, gradient alcohol for dehydration, dimethylbenzene for vitrifying and neutral gum for depositing.
Methylation-Specific PCR
Genomic DNA was extracted from cell lines and translated tumor using TIANamp Genomic DNA kit, DP304. The MethPrimer website (https://www.urogene.org/methprimer/index1.html) was performed to identify CpG islands of Sox2, The methylated primers were 5ʹ-AGTCGTCGGGTTCGTAGTAAATTTC −3ʹ (sense), 5ʹ-AAAACATTCATAAACCGCTTAACGCG −3ʹ (antisense). While the unmethylated primers were 5ʹ-TGAAGTTGTTGGGTTTGTAGTAAATTTT C-3ʹ (sense), 5ʹ-ATAAAAACATTCATAAACCACTTAACACA −3ʹ. The reaction mixture contained 2.0 μL DNA, 0.5 μL of each primer, 12.5 μL 2×PCR TaqMix, 9.5 μL ddH2O, the complete MSP conditions were as follows: 94°C for 5 minutes, followed by 40 cycles of 94°C for 30 seconds, 56°C for 30 seconds, and 72°C for 2 minutes, with a final extension at 72°C for 10 minutes. The analysis was repeated on 3 different days. Finally, the PCR products were subjected to 2% agarose gel electrophoresis at 120 V for 40 minutes.
Cell Proliferation Assay
For the MTT assay, BGC-823 cells were seeded and transfected in a 96-well plate, with three wells in each group. At 0.5, 1, 2, and 4 days, OD at 490 nm was selected to assess the absorbance of each well using an enzyme-linked immunometric meter. Experiments were repeated at least three times.
Scratch-Wound Migration Assay
A pipette was used to draw a horizontal line at the back of the six-well plate after the cells spread over the plate. Then PBS was used to wash the plate, and 0 μmol/L, 1 μmol/L, 10 μmol/L 5-Aza-CdR was added into the culture medium. Finally, an optical microscope was used to observe migration at 0 and 24 hours.
Transwell Invasion Ability
A total of 5×104 cells/mL cells were plated in the upper chamber containing 200 µL serum-free media, while the bottom chamber contained 600 medium supplemented with 10% FBS. After 48 hours, the migrated cells were fixed, stained, dried, and measured.
5-Aza-CdR Inhibited Transplanted Tumors in a Nude Mice Model
A 0.2 mL 1×107 cells/mL cell suspension of BGC-823 cells was injected into the back of nude mice. When the volume had grown into 10 mm3, 40 nude mice were randomly divided into two groups with 20 per group. 2mL PBS, 5-Aza-CdR (10 mol I山mol) was injected into the abdominal cavity. Next, 10 nude mice in each group were selected for calculating volume every 24 hours, tumor volume was monitored and calculated according to the formula: V (mm3) = 0.526 × L (length) × W2 (width) by measuring tumor length and width every 24 hours. At the end of the 15th day, each mouse was euthanized (by cervical dislocation) and the tumor tissues were removed for weighing. Furthermore, the tumor tissues were used for Western blot and Immunohistochemical staining. Finally, the other 10 nude mice in each group were used to record the survival time.
Effect of Different Drug Treatment on Transplanted Tumors in a Nude Mice Model
A total of 0.2 mL 1×107 cells/mL cell suspension of BGC-823 cell was injected into the back of nude mice. When the long diameter of the tumors grew into 0.5 cm, 32 nude mice were randomly divided into four groups, with eight per group. In the control group, PBS was injected into the abdominal cavity on the first and 4th day. In the gemcitabine group, 5 mg/kg gemcitabine was injected into the abdominal cavity on the first day and PBS was injected into the abdominal cavity on the 4th day. In the cisplatin group, PBS was injected into the abdominal cavity on the first day and 6 mg/kg cisplatin was injected into the abdominal cavity on the 4th day. In the cCisplatin+gemcitabine group, 5 mg/kg gemcitabine was injected into the abdominal cavity on the first day, and 6 mg/kg cisplatin was injected into the abdominal cavity on the 4th day. Tumor volume was monitored and calculated according to the formula: V (mm3) = 0.526 × L (length) × W2 (width) by measuring tumor length and width every 24 hours.
Results
Sox2 Has a Significantly Clinicopathological Significance
Sox2 Expression Level in Different Differentiated Gastric and Surrounding Nontumor Tissues
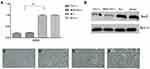
The relationship between Sox2 expression and progression of GC was investigated in cancerous and the surrounding nontumor tissues from 60 surgical specimens, RT-PCR indicated that there was no significant difference between well-differentiated tissues and surrounding nontumor tissues. However, Sox2 mRNA was significantly higher in well-differentiated tissues than moderately differentiated and poorly differentiated tissues (Table 1 and Figure 1A), Meantime, Western-blotting indicated Sox2 protein was obviously higher in well-differentiated tissues than moderately differentiated and poorly differentiated tissues (Figure 1B). In addition, immunohistochemistry was performed to detect the expression level of Sox2 protein in different differentiation degree, well-differentiated tissues had a significantly higher positive rate than moderately differentiated and poorly differentiated tissues, and the positive rate of well-differentiated tissues were similar to the surrounding nontumor tissues (Table 2 and Figure 1C–F).
 |
Table 1 Relative Sox2 mRNA Expression Level in Different Differentiated Gastric Tissues and Surrounding Nontumor Tissues |
 |
Table 2 Sox2 Protein Expression Level in Different Differentiated Gastric Tissues and Surrounding Nontumor Tissues |
The Association of Sox2 Expression with Clinicopathological Parameters
To further investigate the association between Sox2 expression and clinicopathological parameters. 60 samples were divided into two groups according to Sox2 staining intensity, including high Sox2 staining (n=19) and low Sox2 staining (n=41). We found the Sox2 expression had a strong association with depth of invasion (0.0011), lymph node metastasis (0.0013) and TNM stage (0.0002). However, Sox2 expression had no significant correlation with age (0.4311 and gender (0.8960) (Table 3).
 |
Table 3 Correlation of Sox2 Expression with Clinicopathological Characteristics |
DNA Methyltransferase Inhibitor Can Inhibit the Growth, Migration and Invasion of BGC-823 Cell Lines
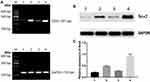
5-Aza-CdR Reversed Methylation Status to Influence Sox2 Expression
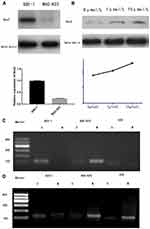
Western-blotting indicated Sox2 protein was obviously lower in BGC-823 than normal gastric mucosa epithelium cell line GES-1 (Figure 2A), and methyltransferase inhibitor 5-Aza-CdR could promote Sox2 expression in a dose-dependent manner, Sox2 expression level was higher in the 10 μmol/L group (0.83±0.14) than in the 1 μmol/L (0.73±0.13) and 0 μmol/L groups (0.65±0.19) (Figure 2B). The MSP revealed that Sox2 gene promotor was in the status of methylation in BGC-823 (Figure 2C), and 5-Aza-CdR reversed status from methylation to nonmethylation status (Figure 2D).
5-Aza-CdR Inhibited the Proliferation of BGC-823 cell Lines
To investigate the effect of 5-Aza-CdR on cell proliferation, MTT assay was performed to assess the cell proliferation rate. In a fixed concentration, the cell proliferation inhibition rate increased with the prolonged time of administration of 5-Aza-CdR. Also, in a fixed time point, cell proliferation inhibition rate increased with increased concentration (Table 4 and Figure 3A).
 |
Table 4 The Effect of Different Concentrations on Proliferation Inhibition Rate of BGC-823 in Different Time Points |
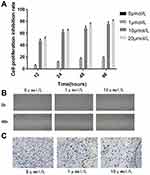 |
Figure 3 5-Aza-CdR could inhibit the proliferation, migration and invasion ability of BGC-823 cell lines.(A) Cell proliferation inhibition rate, (B) Cell migration image, (C) Cell invasion image. |
5-Aza-CdR Inhibited Migration Ability of BGC-823 Cell Lines
The migration distance of the 0 μmol/L group, the 1 μmol/L group and the 10 μmol/L group were 0.268±0.0190 mm, 0.020±0.008 mm, and 0.010±0.001 mm, respectively. The result revealed that the migration distance of 1 μmol/L and 10 μmol/L group was significantly shorter compared with the 0 μmol/L group. Scratch-wound migration assay indicated 5-Aza-CdR could inhibit migration ability (Figure 3B).
5-Aza-CdR Inhibited Invasion Ability of BGC-823 Cell Lines
The number of BGC-823 invading and passing through the basement membrane was 188.60±10.90, 75.20±6.18 and 85.4±8.47, respectively. Compared with the 0 μmol/L group, BGC-823 invading and passing through the basement membrane were significantly decreased in the 1 and 10 μmol/L groups. Transwell invasion assay indicated that 5-Aza-CdR could inhibit invasion ability (Figure 3C).
5-Aza-CdR Inhibited Transplanted Tumor in Nude Mice Model
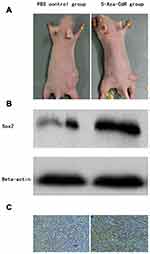
The transplanted tumors in nude mice grew to about 125 mm3. Treatment was implied in the control and 5-Aza-CdR groups, though the 5-Aza-CdR group showed a slower increase in tumor volume compared with the control group. The final weight of transplanted tumor was 694.7±36.1 mg in the control group and 325.2±32.2 mg in the 5-Aza-CdR group, respectively. The tumor inhibition rate of 5-Aza-CdR was 53.2%, which indicated 5-Aza-CdR could inhibit tumor in vivo (Table 5 and Figure 4A).
 |
Table 5 Effect of 5-Aza-CdR on Transplanted Tumors in a Nude Mice Model |
Western-Blotting Detecting Sox2 Protein from Transplanted Tumor
Tumor tissue was taken out from nude mice after treatment. The protein expression level of Sox2 was higher in the 5-Aza-CdR (0.96±0.25) than in the control group (0.73±0.15) (t=16.052, P<0.0001) (Figure 4B).
Immunohistochemistry Detecting Sox2 Protein from Transplanted Tumor
Tumor tissues were taken out from nude mice for immunohistochemistry, and protein expression level of Sox2 was detected by immunohistochemistry. The 5-Aza-CdR group demonstrated strong Sox2 staining while the control group showed weak Sox2 staining. The expression score in the 5-Aza-CdR group was higher than in the control group (Figure 4C).
Gemcitabine and Cisplatin Were Synergistic to Inhibit Tumor Growth Through Sox2 Methylation in BGC-823 Cell Lines
Effect of Different Treatments on Sox2 Methylation in BGC-823 Cell Lines
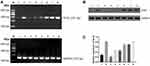
After treating with different drug treatments, BGC-823 was taken out for MSP to assess Sox2 promoter. As shown in Figure 5, it revealed that the methylated band of Sox2 was very strong while the unmethylated band was very weak in the control group. In the 5 mg/kg gemcitabine group, the unmethylated band was obvious, however, the methylated band was relatively weaker than the control group. After treating with different concentrations of cisplatin (1 μM, 10 μM and 100 Μm), the unmethylated band was gradually more obvious as the concentration increased. After treating with gemcitabine and different concentrations of cisplatin (1 μM, 10 μM and 100 μM), the unmethylated band was gradually more obvious and the methylated band was gradually weaker as the centration of cisplatin increased. The result indicated Sox2 promoter was hypermethylated and 5 mg/kg gemcitabine could partly reverse the methylated status. With centration of cisplatin increased, the unmethylated band had an increasing trend. Gemcitabine and different concentrations of cisplatin (1 μM, 10 μM and 100 μM) were synergistic to reverse the methylated status.
 |
Figure 5 Effect of different treatments on Sox2 methylation in BGC-823 cell lines. Marker: 600 bp DNA Ladder Marker. Abbreviations: U, unmethylation; M, methylation. |
Effect of Different Treatments on Sox2 mRNA Expression in BGC-823 Cell Lines
After treating with different drug treatments, BGC-823 cell lines were taken out for RT-PCR to detect Sox2 mRNA expression. As shown in Figure 6A, compared with the control group, Sox2 mRNA expression level markedly increased. After treating with different concentrations of cisplatin (1 μM, 10 μM and 100 μM), Sox2 mRNA expression level demonstrated a trend of a slow rise. THe combination of gemcitabine and different concentrations of cisplatin (1 μM, 10 μM and 100 μM) could further increase SOX2 mRNA expression level.
Effect of Different Treatments on Sox2 Protein in BGC-823 Cell Lines
After treating with different drug treatments, Western blot was performed to detect Sox2 protein. As shown in Figure 6B, Figure 6C and Table 6, the expression of SOX2 protein was hardly detected in the control group. After treating with gemcitabine, Sox2 protein level markedly increased. After treating with different concentrations of cisplatin (1 μM, 10 μM and 100 μM), SOX2 protein demonstrated a trend of slow rise. Combination of gemcitabine and different concentrations of cisplatin (1 μM, 10 μM and 100 μM) could further increase SOX2 protein expression level. Compared with the 5 μM gemcitabine group, SOX2 protein was significantly higher in the 5 μM gemcitabine +1 μM cisplatin group (P<0.05), 5 μM gemcitabine +10 μM cisplatin group (P<0.01) and 5 μM gemcitabine +100 μM cisplatin group (P<0.01). Compared with the 5 μM gemcitabine +10 μM cisplatin group, there was no significant difference in the 5 μM gemcitabine +1 μM cisplatin group (P>0.05) and in the 5 μM gemcitabine +100 μM cisplatin group (P>0.05).
 |
Table 6 The Effect of Different Treatments on Sox2 mRNA Expression Level in BGC-823 |
Gemcitabine and Cisplatin are Synergistic to Inhibit Tumor Growth Through Sox2 Methylation in a Nude Mice Model
Effect of Different Treatments on Transplanted Tumor Growth
During the first 2 weeks, tumors were observed, measured, and recorded every 2 days. Since the 6th day, there was a significant difference in tumor volume between the gemcitabine + cisplatin group and the other three groups. After the 10th day, there was a significant difference in tumor volume between the 5 mg/kg gemcitabine group and the control group. Since the 8th day, the 6 mg/kg cisplatin group had a significant difference compared with the control group (Figure 7).
Effect of Different Treatments on Sox2 Methylation in a Nude Mice Model
Tumor tissues were taken out from nude mice for MSP to assess Sox2 promoter. The result indicated that methylated band of Sox2 was very strong in the control group, while the unmethylated band was very weak. In the 5 mg/kg gemcitabine group, the unmethylated band was obvious, however, the methylated band was relatively weaker than in the control group. Meantime, in the 6 mg/kg cCisplatin group, the unmethylated band was weak and the methylated band was very obvious. Furthermore, in the 5 mg/kg gemcitabine + 6 mg/kg cisplatin group, the unmethylated band was very obvious, while the methylated band was weakened further. The result indicated that the Sox2 promoter was hypermethylated in the control group, and 5 mg/kg gemcitabine could partly reverse the methylated status, while the impact of 6 mg/kg cisplatin on methylated status was limited. However, 5 mg/kg gemcitabine and 6 mg/kg cisplatin could be synergistic to reverse the methylated status (Figure 8).
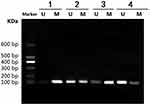 |
Figure 8 Effect of different treatments on Sox2 methylation in a nude mice model. 1) control group; 2) 5 mg/kg decitabine; 3) 6 mg/kg cisplatin; 4) 5 mg/kg decitabine + 6 mg/kg cisplatin. |
Effect of Different Treatments on Sox2 mRNA Expression in a Nude Mice Model
Transplanted tumors were taken out for qRT-PCR to detect Sox2 mRNA. Compared with the control group, Sox2 mRNA was higher in the 6 mg/kg cisplatin group, with no significance (P>0.05), while Sox2 mRNA in the 5 mg/kg gemcitabine group was significantly higher (P<0.01). THe combination of 5 mg/kg gemcitabine + 6 mg/kg cisplatin treatment was marginally higher than the control group (P<0.01), the 6 mg/kg cisplatin group (P<0.01) and the 5 mg/kg gemcitabine group (P<0.01). The result demonstrated that 6 mg/kg cisplatin and 5 mg/kg gemcitabine could promote Sox2 expression, and the combination of 5 mg/kg gemcitabine + 6 mg/kg cisplatin treatment could be synergistic to promote Sox2 mRNA expression (Figure 9A and Table 7).
 |
Table 7 The Effect of Different Treatments on Sox2 mRNA Expression Level in a Nude Mice Model |
Effect of Different Treatments on Sox2 Protein Expression in a Nude Mice Model
Transplanted tumors were taken out for Western blot to detect Sox2 protein. Compared with the control group, there was no significance in the 6 mg/kg cisplatin group, though there was a significant difference between the control group and the 5 mg/kg gemcitabine group. Compared with the single control group, 6 mg/kg cisplatin group (P<0.01) and the 5 mg/kg gemcitabine group, the combination of 5 mg/kg gemcitabine + 6 mg/kg cisplatin treatment revealed a striking difference (Figure 9B, Figure 9C and Table 8).
 |
Table 8 The Effect of Different Treatments on Sox2 Protein Expression Level in a Nude Mice Model |
Discussion
Reversing gene silencing through DNA methylation is an effective anti-tumor therapy, and methylation inhibitors could reactivate various cancer suppressor genes.25,26 Decitabine is the representative methylation inhibitor. However, few studies have focused on the effect of cisplatin on DNA methylation. Meantime, it is not clear whether cisplatin and decitabine are synergistic to promote DNA demethylation. In the present study, we intended to investigate the effect of the combination of decitabine and cisplatin on the DNA methylation status of Sox2 gene in GC.
Sox2 belongs to a member of the Sox (SRY-related HMG-box) gene family, which encodes transcription factors associated with sex determining region Y gene (SRY). Sox2 plays a regulatory role in the development of early embryos and maintaining progenitor cell self-renewal, and plays vital roles in differentiation of gastric mucosa. The aberrant expression is involved in gastritis, intestinal metaplasia and GC.6,27–31 Recently, It is reported that Sox2 is correlated with tumor initiation and progression. Meantime, abnormal overexpression is associated with various cancers, such as lung cancer32 and prostatic cancer.33
We reviewed previous studies and found the discordance between Sox2 expression and clinicopathological features. Some research revealed that Sox2 is up-regulated in gastric cancer cells9,11 and gastric cancer stem cells,10 which indicated Sox2 might be the oncogene to promote the occurrence and progress. Matsuoka et al11 revealed Sox2 overexpression is associated with strong invasiveness, poor prognosis and high TNM grade. However, more studies indicated Sox2 functions as an anti-tumor role. Otsubo et al13 and Zhang et al16 revealed that patients with positive Sox2 are correlated with favorable prognosis, Wang et al17 reported that SOX2 protein expression could be used as an independent prognostic indicator of GC. In consistency with our result, we found Sox2 protein level was significantly higher in well-differentiated tissues than in moderately differentiated and poorly differentiated tissues, and no significant difference has been observed between well-differentiated and surrounding nontumor tissues. Furthermore, Sox2 expression had a strong association with invasion (0.0011), lymph node metastasis (0.0013) and TNM stage (0.0002). Thus it is believed that Sox2 is a cancer suppressor gene in GC. Next, compared with normal gastric mucosa epithelium cell line GES-1, the Sox2 expression level was lower in gastric cancer cell line BGC-82, 5-Aza-CdR could promote Sox2 expression in a dose-dependent manner, which indicated hypermethylation inhibits Sox2 expression in gastric cancer cells. Furthermore, we found several oncology characteristics of BGC-82 remarkably decreased by using 5-Aza-CdR, such as proliferation, migration and invasiveness. Finally, it have been validated in BGC-82 and nude mice model. Therefore, DNMT inhibitor could reactivate Sox2 to inhibit progression of gastric cancer cells in vivo and vitro.
As the representative of a demethylated drug, decitabine has been widely applied to hematological malignant tumors in clinical trials. However, relevant research associated with solid tumor is limited, especially gastric cancer. Tian et al34 have revealed decitabine could inhibit gastric tumor xenografts in a nude mice model because of NES1 promoter methylation. Liang et al35 reported that decitabine could inhibit HepG2 cell xenografts in a nude mice model by reversing T-cadherin expression via demethylating NES1 promoter. Plumb et al36 indicated that decitabine could increase the sensitivity to cisplatin, carboplatin, temozolomide and doxorubicin. Compared with monotherapy, the combination of doxorubicin and histone acetylation inhibitor belinostat significantly reversed MLH1 and MAGE-A1 expression to increase drug sensitivity of the cisplatin-resistant human ovarian cancer cells A2780/CP70 xenografts in a nude mice model.
BGC-823 cell lines whose Sox2 methylation status is easily influence by decitabine, were applied to investigate whether decitabine and cisplatin are synergistic to influence methylated status and expression level of Sox2. Our study revealed that decitabine could promote Sox2 demethylation, and increase mRNA and protein expression level of Sox2. In addition, cisplatin could also reduce methylation level and the combination of cisplatin and decitabine has a more obvious effect. It indicated that cisplatin could reverse methylation status in gastric cancer cell lines, which has not been reported in previous studies. In summary, decitabine, cisplatin and combined therapy could promote Sox2 demethylation and increase mRNA and protein expression, while decitabine and cisplatin play a synergistic effect.
In order to investigate whether dDecitabine and cisplatin are synergistic to inhibit GC, we constructed a nude mouse transplantation model to assess the synergistic effect of decitabine combined with cisplatin on transplanted tumor in a nude mice model. The result showed that the combination of decitabine and cisplatin significantly inhibited tumor growth. Meantime, decitabine and cisplatin could be synergistic to reverse the DNA methylation, and mRNA, protein expression level of Sox2. However, further clinical trials should be carried out to prove the clinical value of decitabine combined with cisplatin on patients with GC.
Conclusion
Cisplatin and decitabine could be synergistic to induce Sox2 DNA demethylation to promote re-expression of the Sox2 gene, which exerts an anti-tumor effect on GC. It may suggest an insight for innovative therapeutics of GC.
Statement of Ethics
This study and all experimental protocols were approved by the Animal Care and Use Committee of Xiamen University and carried out in accordance with the guidelines of the Animal Care and Use Committee of Xiamen University. The SGC-823 cell was approved by the ethics committee of the Ethics Committee of the First Affiliated Hospital of Xiamen University and BGC-823 cells were authenticated by STR profile. The human tissue samples were approved by the ethics committee of the First Affiliated Hospital of Xiamen University, and written informed consent was obtained from the patients (Approval number [KYH 2019–044]).
Acknowledgments
We would like to thank everyone who helped with this study. This work was supported by Xiamen Scientific and Technological Plan (No. 3502Z20194005, 3502Z20184020).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700–713. doi:10.1158/1055-9965.EPI-13-1057
2. Ajani JA, Lee J, Sano T, Janjigian YY, Fan D, Song S. Gastric adenocarcinoma. Nat Rev Dis Primers. 2017;3(1):17036. doi:10.1038/nrdp.2017.36
3. Toiyama Y, Okugawa Y, Goel A. DNA methylation and microRNA biomarkers for noninvasive detection of gastric and colorectal cancer. Biochem Biophys Res Commun. 2014;455(1–2):43–57. doi:10.1016/j.bbrc.2014.08.001
4. Li L, Ying J, Li H, et al. The human cadherin 11 is a pro-apoptotic tumor suppressor modulating cell stemness through Wnt/beta-catenin signaling and silenced in common carcinomas. Oncogene. 2012;31(34):3901–3912. doi:10.1038/onc.2011.541
5. Esteller M, Fraga MF, Guo M, et al. DNA methylation patterns in hereditary human cancers mimic sporadic tumorigenesis. Hum Mol Genet. 2001;10(26):3001–3007. doi:10.1093/hmg/10.26.3001
6. Esteller M, Corn PG, Baylin SB, Herman JG. A gene hypermethylation profile of human cancer. Cancer Res. 2001;61(8):3225–3229.
7. Tsukamoto T, Inada K, Tanaka H, et al. Down-regulation of a gastric transcription factor, Sox2, and ectopic expression of intestinal homeobox genes, Cdx1 and Cdx2: inverse correlation during progression from gastric/intestinal-mixed to complete intestinal metaplasia. J Cancer Res Clin Oncol. 2004;130(3):135–145. doi:10.1007/s00432-003-0519-6
8. Tsukamoto T, Mizoshita T, Mihara M, et al. Sox2 expression in human stomach adenocarcinomas with gastric and gastric-and-intestinal-mixed phenotypes. Histopathology. 2005;46(6):649–658. doi:10.1111/j.1365-2559.2005.02170.x
9. Hutz K, Mejias-Luque R, Farsakova K, et al. The stem cell factor SOX2 regulates the tumorigenic potential in human gastric cancer cells. Carcinogenesis. 2014;35(4):942–950. doi:10.1093/carcin/bgt410
10. Tian T, Zhang Y, Wang S, Zhou J, Xu S. Sox2 enhances the tumorigenicity and chemoresistance of cancer stem-like cells derived from gastric cancer. J Biomed Res. 2012;26(5):336–345. doi:10.7555/JBR.26.20120045
11. Matsuoka J, Yashiro M, Sakurai K, et al. Role of the stemness factors sox2, oct3/4, and nanog in gastric carcinoma. J Surg Res. 2012;174(1):130–135. doi:10.1016/j.jss.2010.11.903
12. Li XL, Eishi Y, Bai YQ, et al. Expression of the SRY-related HMG box protein SOX2 in human gastric carcinoma. Int J Oncol. 2004;24(2):257–263.
13. Otsubo T, Akiyama Y, Yanagihara K, Yuasa Y. SOX2 is frequently downregulated in gastric cancers and inhibits cell growth through cell-cycle arrest and apoptosis. Br J Cancer. 2008;98(4):824–831. doi:10.1038/sj.bjc.6604193
14. Otsubo T, Akiyama Y, Hashimoto Y, Shimada S, Goto K, Yuasa Y. MicroRNA-126 inhibits SOX2 expression and contributes to gastric carcinogenesis. PLoS One. 2011;6(1):e16617. doi:10.1371/journal.pone.0016617
15. Camilo V, Barros R, Sousa S, et al. Helicobacter pylori and the BMP pathway regulate CDX2 and SOX2 expression in gastric cells. Carcinogenesis. 2012;33(10):1985–1992. doi:10.1093/carcin/bgs233
16. Zhang X, Yu H, Yang Y, et al. SOX2 in gastric carcinoma, but not Hath1, is related to patients’ clinicopathological features and prognosis. J Gastrointest Surg. 2010;14(8):1220–1226. doi:10.1007/s11605-010-1246-3
17. Wang S, Tie J, Wang R, et al. SOX2, a predictor of survival in gastric cancer, inhibits cell proliferation and metastasis by regulating PTEN. Cancer Lett. 2015;358(2):210–219. doi:10.1016/j.canlet.2014.12.045
18. Sartorius UA, Krammer PH. Upregulation of Bcl-2 is involved in the mediation of chemotherapy resistance in human small cell lung cancer cell lines. Int J Cancer. 2002;97(5):584–592. doi:10.1002/ijc.10096
19. Helmbach H, Kern MA, Rossmann E, et al. Drug resistance towards etoposide and cisplatin in human melanoma cells is associated with drug-dependent apoptosis deficiency. J Invest Dermatol. 2002;118(6):923–932. doi:10.1046/j.1523-1747.2002.01786.x
20. Sussman RT, Stanek TJ, Esteso P, Gearhart JD, Knudsen KE, McMahon SB. The epigenetic modifier ubiquitin-specific protease 22 (USP22) regulates embryonic stem cell differentiation via transcriptional repression of sex-determining region Y-box 2 (SOX2). J Biol Chem. 2013;288(33):24234–24246. doi:10.1074/jbc.M113.469783
21. Momparler RL. Epigenetic therapy of non-small cell lung cancer using decitabine (5-aza-2ʹ-deoxycytidine). Front Oncol. 2013;3:188. doi:10.3389/fonc.2013.00188
22. Albertus DL, Seder CW, Chen G, et al. AZGP1 autoantibody predicts survival and histone deacetylase inhibitors increase expression in lung adenocarcinoma. J Thorac Oncol. 2008;3(11):1236–1244. doi:10.1097/JTO.0b013e318189f5ec
23. Gravina GL, Marampon F, Di Staso M, et al. 5-Azacitidine restores and amplifies the bicalutamide response on preclinical models of androgen receptor expressing or deficient prostate tumors. Prostate. 2010;70(11):1166–1178. doi:10.1002/pros.21151
24. Viet CT, Dang D, Achdjian S, Ye Y, Katz SG, Schmidt BL. Decitabine rescues cisplatin resistance in head and neck squamous cell carcinoma. PLoS One. 2014;9(11):e112880. doi:10.1371/journal.pone.0112880
25. Balch C, Yan P, Craft T, et al. Antimitogenic and chemosensitizing effects of the methylation inhibitor zebularine in ovarian cancer. Mol Cancer Ther. 2005;4(10):1505–1514. doi:10.1158/1535-7163.MCT-05-0216
26. Momparler RL. Cancer epigenetics. Oncogene. 2003;22(42):6479–6483. doi:10.1038/sj.onc.1206774
27. Wei Z, Yang Y, Zhang P, et al. Klf4 interacts directly with Oct4 and Sox2 to promote reprogramming. Stem Cells. 2009;27(12):2969–2978. doi:10.1002/stem.231
28. van den Berg DL, Snoek T, Mullin NP, et al. An Oct4-centered protein interaction network in embryonic stem cells. Stem Cell. 2010;6(4):369–381. doi:10.1016/j.stem.2010.02.014
29. Driessens G, Blanpain C. Long live sox2: sox2 lasts a lifetime. Stem Cell. 2011;9(4):283–284. doi:10.1016/j.stem.2011.09.007
30. Masui S, Nakatake Y, Toyooka Y, et al. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol. 2007;9(6):625–635. doi:10.1038/ncb1589
31. Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17(1):126–140. doi:10.1101/gad.224503
32. Wang SQ, Liu J, Qin J, et al. CAMK2A supported tumor initiating cells of lung adenocarcinoma by upregulating SOX2 through EZH2 phosphorylation. Cell Death Dis. 2020;11(6):410. doi:10.1038/s41419-020-2553-6
33. Rybak AP, Tang D. SOX2 plays a critical role in EGFR-mediated self-renewal of human prostate cancer stem-like cells. Cell Signal. 2013;25(12):2734–2742. doi:10.1016/j.cellsig.2013.08.041
34. Xianglong Tian JZ, Biao L. T-cadherin expression induced by 5-azo-2 ‘-deoxycytidine in nude mouse HepG2 tumor cells and its inhibitory effect on tumor growth. J Shanghai Jiao Tong Univ. 2007;27(5):533–536.
35. Zhilun Liang ZH, Chen X. T-cadherin expression induced by 5-azo-2 ‘-deoxycytidine in nude mouse HepG2 tumor cells and its inhibitory effect on tumor growth. World Chin Dig J. 2008;16(16):1741–1745. doi:10.11569/wcjd.v16.i16.1741
36. Plumb JA, Strathdee G, Sludden J, Kaye SB, Brown R. Reversal of drug resistance in human tumor xenografts by 2ʹ-deoxy-5-azacytidine-induced demethylation of the hMLH1 gene promoter. Cancer Res. 2000;60(21):6039–6044.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.