Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Day and Night Control of COPD and Role of Pharmacotherapy: A Review
Authors Braghiroli A , Braido F , Piraino A , Rogliani P , Santus P, Scichilone N
Received 26 November 2019
Accepted for publication 3 May 2020
Published 4 June 2020 Volume 2020:15 Pages 1269—1285
DOI https://doi.org/10.2147/COPD.S240033
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Video abstract presented by Fulvio Braido.
Views: 384
Alberto Braghiroli,1 Fulvio Braido,2 Alessio Piraino,3 Paola Rogliani,4 Pierachille Santus,5 Nicola Scichilone6
1Department of Pulmonary Rehabilitation, Sleep Laboratory, Istituti Clinici Scientifici Maugeri, IRCCS, Veruno, NO, Italy; 2Department of Internal Medicine, Respiratory Diseases and Allergy Clinic, University of Genoa, Azienda Policlinico IRCCS San Martino, Genoa, Italy; 3Respiratory Area, Medical Affairs Chiesi Italia, Parma, Italy; 4Respiratory Unit, Department of Experimental Medicine, University of Rome “Tor Vergata”, Rome, Italy; 5Pierachille Santus, Department of Biomedical and Clinical Sciences (DIBIC), University of Milan, Milan, Italy; 6Department of Biomedicine and Internal and Specialistic Medicine (DIBIMIS), University of Palermo, Palermo, Italy
Correspondence: Fulvio Braido
Department of Internal Medicine, IRCCS San Martino Genoa University Hospital, Genoa, Italy
Email [email protected]
Abstract: The topic of 24-hour management of COPD is related to day-to-night symptoms management, specific follow-up and patients’ adherence to therapy. COPD symptoms strongly vary during day and night, being worse in the night and early morning. This variability is not always adequately considered in the trials. Night-time symptoms are predictive of higher mortality and more frequent exacerbations; therefore, they should be a target of therapy. During night-time, in COPD patients the supine position is responsible for a different thoracic physiology; moreover, during some sleep phases the vagal stimulation determines increased bronchial secretions, increased blood flow in the bronchial circulation (enhancing inflammation) and increased airway resistance (broncho-motor tone). Moreover, in COPD patients the circadian rhythm may be impaired. The role of pharmacotherapy in this regard is still poorly investigated. Symptoms can be grossly differentiated according to the different phenotypes of the disease: wheezing recalls asthma, while dyspnea is strongly related to emphysema (dynamic hyperinflation) or obstructive bronchiolitis (secretions). Those symptoms may be different targets of therapy. In this regard, GOLD recommendations for the first time introduced the concept of phenotype distinction suggesting the use of inhaled corticosteroids (ICS) particularly when an asthmatic pattern or eosiophilic inflammations are present, and hypothesized different approaches to target symptoms (ie, dyspnea) or exacerbations. Pharmacotherapy should be evaluated and possibly directed on the basis of circadian variations, for instance, supporting the use of twice-daily rapid-action bronchodilators and evening dose of ICS. Recommendations on day and night symptoms monitoring strategies and choice of the specific drug according to patient’s profile are still not systematically investigated or established. This review is the summary of an advisory board on the topic “ 24-hour control of COPD and role of pharmacotherapy”, held by five pulmonologists, experts in respiratory pathophysiology, pharmacology and sleep medicine.
Keywords: COPD, symptoms, dyspnea, night, sleep, follow-up, adherence, circadian LAMA, LABA, ICS
Introduction
Chronic obstructive pulmonary disease (COPD) is a major global health problem, being the fourth cause of death worldwide.1 Among other conditions, it has been associated with chronic airways and lung parenchyma inflammation. At present, available therapies are not capable of reversing the progression or completely suppressing the inflammation associated with COPD itself. Therefore, there is a pressing need to find new pathophysiological bases for treatments, as expressed in the most recent 2020 Global initiative for chronic Obstructive Lung Disease (GOLD) recommendations.2 In particular, the pattern of symptoms and long-term disease control are still unmet needs.
The present review is taken from a meeting in which five pulmonologists, expert in respiratory pathophysiology, pharmacology and sleep medicine discussed the topic “24-hour control of COPD and the role of pharmacotherapy” from different points of view by means of lectures and plenary debate. The areas covered by the discussion and presented in this article refer to the stable patient and can be resumed in: circadian variation of COPD symptoms and its pathophysiology, disease management during follow-up, pharmacotherapy and 24-hour symptoms control, patients’ adherence.
COPD Symptoms: Circadian Variation and Prognostic Impact
COPD is characterized by a lot of symptoms including dyspnea, cough, wheezing, fatigue and phlegm.3 Symptoms worsening is crucial since it precedes exacerbations. To reduce symptoms is one of the immediate aims of COPD therapy according to the GOLD document.2 They suggest assessing the impact of COPD on a patient’s life by means of the COPD Assessment Test (CAT) questionnaire, which includes dyspnea as well as cough and phlegm. However, apart from dyspnea, the other symptoms (eg, wheezing, fatigue, phlegm, cough) are generally lacking in studies and scores. New domains must be explored to better characterize patients. Indeed, the symptom with the worst impact on patients’ disability is dyspnea, principally in those with moderate COPD.4 The variability of COPD symptoms during day and night is well known. In particular, they are worse during night and in the morning, especially when COPD is severe (Figure 1).4 37% of all COPD patients and 73% of those with severe disease reported as troublesome the routine early morning activities.5 The prevalence of night-time symptoms varies according to GOLD classes, with the greater step up percentage between GOLD 2 and 3.6 Circadian variations are more expressed at severe stages, showing a progressive increase in the prevalence of both morning and night symptoms and depends on age, dyspnea severity, intensity of therapy, physical activity. Moreover, night-time symptoms are predictive of higher mortality and more frequent exacerbations.6,7 They are also partly responsible for early morning symptoms. On the other hand, morning symptoms increased the odds of poor health status at follow-up.7 In addition, early morning symptoms can also be linked to a mechanical issue, since the patients start to move. Night-time symptoms can be assessed by specific questionnaires, but they should be answered early after the awakening, otherwise they lose sensibility, particularly in the elderly. Recently, the LEOSound system has been developed and validated, in order to register nocturnal wheezing and cough, which remain otherwise unnoticed in COPD patients.8 It is a mobile system used for automated long-term recording and objective analysis of respiratory sounds, like cough and wheezing, composed by a trachea microphone and two lungs microphones. An internal ambient microphone helps distinguishing lung sounds from environment noise.9 The authors found that these symptoms were common, particularly in smokers.8 The same device has recently revealed a high rate of nocturnal cough epochs in GOLD 3 than 2 or 4 (probably because stage 4 might be associated with emphysema). In patients who smoke cough is generally productive, while in the others it is more frequently “normal”.10 New tools are now available to objectively assess nocturnal symptoms (instead of questionnaires). They provide quality data, differentiating cough from snoring. Daytime cough is rarely referred by patients because it is considered normal, particularly in smokers. Symptoms can be prevalent in different phenotypes of COPD: wheezing is related to asthma or to a bronchospastic condition (for instance at a certain phases of exacerbation), while dyspnea is particularly associated with emphysema (dynamic hyperinflation) or obstructive bronchiolitis (secretions). All these symptoms may be different target of therapy. However, nocturnal symptoms related to COPD must be differentiated to those related to obstructive sleep apnea (OSA) syndrome, which can overlap with COPD. Nevertheless, the quality of sleep is often inadequate in COPD patients, irrespective of the presence or absence of OSA. In addition, it strongly impacts on quality of life. Daytime vitality is worse in patients suffering from both COPD and OSA.11
 |
Figure 1 Circadian variation of COPD symptoms: *p<0.001 versus “midday”, “afternoon”, “evening”, “night” and “difficult to say” groups; p=0.006 versus “no particular time of day” (all COPD patients); †p<0.001 versus “midday”. Patient insight into the impact of chronic obstructive pulmonary disease in the morning: an internet survey. Partridge MR, Karlsson N, Small IR, et al. Curr Med Res Opin. 2009;25:2043–2048. Informa UK Ltd, trading as Taylor & Francis Group, reprinted by permission of the publisher (Taylor & Francis Ltd, http://www.tandfonline.com).4 |
Discrepancy exists between what patients refer and what the physician measures in COPD. 90% of patients with moderate COPD refer symptoms, but only 20% receive a diagnosis and 25% are treated. Vice versa, all severe COPD patients are symptomatic, with 50% receiving a diagnosis and 60–70% a treatment.12 Patients suffering from COPD define dyspnea as inspiratory difficulty or unsatisfied inspiration, because of reduced displacement ratio per breath with higher mechanical load.13 Beyond dyspnea, other COPD symptoms vary consistently during day and night and in the seasons. In the early morning, they are worse due to a reduced airway clearance after the night,14 particularly in severe COPD (Figure 1).4 This aspect could be well targeted by the twice-daily bronchodilators administration: the evening dose acting on night-time and early morning symptoms. In general, COPD symptoms are strongly related to inactivity and deconditioning.15 Deconditioning, on the other hand, determines a reduced symptom perception representing another element of variability.
COPD Pathophysiology in Determining 24-Hour Symptom Variability (Figure 2)
Mechanical Issues
At a mechanical level, COPD is characterized by airways flow limitation and hyperinflation, which are major determinants of clinical outcomes including nocturnal symptoms, impaired quality of life, exacerbations, reduced physical activity, and, importantly, increased mortality.16 a) At rest, COPD patients with moderate disease experience an altered mechanical condition in terms of volumes and respiratory rate, similarly to healthy controls during exercise. b) During exercise, these items worsen and the exercise itself is shorter. An impaired respiratory mechanics is on the basis of these phenomena, particularly referring to reduced compliance, increased resistance and PEEPi. c) Activity-related dyspnea is one of the most distressing symptoms experienced by COPD patients, widely and variably occurring across the spectrum of disease severity17 and particularly impactful in the early morning as a consequence of nocturnal alterations of respiratory mechanics. d) In addition, activities of daily living are reduced according to the severity of the disease, as well as breathing discomfort.18 e) During night-time, the supine position is responsible for a different thoracic physiology. In particular, the recumbent surface reduces its ventilatory ability as well as the chest wall outward elastic recoil, Secretions accumulate in the lower part of the lungs, as blood itself does, enhancing the ventilation/perfusion mismatch.19,20
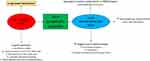 |
Figure 2 COPD pathophysiology in determining nocturnal symptom variability. |
Considering that COPD is heterogeneous in the lung parenchyma, this issue is variable. In addition, the cephalad force of abdomen and the reduced diaphragm excursion due to hyperinflation contribute to the pathophysiologic alterations related to the supine position in COPD patients.
Comparing COPD patients and healthy controls by means of pressure-volumes curves, Pecchiari et al demonstrated that the closing volume was different in the two groups and in 50% of COPD patients it was present at rest, meaning a 24-hour unfavorable breathing.21 Moreover, the airway cycling opening and closing should cause mechanical damage and stimulate non-traditional inflammation.21 The acute positive effects of different classes of bronchodilators (namely, tiotropium vs indacaterol) on small airways (ie, closing volume) and ventilation inhomogeneity were compared in patients with moderate-severe COPD, finding no interclass difference.22 In addition, bronchodilation improved the vital capacity and the closing volume, without critical closing pressure modification. After bronchodilators, the number of patients breathing over their closing volume increased.22 This should reduce the risk of small-airway impairment and positively affect gas exchange. Key elements in the pathogenesis of COPD are peripheral airway inflammation and dysfunction. The exhaled alveolar fraction of nitric oxide (CANO) indirectly reflects lung peripheral inflammation, correlating with airway resistances, RV/TLC (Motley index), transfer factor and FEV1.23 CANO was significantly reduced at 60 and 180 minutes compared to baseline for both formoterol and salmeterol, but higher with formoterol, exhibiting a more rapid onset of action. Changes in CANO were correlated with changes in vital capacity and RV/TLC, but not FEV1. The reduction of CANO was associated with amelioration in functional parameters reflecting air trapping. Indeed, long-acting β2-agonists (LABA) acutely improve lung ventilation inhomogeneity (which is enhanced in COPD during sleep) and favor lung volume recruitment.23 The dynamic lung hyperinflation has anatomical consequences on the heart.24 In fact, COPD is a comorbidity that increase mortality for heart failure with both reduced and preserved ejection fraction.25 Lung hyperinflation, increasing intrathoracic pressure, impairs biventricular diastolic function. Considering that coronary arteries are perfused in diastole, left ventricular diastolic dysfunction has negative effects on heart tissue perfusion. Consequently, lung deflation has acute and chronic effects on both left and right heart geometry and performance, either studied by echocardiography or cardiac magnetic resonance imaging.26–28 This aspect may contribute to daytime activity-related symptoms (eg dyspnea). Moreover, lung congestion and/or pulmonary hypertension related to left heart remodeling can also worsen night-time air trapping in the supine position. The functional characteristics of COPD have a distribution that diverge from different GOLD stage and between different classification over time. Particularly, in a multicenter observational study has been reported that the functional GOLD 2007 classification better reflected the pathophysiological COPD abnormalities compared to the GOLD ABCD criteria; the transition from GOLD 2011 to GOLD 2017 led to a significant shift from high risk (C and D) to low risk categories (A and B) and that the ABCD criteria poorly reflected the clinical heterogeneity (the presence of chronic bronchitis and/or emphysema) of COPD patients. Moreover, the clinical phenotype assessment recognizes that the most frequent phenotype was the emphysema, followed by the mixed phenotype and lastly chronic bronchitis. Furthermore, the phenotype distribution in the GOLD 2017 classification was similar across the ABCD subgroups.29 We can argue that all the mechanical issues worsen in parallel with COPD itself.
Sleep and Circadian Rhythm Regulation
Sleep is not safe for COPD patients. In the rapid eye movement (REM) sleep phase the diaphragm is the only muscle able to move, so that it has to substitute the other respiratory muscles.30 The flatter the diaphragm is (due to the cephalad force of abdomen), the less it contracts, determining hypoventilation events. Consequently, these events are more frequent in the second part of the night, when REM phases are prevalent.31,32 Delta waves phases are at higher risk, when the vagal stimulation (by means of the activation of the rostral portion of the nucleus ambiguous and the dorsal motor nucleus of the vagus) determines increased bronchial secretions, blood flow increase in the bronchial circulation (enhancing inflammation) and airway resistance (broncho-motor tone). This is why in the asthmatic patient, bronchospasm improves when he is awake.33 The circadian rhythm influences the vagal activity on broncho-motor tone.34 This is a possible explanation about the worsening of COPD symptoms night-time and at awakening. In COPD patients, the circadian rhythm may be impaired. The output from the central circadian clock in the cerebral suprachiasmatic nucleus is a source of timing information to let peripheral clocks to identify day and night, as they lack light input. The molecular circadian clock consists of interlocking transcriptional and translational feedback loops, culminating in the rhythmic expression of core clock genes. Clara cells are the peripheral clocks in the lungs.35 For instance, hematogenous inflammation is increased during night. As a consequence, the timing of administration of both inhaled and systemic drugs is pivotal for an efficacious treatment. In asthma, parenchymal and bronchial eosinophils have a circadian rhythm, increasing during night.36 In COPD, the oxidative stress of tobacco smoke enhances the activity of kinases and reduces that of phosphatases, thus determining abnormal clock protein rhythms. The role of pharmacotherapy in this regard is unknown but inhaled corticosteroids (ICS), for instance, could be useful during night. Moreover, the role of bronchodilators on the circadian alterations has not been studied yet: the twice a day administration may be useful to restore, locally, the circadian rhythm. In this regard, a possible explanation of the FEV1 reduction without exacerbations in some patients switching from ICS/LABA to long-acting muscarinic agonists (LAMA)/LABA can be linked to these considerations. Different patterns of bronchodilator response in COPD have been described independently from age, gender and ICS use: flow response with increased expiratory flow reserve in chronic bronchiolitis, volume response with reduced hyperinflation in emphysema. Accordingly, FEV1 is increased in both categories, while FEV1/VC only in the first one.37–39 Moreover, small airways remodeling and narrowing is responsible for a low ventilatory/perfusion ratio in chronic bronchiolitis, while capillary network reduction, related to alveolar wall destruction, determines a high ratio in pure emphysema.
How to Monitor Disease Control: From Guidelines to Clinical Practice
There are 18 clinical practice guidelines on COPD published or updated after 2000: only 12 include a separate paragraph on disease monitoring and 9, quality of life.40 In fact, the follow-up of lung function parameters is the most frequently recommended monitoring routine (89% of them, using FEV1 or FEV1/FVC), even if lung function and symptoms do not necessarily correlate. In general, symptoms monitoring, exercise tolerance, comorbidity, and smoking habits are recommended regularly. Usually, the monitoring strategy is set up according to disease severity. 6 guidelines do not provide any information, 3 suggest that frequency should be based on the local health care system, 9 that visits should be calibrated on the disease gravity and progression, and 8 provide suggestions for frequency of monitoring visits that varies from monthly to yearly. However, no guidelines recommended an evidence-based monitoring strategy.40 No mention is usually present on the monitoring of symptoms variability as mentioned above, new domains must be explored to better characterize patients. According to MacNee, assessing and treating of chronic diseases should be based on: severity, activity and impact. The severity of a chronic disease (including COPD) is inversely related to the functional reserve left to the damaged organ (regarding COPD it can be measured by FEV1, IC/TLC, PaO2, 6MWD, comorbidities). The degree of activity reflects the intensity of the causal biological mechanisms: in COPD, it can be measured by smoking, FEV1 decline, exacerbations, weight, and biomarkers. Notably, severity and activity do not always run in parallel. Finally, the impact of a disease depends on the patient’s perception of both the activity and severity of the disease: it is what patients report, eventually measurable, for example, with mMRC, daily activity, CAT score.41 Several questionnaires have been elaborated in the recent years about COPD control: Capacity of Daily Living during the Morning (CDLM) by Partridge et al42 and CAT by Jones et al43 are commonly used. CAT includes also symptoms and quality of sleep. The CAT score is composed of 8 items, namely: cough, phlegm, chest tightness, breathlessness, activities, confidence, sleep and energy. Interestingly, symptoms other from dyspnea, sleep quality and daytime activities are combined in a simple qualitative instrument to globally assess the COPD patient and also to monitor the disease activity over time.43 In a pooled analysis of two 6-month Phase III studies about aclidinium bromide authors analyzed the impact of this twice-daily drug through the Evaluating Respiratory Symptoms in COPD (E-RS(™): COPD) scale (formerly EXACT-RS).44 This tool was developed to meet the need for a standardized respiratory symptom diary: it is an 11-item score assessing both overall daily respiratory COPD symptoms (RS-Total score) and specific respiratory symptoms by means of three subscales (RS-Breathlessness, RS-Cough & Sputum and RS-Chest Symptoms).45 Interestingly, aclidinium, given its twice-daily posology, improved the score and its domains irrespective of the GOLD degree. Moreover, the E-RS scores were significantly correlated with other clinical outcomes, including health status, relief-medication use and alternate measures of symptom severity.44 Recently, Soler-Cataluña et al created a new tool to evaluate COPD control, namely the “modified” control criteria (MCC). Control was defined as the presence of low clinical impact and clinical stability. To assess clinical impact, the following clinical parameters were evaluated: the degree of dyspnea, the use of rescue medication, the physical activity, and the sputum color. Stability was calculated by clinical changes and exacerbations in the last 3 months. The authors found that MCC is predictive of the combined event (emergency visit, hospitalization, or death) and identifies patients with a better quality of life.46 Consequently, the concept of control and the new MCC could usefully help in treatment optimization. The stability is a good parameter, meaning inhibition of deterioration. However, the term “control” is too much related to asthma, it would be better to use “wellness”. Recently a questionnaire about the awareness of COPD, similar to the CAT score, has been developed. The authors found that among patients, the awareness is suboptimal and it is associated with care/cure satisfaction.47
Sleep quality is related to health status.48 From questionnaires about the quality of sleep in COPD patients, we have learnt that nocturnal symptoms have a prognostic role. Omachi et al demonstrated that the more disturbed is sleep and the worse is COPD (but not FEV1), being longitudinally predictive of COPD exacerbations, emergency health care utilization, and mortality.49,50 Moreover, in patients with overlap syndrome (COPD + OSA) the increased severity of hyperinflation correlates with worse sleep efficiency, independently from apnea and night-time hypoxemia.51
A recent publication has also showed, in COPD patients, a correlation between higher CASIS score and a greater small airways impairment, evaluated by impulse oscillometry.52 CASIS score is a very practical, self-administered 7-item scale assessing sleeping problems associated specifically with COPD and asthma in the previous week. Five items refer to disturbance falling asleep or staying awake during the day. The other two items regard sleep quality. A first raw score is calculated from the sum of the seven items, which is then linearly transformed to a scale ranging from 0 to 100. The higher the score and the greater is sleep deterioration in the previous week. Internal consistency (Cronbach’s alpha 0.91), test–retest reproducibility (intraclass coefficient 0.84), and concurrent validity (correlated with the St George’s Respiratory Questionnaire, r=0.68) were good. In COPD, CASIS score correlates with SGRQ.53
Patients and physicians have different hopes regarding COPD therapy. Patients think about symptoms reduction, increased exercise capacity, reduced use of emergency drugs, reduced exacerbations, and improved quality of life. Physicians, instead, aim at increased respiratory function, reduction of functional decline, and few adverse events. The last scope of COPD therapy is the reduction of mortality and the increased life expectancy, as reached by smoke cessation, oxygen therapy and CPAP in those with overlap syndrome. No drug was definitely demonstrated to have this ability, although in the TORCH study an ICS-LABA combination was able to promisingly reduce the risk of death of 17.5%.54 In addition to the prescribed therapy, accurate follow-up and adequate disease monitoring are also important to COPD control. Notably, as reported above, monitoring some parameters can be seen as a tool to verify the therapy efficacy. However, apart from FEV1, tools evaluating how ventilation changes over time are lacking so far. Only nocturnal cardio-respiratory monitoring gives simple information about dynamic hyperinflation, hypoventilation and hypoxemia: these devices are dynamic and sensitive. The LEOSound (Löwenstein Medical GmbH & Co. KG, Bad Ems, Germany) cited above automatically records respiratory sounds.9 However, to date, cardio-respiratory monitoring systems are the most reliable but they have been employed with aims different than pharmacological therapy efficacy monitoring. The ongoing “STudio Osservazionale sulla caratteRizzazione dei sIntomi delle 24 ore nei pazienti con BPCO” (STORICO) trial aims at providing effective and patient-oriented care, evaluating patients on the basis of lung function, frequency of symptoms (including sleep) and patient-perceived impact of symptoms on their lives. On this basis, treatment decisions are made case-by-case.55 Another ongoing study is “choose your outcome”, which considers only outcome related to exercise/movement.47
New tools, non-invasively monitoring respiratory rate and ventilation, are incoming. They could be useful to better characterize patients and to easily study drug response. In the most commonly used questionnaires, the identification of the key symptomatology for each patient is lacking: symptoms and their daily variability, retain their loyalty and indicate the effects before and after therapy.
For completeness, in Table 1 are reported, the main instruments found by the authors that consider, at least partially, symptoms in different moments of the day or over the time. Their diffusion and their appropriate validation to assess therapy-response in COPD is highly variable.
 |
Table 1 Tools to Assess COPD Symptoms Referring to Daily Variability |
Tailored Therapy: Pharmacologic Characteristics Related to the Day and Night Therapeutic Effect
From Pathophysiology to Clinical Impact
Regarding tailored therapy and symptoms variability, nocturnal symptoms and small airways involvement are very important. Bronchiolitis and emphysema are also relevant pathological conditions. 62.7% of symptomatic patients reported daily and/or weekly symptom variability. Patient-perceived COPD symptoms vary daily and weekly, with variable impact on everyday activities; morning being the worst time of the day.14 The SARA study analyzed different patterns of sleep disturbances in the geriatric population with COPD or asthma: morning tiredness and early awakenings were more prevalent.56 Omachi et al demonstrated that the more disturbed is sleep and the worse is COPD (but not FEV1), being longitudinally predictive of COPD exacerbations, emergency health care utilization, and mortality.49 Indeed, the more severe is COPD and the more variable are symptoms during night and day. As already mentioned, by means of the COPD and Asthma Sleep Impact Scale (CASIS) score Basile et al found that nocturnal symptoms in COPD are associated with peripheral airways alterations and with air trappers vs non-trappers, but independent of the severity of FEV1.52 A sub-analysis of the STORICO study revealed that the circadian rhythm of respiratory symptoms varies according to clinical phenotypes: bronchiolitis is characterized by higher circadian variability and more evident nocturnal symptoms.52 Probably, small airways can be involved by the increased cholinergic tone and the supine position during night. This could lead to a different therapeutic approach preference.
Pharmacology of LAMAs and LABAs: Rationale for Combination Therapy
Data about pharmacology and its implication regarding the clinical issues cited above are sparse. GOLD document recommends classes of drugs (eg, LABA, LAMA, ICS, etc.) and not molecules, according to the demonstrated effects on two major outcomes.2 The aim of the therapy is to reduce symptoms and risks (ie, disease progression, exacerbations, mortality). Bronchodilators (namely, LABA, LAMA, theophylline or their combinations) are pivotal to control symptoms. In moderate-to-severe COPD, associating an ICS can further increase the efficacy of bronchodilators, especially considering exacerbations. New molecules of these classes have been developed in the recent years. Airways innervation is fundamental to understand the mechanism of action of bronchodilators. The vagus nerves are tonically active, in particular at night and produce a stable, promptly reversible basal tone of the airway smooth muscle, the so-called bronchomotor tone.57 LAMAs block M1 and M3 receptors preventing their binding to acetylcholine and airway smooth muscle contraction. Vice versa, the stimulation of β2-adrenergic receptors activates adenylyl cyclase and increases intracellular cAMP, relaxing airway smooth muscle.58 These two systems are widely linked both at pre-synaptic and post-synaptic levels,59 thus justifying the combination of the two classes. The combination of LAMA/LABA can be clinically relevant to improve air trapping which has been already described as cause of symptoms variability in the previous chapters. The first study to analyze the interaction between LAMA and LABA is that of Cazzola et al, who demonstrated that the combined inhalation of indacaterol and glycopyrronium significantly anticipated at 15 minutes the mean peak of bronchodilatory effect post-administration, than the two molecules alone, afterwards there is maximal efficacy.60 However, the various available associations differ in terms of pharmacodynamic and thus administration. Notably, in COPD patients, the presence of small-airway dysfunction increases in parallel with GOLD classifications and it is closely related to a higher impact of the disease on health status.61 β2-adrenergic receptors are more expressed in peripheral airways, while muscarinic ones at proximal levels. However, muscarinic receptors are also expressed in periphery on nonstructural cells (lymphocytes, dendritic cells, endothelial cells) and activated by the so-called “non-neuronal” acetylcholine in autocrine way released by epithelial cell in response to inflammatory stimuli. This “non-neuronal” acetylcholine has inflammatory properties on neutrophils, lymphocytes, macrophages and fibroblasts.62 Consequently, some authors have discussed a possible role of LAMA in modulating inflammation. Profita et al demonstrated that LABA and LAMA (namely, olodaterol and tiotropium) control the bronchoconstriction and TGF-β1-mediated neutrophilic inflammation in COPD in induced sputum supernatants.63 While co-administration of glycopyrronium/indacaterol induces bronchodilation with synergistic improvement as evidenced by the increase in cAMP concentrations both airways smooth muscle and bronchial epithelium associated with a decreased release of acetylcholine from the epithelium; especially when these drugs are administered at low concentrations.64
The effectiveness of theophylline has long been thought to be due primarily to bronchodilatation, although it is now known that these drugs also exhibit anti-inflammatory actions. The predominant mechanism of action of theophylline has traditionally been ascribed to non-selective inhibition of phosphodiesterase enzymes (PDE), but there is increasing evidence that some of the clinical effects of theophylline might be due to their mechanisms of action such as increasing histone deacetylase enzyme(s) activity (HDAC), or interference with certain intracellular kinases. Theophylline has the distinct advantage of being able to be administered orally and is still used in the management of patients with obstructive pulmonary disease steroid resistant, as a matter of act it has been demonstrated additional benefit from combined theophylline and glucocorticosteroid therapy rather than increasing the dose of glucocorticosteroids.65–69
The rapid onset of action of bronchodilators can be a critical aspect to consider when choosing a therapeutic option. Different drugs in the same class have variable pharmacological properties. Considering treatment with LABA or LAMA, the fastest products in terms of onset of action, are represented, respectively, by formoterol70 and glycopirronium.71,72 On the other side, those two active principles present a pharmacokinetic profile making them suitable for a twice-daily administration. Both these two features (ie, fast action and twice-daily administration) may represent a reason of choice in patients requiring rapid symptoms relief in the morning and in the evening.
Role of ICS in Dual and Triple Therapy: Pharmacology and Outcomes
2020 GOLD recommendations suggest a management algorithm for COPD patients, characterized by “review (symptoms)”, “assess (adherence)” and “adjust (therapy/device)”.2 Practically, they suggest an initial therapy based on GOLD classes and a subsequent re-evaluation according to dyspnea and exacerbations. Interestingly, for the first time they introduce the concept of phenotype distinction, giving a very important role to ICS in case of elevated blood eosinophils.2 Indeed, Pascoe et al recently found that blood eosinophil count is a promising biomarker of response to ICS in patients with COPD. It may be used to stratify patients for different strategies to reduce exacerbation rate.73 Regarding phenotyping, night-time symptoms and their variability should as well represent relevant aspect to guide a tailored therapy, even if they are not considered in GOLD document. ICS drugs are mainly discussed in the contest of a specific outcomes control: COPD exacerbations. As discussed further in this article, studies regarding fixed triple therapies have confirmed the role of ICS in exacerbations prevention compared to bronchodilators alone.74,75 The WISDOM trial found that descaling from LABA/LAMA/ICS to LAMA/LABA in complex patients with severe COPD did not increase exacerbations but worsened dyspnea and reduced FEV1.76 Supporting these results, Calzetta et al performed a meta-analysis which revealed that ICS withdrawal did not significantly increase the overall rate of COPD exacerbation, but impaired both lung function and quality of life, albeit in a non-clinically relevant way. A strong clinical need in understanding what is the real impact of ICS withdrawal in COPD persists.77 Importantly, the correlation between FEV1 and symptoms is weak, and FEV1 gives only a partial patient’s description. It could be useful to differentiate patients whose FEV1 remains stable vs those whose FEV1 declines in order to understand which subgroup benefits from LAMA/LABA and which from LAMA/LABA/ICS to control this important aspect of COPD prognosis. According to Reilly, ICS continuation in patients assuming bronchodilators should be guided on symptoms rather than on exacerbations.78 Indeed, the SUNSET study about de-escalation of ICS to indacaterol/glycopirronium in non-frequently exacerbating patients with moderate-severe COPD confirmed a small, statistically relevant reduction in lung function, without difference in exacerbations. However, the higher exacerbation risk found in the same study, in patients with ≥300 blood eosinophils/μL withdrawing from ICS, suggests to apply a careful follow-up and to avoid triple therapy discontinuation.79 In addition, three different meta-analyses suggested a role of ICS/LABA in reducing mortality, without definitive conclusions.80–82 Manoharan et al analyzed 4133 COPD patients and found that in those exposed to ICS, co-use of LAMA (dual therapy) or in combination with LABA (triple therapy) were associated with reduced all-cause mortality, while concomitant use of LABA without LAMA provided no reduction. In addition, triple therapy alone conferred benefits on cardiovascular mortality.83 Recent data on two fixed-triple therapies confirmed a positive effect on mortality75,84 and on the delay of clinical important deterioration.85 The improvement in outcomes can be related to inflammation reduction as suggested by a recent meta-analysis:86 this can be a therapeutic target. Long-term ICS treatment partially influences the composition of extracellular matrix in moderate-to-severe COPD. This is linked to increased lung function, suggesting that long-term ICS modulates airway remodeling thereby potentially preventing airway collapse in COPD.87 These data agree with Tantucci et al, who demonstrated that the loss of lung function, assessed as FEV1 reduction, seems more rapid and so more important in the initial phases of COPD. Consequently, to have an impact on the natural history of COPD, it is logical to look at the effects of treatment in the earlier stages.88 Finally, considering the combination of three active principles, the TRIBUTE trial compared beclometasone/formoterol/glycopirronium with indacaterol/glycopirronium in severe and very severe symptomatic COPD with exacerbation history, finding that triple therapy significantly reduced the rate of moderate-to-severe exacerbations, without increasing the risk of pneumonia, and improved FEV1 and quality of life.74 These results do not directly refer to mild COPD, nevertheless a sub-analysis of the study confirmed the same effect of the beclometasone/formoterol/glycopirronium extrafine fixed triple combination in preventing exacerbations and improving both lung function and quality of life, in patients with a lower risk of exacerbations, without increasing the risk of pneumonia.89 Positive results in mild patients treated with a fixed-triple combination were also confirmed by the KRONOS study.90 The characteristics of the reported trials are summarized in Table 2.
 |
Table 2 Charactestistics of the Main Trial Reported by the Board |
GOLD recommendations, therefore, currently suggest the use of triple therapy for the follow-up of the patient, independently of the severity of the disease, but mainly based on exacerbation recurrence despite maintenance treatment, with an increasing effect from the blood eosinophils count cut-off of 100/µL.2 A meta-analysis, undertaken to compare triple therapy vs LABA/LAMA or single bronchodilators, suggested that patients on single long-acting LABA or LAMA therapy or LABA/LAMA combination, who still have exacerbations and have blood eosinophil count ≥300/µL, could benefit from ICS/LABA/LAMA combination: the combination of efficacy and security favors LABA/LAMA, but efficacy favors triple therapy. Moreover, for triple therapy, the number needed to harm regarding pneumonia was 195, and it decreased to 34 (ie, an increased risk of pneumonia) when considering only the studies that included fluticasone in the triple combination.91 Notably, ICS are not similar, nor the patients. Considering administration, all ICS are effective with once-daily dosing, but they are more effective when dosed twice daily.92 Fluticasone furoate, randomly tested in 5 different regimens for asthma vs placebo, was similarly effective (non-inferiority) on 8-week pre-dose FEV1 either when administered twice daily or once daily in the evening.93 The same non-inferiority was found for a halved dose and at a 4-week follow-up.94
Twice Daily or Once Daily Administration
As reported above, dyspnea and other COPD symptoms vary consistently during day and night and over seasons. In the early morning, they are worse due to a reduced airway clearance after the night and the clinostatic position,14 particularly in severe COPD.5 This issue may support the twice-daily bronchodilators administration: the evening dose can act on night-time and early morning symptoms.
Use of reliever medication, in COPD, is not considered a strong indicator of disease control, as it is in asthma. Speculatively, the normally repeated dose of a twice-daily fast-acting maintenance drug could potentially work at the same time as potential reliever, possibly reducing the need of rescue medications. In asthma, the last GINA document has clearly identified the role of ICS-formoterol as the preferred reliever therapy (off-label at the time the document was released), and supports the use of the maintenance and reliever therapy.95 This is mainly to reduce possible disease exacerbations. In COPD this concept maybe quite undue, but can be intriguing, suggesting to better consider the effect of the drugs on the use of rescue medications.
Another mechanical reason supporting the twice-daily administration of bronchodilators is given by the presence of closing volume at rest in at least 50% of COPD patients:21 in clinostatism, the cephalad force of abdomen, and the enhanced ventilation/perfusion mismatch during sleep may aggravate this phenomenon, so that the evening administration of a rapid-action, long-acting bronchodilator may be beneficial during night and have positive consequences on the early morning symptoms.22 Moreover, the small airways can be involved by the increased cholinergic tone and the supine position during night: rapid-action bronchodilators administered in the evening can reduce air trapping and cholinergic tone before sleeping, with the same positive consequences.
In COPD patients, the circadian rhythm influencing the broncho-motor tone may be impaired34 while Clara cells act as peripheral clocks in the lungs.35 Considering that hematogenous inflammation is increased during night as well as parenchymal and bronchial eosinophils in the asthmatic patient,36 the timing of administration of drugs may be pivotal. There are no specific evidences in this regard but, for instance, ICS administration could be useful during night. Moreover, the role of bronchodilators on the circadian alterations has not been studied yet: the twice daily administration may be useful to restore the circadian rhythm.
A study considering bid vs qd administration is that of Beier et al, who compared aclidinium twice-daily and tiotropium once-daily. In the study, aclidinium provided additional improvements in bronchodilation, particularly during the night-time, early-morning and night-time symptoms, early-morning limitation of activity.96 In a meta-analysis, tiotropium administration in the evening vs in the morning did not differ according to night-time awakenings.97 This meta-analysis has several limitations, including the self-reported outcomes by patients and the inclusion of studies started in 1997. Moreover, the main issue is not related to the best moment of administration of a 24h lasting drug, but to the preference of a twice daily posology. Moving on to pure pharmacology, the choice of the available different formulations of active principles should also consider the best profile of the drug. For instance, glycopirronium shows a profile more suitable for a twice-daily administration in order to guarantee a best coverage in terms of bronchodilation over the 24 hours.98 Notably, apart from the US market where glycopirronium is available as single agent to be administered twice daily, in Europe it is available in this posology, only in the triple combination beclometasone/formoterol/glycopyrronium. Some data demonstrated that Tiotropium produced sustained bronchodilation throughout the 24 hour day without necessarily abolishing circadian variation in airway calibre. Particularly, this findings show that tiotropium once daily, whether administered in the morning or evening, results in sustained improvements in spirometric indices throughout the 24 hours, including improvement in the early morning nadir in spirometric values, without necessarily affecting circadian variability;99 this could be probably due to its prolonged pharmacologic effect.
Despite all, posology itself is seldom considered in the experiences available in the literature, and consequently in the different guidelines. Even if posology does not seem to directly affect the efficacy of the drug on the commonest considered outcomes, the type of patient’s symptomatology and variability has never been used as inclusion or selection criteria of clinical trials. Therefore, the potential of a double administration should not be neglected when considering patient’s needs or disease variability. If we consider the actual situation, in a recent observational study, the inhaled therapy distribution has been evaluated across GOLD stages showing a 43.6% of the entire study population being treated with LABA/ICS/LAMA, 21.4% with LABA/LAMA, 12.1% with LABA/ICS, 18.6 and 4.3% with LABA or LAMA alone, respectively. The patients with reported frequent exacerbations were more frequently on triple therapy (P=0.003); while the LAMA monotherapy was the most frequently administered regimen in the patients with mild disease, whereas for those ranked GOLD stage ≥ 2, the most frequently prescribed regimen was triple combination therapy.29
Compliance, Administration Modalities and Particles Dimension
Many respiratory diseases are chronic and so strongly linked to patient–doctor relationship, disease perception and adherence to therapy. 18% of patients spontaneously discontinue the therapy, considering it too complex.100 A reduced adherence is related to increased hospitalization and healthcare costs,101 as well as reduced 5-year survival.102 In COPD patients, adherence ranges from 10% to 40%.103–109 Inhaler mishandling remains common in real life and is associated with reduced disease control, including emergency department visits and hospitalizations.110 A meta-analysis of 62 trials demonstrated that reduced out-of-pocket expenses, case management, and patient education with behavioral support all improved medication adherence.111 In COPD adherence to therapy is determined by several factors related to patients (health beliefs, cognitive abilities, self-efficacy, comorbidities, psychological profile, conscientiousness), physicians (method of administration, dosing regimen, polypharmacy, side effects), and society (patient–prescriber relationship, social support, access to medication, device training, follow-up).106 Evidence is still lacking regarding administration modalities in COPD.
In the same context, a small study suggested that switching from twice-daily to once-daily asthma treatments may improve compliance,112 whereas a randomized trial of the two administration modalities confirmed greater mean adherence rates with a once-daily dose regimen, without a significant difference in clinical outcomes.113 Indeed, this kind of studies is often complicated by several factors, including close patient monitoring, resulting in higher adherence rates than clinical practice.102,114 In addition, adherence in trials is generally tested for shorter periods than real life. However, again in the asthma scenario, both once-daily and twice-daily modalities show a continuous and significant reduction in adherence after 3 months of therapy.115 In COPD, a study aiming to assess the degree of adherence for once-daily and twice-daily regimens for LAMAs administration in an area with a centralized control of prescriptions did not find that patients who used twice-daily medication had a lower adherence.116 On the contrary, few evidences support the idea that once-daily administration favors patients’ adherence, but with low quality of data.117,118 In a certain sense, while once-daily posology is preferred by patients with a higher probability to be not adherent,112,113 twice-daily posology may be preferred by patients who need reassurance about being well controlled. Both strategies can be worth it to guarantee optimal management of the adherence.118
Regarding administration modalities and compliance it is important also to choose the correct inhaler. Several inhalers exist, of complex usage. In addition, COPD patient is complex, due to comorbidities.119 Both the aspects can impact on the wrong use of inhalers. Some comorbidities are equally present from GOLD 1 to 4.120 Different devices (ie, pressurized metered dose inhaler, dry powered inhaler or nebulizer) have typical advantages and limitations, which should be considered according to patient’s characteristics.121,122 Handling errors increase with age and severity of COPD.123 Coordination errors occur in 33% of patients,124 while 15–25% does not shake the pMDI inhaler correctly.125 In conclusion, inhalers are definitely equally efficacious if properly used and chosen.126
According to GOLD recommendations, particles dimension influences the total respirable fraction, the dose delivery and the target site reached.2 Small-particle aerosol formulations better target the distal lung, enhancing the drug delivery efficiency,127 thus they target specific disease or receptor locations, decreasing drug exposure and adverse effects. Therefore, new extrafine formulations may provide important clinical benefit as regards symptoms, exacerbations, quality of life, prognosis.61,127–129 Individual patient’s compliance to the chosen device could be, finally, strongly related to the maximization of the disease control achievable with the combination of the inhalation technique, the intrinsic characteristics of the formulation (influencing drug delivery) and the active principles’ efficacy.
Future Directions and Conclusions
COPD is a severe disease, highly disabling and impacting on quality of life and survival. Symptoms are heterogeneous partly along with phenotypes, namely emphysema, obstructive bronchiolitis, overlap with asthma. Dyspnea is the most referred symptom, but also others are present and should be considered in disease assessment and monitoring (eg, cough, wheezing, fatigue, phlegm). Notably, independently from the GOLD class, these symptoms also vary during night and day, generally being worse in night-time and after the awakening, and impacting on the quality of sleep and ultimately on exacerbations and survival. The cyclic variation of COPD symptoms is related to mechanical issues (clinostatism, reduced respiratory surface, cephalad force of the abdomen, increase ventilatory/perfusion ratio) acting on the closing volume at rest and on small airways, favouring air trapping. Additionally, also biochemical alterations on the circadian regulation due to cholinergic system and Clara cells action on the broncho-motor tone and to hematogenous inflammation (particularly eosinophilic), have a role in the genesis of night-time symptoms. Consequently, this board argues that pharmacotherapy should be studied and directed towards these aspects, for instance supporting the use of twice-daily rapid-action bronchodilators and evening dose of ICS. The concept of a tailored therapy based on the patients’ needs, including the daily variability of symptoms, is intriguing and requires dedicated studies. In addition, the use in clinical practice or in research field of instruments such as questionnaires, cardiorespiratory recording devices or new digital tools is advocated for disease monitoring, providing also objective data to support the therapeutical choice.
Abbreviations
CANO, Peripheral airway/alveolar nitric oxide concentration; CASIS, COPD and Asthma Sleep Impact Scale; CAT, COPD Assessment Test; CDML, Capacity of Daily Living during the Morning; COPD, Chronic obstructive pulmonary disease; GOLD, Global initiative for chronic Obstructive Lung Disease; ICS, Inhaled corticosteroids; LABA, Long-acting â2-agonists; LAMA, Long-acting muscarinic antagonists; OSA, Obstructive sleep apnea; PEEPi, Intrinsic Positive End-Expiratory Pressure; PRO, Patient Reported Outcomes; REM, Rapid Eye Movement.
Disclosure
The advisory board was sponsored by Chiesi Farmaceutici (Parma, Italy). Dr Alessio Piraino is an employee of Chiesi Farmaceutici S.p.A. Professor Pierachille Santus reports personal fees from GSK, Guidotti, Menarini International, ALK Abellò, and Berlin Chemie; grants, personal fees from AstraZeneca, Novartis, and Boehringer Ingelheim; grants from Chiesi Farmaceutici, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi:10.1016/S0140-6736(12)61728-0
2. Available from: https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.0wms.pdf.
3. Jones P, Miravitlles M, van der Molen T, Kulich K. Beyond FEV1 in COPD: a review of patient-reported outcomes and their measurement. Int J Chron Obstruct Pulmon Dis. 2012;7:697–709. doi:10.2147/COPD.S32675
4. Partridge MR, Karlsson N, Small IR, et al. Patient insight into the impact of chronic obstructive pulmonary disease in the morning: an internet survey. Curr Med Res Opin. 2009;25:2043–2048. doi:10.1185/03007990903103006
5. Braido F, Baiardini I, Scichilone N, et al. Disability in moderate chronic obstructive pulmonary disease: prevalence, burden and assessment - results from a real-life study. Respiration. 2015;89:100–106. doi:10.1159/000368365
6. Agusti A, Hedner J, Marin JM, Barbé F, Cazzola M, Rennard S. Night-time symptoms: a forgotten dimension of COPD. Eur Respir Rev. 2011;20:183–194. doi:10.1183/09059180.00004311
7. Tsiligianni I, Metting E, van der Molen T, Chavannes N, Kocks J. Morning and night symptoms in primary care COPD patients: a cross-sectional and longitudinal study. An UNLOCK study from the IPCRG. NPJ Prim Care Respir Med. 2016;21(26):16040. doi:10.1038/npjpcrm.2016.40
8. Krönig J, Hildebrandt O, Weissflog A, et al. Long-term recording of night-time respiratory symptoms in patients with stable COPD II-IV. COPD. 2017;14:498–503. doi:10.1080/15412555.2017.1338681
9. Koehler U, Brandenburg U, Weissflog A, Sohrabi K, Groß V.[LEOSound, an innovative procedure for acoustic long-term monitoring of asthma symptoms (wheezing and coughing) in children and adults]. Pneumologie.2014;68:277–281. doi:10.1055/s-0034-1365156. German.
10. Fischer P, Gross V, Kroenig J, et al. Description of nighttime cough epochs in patients with stable COPD GOLD II-IV. Int J Chron Obstruct Pulmon Dis. 2018;13:1071–1078. doi:10.2147/COPD.S154539
11. Akinci B, Aslan GK, Kiyan E. Sleep quality and quality of life in patients with moderate to very severe chronic obstructive pulmonary disease. Clin Respir J. 2018;12:1739–1746. doi:10.1111/crj.12738
12. Lindberg A, Bjerg A, Rönmark E, Larsson LG, Lundbäck B. Prevalence and underdiagnosis of COPD by disease severity and the attributable fraction of smoking report from the obstructive lung disease in Northern Sweden Studies. Respir Med. 2006;100:264–272.
13. O’Donnell DE, Bertley JC, Chau LK, Webb KA. Qualitative aspects of exertional breathlessness in chronic airflow limitation: pathophysiologic mechanisms. Am J Respir Crit Care Med. 1997;155:109–115. doi:10.1164/ajrccm.155.1.9001298
14. Kessler R, Partridge MR, Miravitlles M, et al. Symptom variability in patients with severe COPD: a pan-European cross-sectional study. Eur Respir J. 2011;37:264–272. doi:10.1183/09031936.00051110
15. Decramer M, Rennard S, Troosters T, et al. COPD as a lung disease with systemic consequences–clinical impact, mechanisms, and potential for early intervention. COPD. 2008;5:235–256. doi:10.1080/15412550802237531
16. Contoli M, Solidoro P, Di Marco F, et al. Effects of aclidinium on determinants of COPD severity: symptoms and quality of life. Int J Chron Obstruct Pulmon Dis. 2016;11:3043–3050. doi:10.2147/COPD.S122433
17. O’Donnell DE, Elbehairy AF, Faisal A, Webb KA, Neder JA, Mahler DA. Exertional dyspnoea in COPD: the clinical utility of cardiopulmonary exercise testing. Eur Respir Rev. 2016;25:333–347. doi:10.1183/16000617.0054-2016
18. Hannink JD, van Helvoort HA, Dekhuijzen PN, Heijdra YF. Dynamic hyperinflation during daily activities: does COPD global initiative for chronic obstructive lung disease stage matter? Chest. 2010;137:1116–1121. doi:10.1378/chest.09-1847
19. Katz S, Arish N, Rokach A, Zaltzman Y, Marcus E-L. The effect of body position on pulmonary function: a systematic review. BMC Pulm Med. 2018;18(1):159. doi:10.1186/s12890-018-0723-4
20. Kang W, Clark AR, Tawhai MH. Gravity outweighs the contribution of structure to passive ventilation-perfusion matching in the supine adult human lung. J Appl Physiol (1985). 2018;124(1):23–33. doi:10.1152/japplphysiol.00791.2016
21. Pecchiari M, Radovanovic D, Santus P, D’Angelo E. Airway occlusion assessed by single breath N2 test and lung P-V curve in healthy subjects and COPD patients. Respir Physiol Neurobiol. 2016;234:60–68. doi:10.1016/j.resp.2016.09.006
22. Pecchiari M, Santus P, Radovanovic D, D’Angelo E. Acute effects of long-acting bronchodilators on small airways detected in COPD patients by single-breath N2 test and lung P-V curve. J Appl Physiol. 1985;2017(123):1266–1275.
23. Santus P, Radovanovic D, Mascetti S, et al. Effects of bronchodilation on biomarkers of peripheral airway inflammation in COPD. Pharmacol Res. 2018;133:160–169. doi:10.1016/j.phrs.2018.05.010
24. Watz H. Chronic obstructive pulmonary disease: when pulmonologists do something good for the heart. Am J Respir Crit Care Med. 2016;193:703–704. doi:10.1164/rccm.201512-2340ED
25. Ather S, Chan W, Bozkurt B, et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59:998–1005. doi:10.1016/j.jacc.2011.11.040
26. Santus P, Radovanovic D, Di Marco S, et al. Effect of indacaterol on lung deflation improves cardiac performance in hyperinflated COPD patients: an interventional, randomized, double-blind clinical trial. Int J Chron Obstruct Pulmon Dis. 2015;10:1917–1923. doi:10.2147/COPD.S91684
27. Stone IS, Barnes NC, James WY, et al. Lung deflation and cardiovascular structure and function in chronic obstructive pulmonary disease. A randomized controlled trial. Am J Respir Crit Care Med. 2016;193:717–726. doi:10.1164/rccm.201508-1647OC
28. Hohlfeld JM, Vogel-Claussen J, Biller H, et al. Effect of lung deflation with indacaterol plus glycopyrronium on ventricular filling in patients with hyperinflation and COPD (CLAIM): a double-blind, randomised, crossover, placebo-controlled, single-centre trial. Lancet Respir Med. 2018;6:368–378. doi:10.1016/S2213-2600(18)30054-7
29. Radovanovic D, Contoli M, Marco FD, et al. Functional characteristics of COPD patients across GOLD classifications: results of a multicenter observational study. COPD. 2019;16(3–4):215–226. doi:10.1080/15412555.2019.1659760
30. Tabachnik E, Muller NL, Bryan AC, Levison H. Changes in ventilation and chest wall mechanics during sleep in normal adolescents. J Appl Physiol Respir Environ Exerc Physiol. 1981;51(3):557–564. doi:10.1152/jappl.1981.51.3.557
31. Marrone O, Salvaggio A, Insalaco G. Respiratory disorders during sleep in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2006;1:363–372. doi:10.2147/copd.2006.1.4.363
32. O’Donoghue FJ, Catcheside PG, Ellis EE, et al. Australian trial of noninvasive ventilation in chronic airflow limitation investigators. Sleep hypoventilation in hypercapnic chronic obstructive pulmonary disease: prevalence and associated factors. Eur Respir J. 2003;21:977–984.
33. Catterall JR, Rhind GB, Stewart IC, Whyte KF, Shapiro CM, Douglas NJ. Effect of sleep deprivation on overnight bronchoconstriction in nocturnal asthma. Thorax. 1986;41:676–680. doi:10.1136/thx.41.9.676
34. Morrison JF, Pearson SB. The effect of the circadian rhythm of vagal activity on bronchomotor tone in asthma. Br J Clin Pharmacol. 1989;28:545–549. doi:10.1111/j.1365-2125.1989.tb03540.x
35. Durrington HJ, Farrow SN, Loudon AS, Ray DW. The circadian clock and asthma. Thorax. 2014;69:90–92. doi:10.1136/thoraxjnl-2013-203482
36. Martin RJ. Small airway and alveolar tissue changes in nocturnal asthma. Am J Respir Crit Care Med. 1998;157(5 Pt 2):S188–S190. doi:10.1164/ajrccm.157.5.rsaa-4
37. Bateman ED, Ferguson GT, Barnes N, et al. Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur Respir J. 2013;42:1484–1494. doi:10.1183/09031936.00200212
38. Lee JS, Huh JW, Chae EJ, et al. Response patterns to bronchodilator and quantitative computed tomography in chronic obstructive pulmonary disease. Clin Physiol Funct Imaging. 2012;32:12–18. doi:10.1111/j.1475-097X.2011.01046.x
39. Iqbal A, Barnes NC, Brooks J. Is blood eosinophil count a predictor of response to bronchodilators in chronic obstructive pulmonary disease? Results from post hoc subgroup analyses. Clin Drug Investig. 2015;35:685–688. doi:10.1007/s40261-015-0322-6
40. van den Bemt L, Schermer T, Smeele I, et al. Monitoring of patients with COPD: a review of current guidelines’ recommendations. Respir Med. 2008;102:633–641. doi:10.1016/j.rmed.2007.12.014
41. Agusti A. The path to personalised medicine in COPD. Thorax. 2014;69(9):857–864. doi:10.1136/thoraxjnl-2014-205507
42. Partridge MR, Miravitlles M, Ståhl E, Karlsson N, Svensson K, Welte T. Development and validation of the capacity of daily living during the morning questionnaire and the Global Chest Symptoms Questionnaire in COPD. Eur Respir J. 2010;36:96–104. doi:10.1183/09031936.00123709
43. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34:648–654. doi:10.1183/09031936.00102509
44. Jones PW, Leidy NK, Hareendran A, Lamarca R, Chuecos F, Garcia Gil E. The effect of aclidinium bromide on daily respiratory symptoms of COPD, measured using the Evaluating Respiratory Symptoms in COPD (E-RS: COPD) diary: pooled analysis of two 6-month Phase III studies. Respir Res. 2016;17:61. doi:10.1186/s12931-016-0372-1
45. Leidy NK, Murray LT, Monz BU, et al. Measuring respiratory symptoms of COPD: performance of the EXACT-Respiratory Symptoms Tool (E-RS) in three clinical trials. Respir Res. 2014;15:124. doi:10.1186/s12931-014-0124-z
46. Soler-Cataluña JJ, Marzo M, Catalán P, Miralles C, Alcazar B, Miravitlles M. Validation of clinical control in COPD as a new tool for optimizing treatment. Int J Chron Obstruct Pulmon Dis. 2018;13:3719–3731. doi:10.2147/COPD.S178149
47. Braido F, Baiardini I, Molinengo G, et al. Choose your outcomes: from the mean to the personalized assessment of outcomes in COPD. An exploratory pragmatic survey. Eur J Intern Med. 2016;34:85–88. doi:10.1016/j.ejim.2016.05.030
48. Nunes DM, Mota RM, de Pontes Neto OL, Pereira ED, de Bruin VM, de Bruin PF. Impaired sleep reduces quality of life in chronic obstructive pulmonary disease. Lung. 2009;187:159–163. doi:10.1007/s00408-009-9147-5
49. Omachi TA, Blanc PD, Claman DM, et al. Disturbed sleep among COPD patients is longitudinally associated with mortality and adverse COPD outcomes. Sleep Med. 2012;13:476–483. doi:10.1016/j.sleep.2011.12.007
50. Lange P, Marott JL, Vestbo J, Nordestgaard BG. Prevalence of night-time dyspnoea in COPD and its implications for prognosis. Eur Respir J. 2014;43:1590–1598. doi:10.1183/09031936.00196713
51. Kwon JS, Wolfe LF, Lu BS, Kalhan R. Hyperinflation is associated with lower sleep efficiency in COPD with co-existent obstructive sleep apnea. COPD. 2009;6:441–445. doi:10.3109/15412550903433000
52. Basile M, Baiamonte P, Mazzuca E, et al. Sleep disturbances in COPD are associated with heterogeneity of airway obstruction. COPD. 2018;15:350–354. doi:10.1080/15412555.2018.1504015
53. Pokrzywinski RF, Meads DM, McKenna SP, Glendenning GA, Revicki DA. Development and psychometric assessment of the COPD and Asthma Sleep Impact Scale (CASIS). Health Qual Life Outcomes. 2009;7:98. doi:10.1186/1477-7525-7-98
54. Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–789. doi:10.1056/NEJMoa063070
55. Canonica GW, Blasi F, Scichilone N, et al.; STORICO study group. Characterization of circadian COPD symptoms by phenotype: methodology of the STORICO observational study. Eur J Intern Med. 2017;43:62–68.
56. Bellia V, Catalano F, Scichilone N, et al. Sleep disorders in the elderly with and without chronic airflow obstruction: the SARA study. Sleep. 2003;26:318–323. doi:10.1093/sleep/26.3.318
57. Cazzola M, Page CP, Calzetta L, Matera MG. Pharmacology and therapeutics of bronchodilators. Pharmacol Rev. 2012;64:450–504.
58. Tashkin DP, Fabbri LM. Long-acting beta-agonists in the management of chronic obstructive pulmonary disease: current and future agents. Respir Res. 2010;11:149. doi:10.1186/1465-9921-11-149
59. Calzetta L, Matera MG, Cazzola M. Pharmacological interaction between LABAs and LAMAs in the airways: optimizing synergy. Eur J Pharmacol. 2015;761:168–173. doi:10.1016/j.ejphar.2015.05.020
60. Cazzola M, Calzetta L, Segreti A, Facciolo F, Rogliani P, Matera MG. Translational study searching for synergy between glycopyrronium and indacaterol. COPD. 2015;12:175–181. doi:10.3109/15412555.2014.922172
61. Crisafulli E, Pisi R, Aiello M, et al. Prevalence of small-airway dysfunction among COPD patients with different GOLD stages and its role in the impact of disease. Respiration. 2017;93:32–41. doi:10.1159/000452479
62. Wessler I, Kirkpatrick CJ. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br J Pharmacol. 2008;154:1558–1571. doi:10.1038/bjp.2008.185
63. Profita M, Bonanno A, Montalbano AM, et al. β₂ long-acting and anticholinergic drugs control TGF-β1-mediated neutrophilic inflammation in COPD. Biochim Biophys Acta. 2012;1822:1079–1089. doi:10.1016/j.bbadis.2012.03.002
64. Cazzola M, Calzetta L, Puxeddu E, et al. Pharmacological characterisation of the interaction between glycopyrronium bromide and indacaterol fumarate in human isolated bronchi, small airways and bronchial epithelial cells. Respir Res. 2016;17:70. doi:10.1186/s12931-016-0386-8
65. Sullivan P, Bekir S, Jaffar Z, Page C, Jeffery P, Costello J. Anti-inflammatory effects of low-dose oral theophylline in atopic asthma. Lancet. 1994;343(8904):1006–1008. doi:10.1016/S0140-6736(94)90127-9
66. Nicholson CD, Shahid M. Inhibitors of cyclic nucleotide phosphodiesterase isoenzymes–their potential utility in the therapy of asthma. Pulm Pharmacol. 1994;7(1):1–17. doi:10.1006/pulp.1994.1001
67. Ito K, Lim S, Caramori G, et al. A molecular mechanism of action of theophylline: induction of histone deacetylase activity to decrease inflammatory gene expression. Proc Natl Acad Sci U S A. 2002;99(13):8921–8926. doi:10.1073/pnas.132556899
68. Lim S, Jatakanon A, Gordon D, Macdonald C, Chung KF, Barnes PJ. Comparison of high dose inhaled steroids, low dose inhaled steroids plus low dose theophylline, and low dose inhaled steroids alone in chronic asthma in general practice. Thorax. 2000;55(10):837–841. doi:10.1136/thorax.55.10.837
69. Ukena D, Harnest U, Sakalauskas R, et al. Comparison of addition of theophylline to inhaled steroid with doubling of the dose of inhaled steroid in asthma. Eur Respir J. 1997;10(12):2754–2760. doi:10.1183/09031936.97.10122754
70. Slack RJ, Barrett VJ, Morrison VS, et al. In vitro pharmacological characterization of vilanterol, a novel long-acting β2-adrenoceptor agonist with 24-hour duration of action. J Pharmacol Exp Ther. 2013;344(1):218–230. doi:10.1124/jpet.112.198481
71. Calzetta L, Rogliani P, Facciolo F, Rendina E, Cazzola M, Matera MG. Pharmacological characterization of the interaction between umeclidinium and vilanterol in human bronchi. Eur J Pharmacol. 2017;812:147–154. doi:10.1016/j.ejphar.2017.07.026
72. Naline E, Grassin Delyle S, Salvator H, et al. Comparison of the in vitro pharmacological profiles of long-acting muscarinic antagonists in human bronchus. Pulm Pharmacol Ther. 2018;49:46–53. doi:10.1016/j.pupt.2018.01.003
73. Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3:435–442. doi:10.1016/S2213-2600(15)00106-X
74. Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 2018;391:1076–1084. doi:10.1016/S0140-6736(18)30206-X
75. Lipson DA, Barnhart F, Brealey N, et al.; IMPACT Investigators. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378:1671–1680.
76. Magnussen H, Disse B, Rodriguez-Roisin R, et al.; WISDOM Investigators. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371:1285–1294.
77. Calzetta L, Matera MG, Braido F, et al. Withdrawal of inhaled corticosteroids in COPD: a meta-analysis. Pulm Pharmacol Ther. 2017;45:148–158. doi:10.1016/j.pupt.2017.06.002
78. Reilly JJ. Stepping down therapy in COPD. N Engl J Med. 2014;371:1340–1341. doi:10.1056/NEJMe1409219
79. Chapman KR, Hurst JR, Frent SM, et al. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med. 2018;198:329–339. doi:10.1164/rccm.201803-0405OC
80. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9:CD006829.
81. Nannini LJ, Cates CJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta-agonist in one inhaler versus inhaled steroids for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;8:CD006826.
82. Nannini LJ, Poole P, Milan SJ, Holmes R, Normansell R. Combined corticosteroid and long-acting beta₂-agonist in one inhaler versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;11:CD003794.
83. Manoharan A, Short PM, Anderson WJ, Lipworth BJ. Impact of long-acting bronchodilators and exposure to inhaled corticosteroids on mortality in COPD: a real-life retrospective cohort study. Lung. 2014;192:649–652. doi:10.1007/s00408-014-9611-8
84. Vestbo J, Fabbri L, Papi A, et al. Inhaled corticosteroid containing combinations and mortality in COPD. Eur Respir J. 2018;52.
85. Singh D, Fabbri LM, Vezzoli S, Petruzzelli S, Papi A. Extrafine triple therapy delays COPD clinically important deterioration vs ICS/LABA, LAMA, or LABA/LAMA. Int J Chron Obstruct Pulmon Dis. 2019;28(14):531–546. doi:10.2147/COPD.S196383
86. Jen R, Rennard SI, Sin DD. Effects of inhaled corticosteroids on airway inflammation in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2012;7:587–595. doi:10.2147/COPD.S32765
87. Kunz LI, Strebus J, Budulac SE, et al; GLUCOLD (Groningen Leiden Universities Corticosteroids in Obstructive Lung Disease) Study Group. Inhaled steroids modulate extracellular matrix composition in bronchial biopsies of COPD patients: a randomized, controlled trial. PLoS One. 2013;8(5):e63430.
88. Tantucci C, Modina D. Lung function decline in COPD. Int J Chron Obstruct Pulmon Dis. 2012;7:95–99. doi:10.2147/COPD.S27480
89. Singh D, Fabbri LM, Corradi M, et al. Extrafine triple therapy in patients with symptomatic COPD and history of one moderate exacerbation. Eur Respir J. 2019;53.
90. Ferguson GT, Rabe KF, Martinez FJ, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, Phase 3 randomised controlled trial. Lancet Respir Med. 2018;6(10):747–758. doi:10.1016/S2213-2600(18)30327-8
91. Cazzola M, Rogliani P, Calzetta L, Matera MG. Triple therapy versus single and dual long-acting bronchodilator therapy in COPD: a systematic review and meta-analysis. Eur Respir J. 2018;52(6):1801586. doi:10.1183/13993003.01586-2018
92. Kelly HW. Comparison of inhaled corticosteroids: an update. Ann Pharmacother. 2009;43(3):519–527. doi:10.1345/aph.1L546
93. Woodcock A, Bateman ED, Busse WW, et al. Efficacy in asthma of once-daily treatment with fluticasone furoate: a randomized, placebo-controlled trial. Respir Res. 2011;12:132. doi:10.1186/1465-9921-12-132
94. Woodcock A, Bleecker ER, Busse WW, et al. Fluticasone furoate: once-daily evening treatment versus twice-daily treatment in moderate asthma. Respir Res. 2011;12:160. doi:10.1186/1465-9921-12-160
95. Global Initiative for Asthma; 2019. Available from: https://ginasthma.org/.
96. Beier J, Mroz R, Kirsten AM, Chuecos F, Gil EG. Improvement in 24-hour bronchodilation and symptom control with aclidinium bromide versus tiotropium and placebo in symptomatic patients with COPD: post hoc analysis of a Phase IIIb study. Int J Chron Obstruct Pulmon Dis. 2017;12:1731–1740. doi:10.2147/COPD.S121723
97. Calverley PM, Rennard SI, Clerisme-Beaty E, Metzdorf N, Zubek VB, ZuWallack R. Effect of tiotropium on night-time awakening and daily rescue medication use in patients with COPD. Respir Res. 2016;17(1):27. doi:10.1186/s12931-016-0340-9
98. Arievich H, Overend T, Renard D, et al. A novel model-based approach for dose determination of glycopyrronium bromide in COPD. BMC Pulm Med. 2012;12:74. doi:10.1186/1471-2466-12-74
99. Calverley PMA, Lee A, Towse L, van Noord J, Witek TJ, Kelsen S. Effect of tiotropium bromide on circadian variation in airflow limitation in chronic obstructive pulmonary disease. Thorax. 2003;58:855–860. doi:10.1136/thorax.58.10.855
100. Santus P, Picciolo S, Proietto A, et al. Doctor-patient relationship: a resource to improve respiratory diseases management. Eur J Intern Med. 2012;23:442–446. doi:10.1016/j.ejim.2012.04.004
101. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi:10.1056/NEJMra050100
102. Belleudi V, Di Martino M, Cascini S, et al. OUTPUL Study Group. The impact of adherence to inhaled drugs on 5-year survival in COPD patients: a time dependent approach. Pharmacoepidemiol Drug Saf. 2016;25:1295–1304. doi:10.1002/pds.4059
103. George J, Kong DC, Thoman R, Stewart K. Factors associated with medication nonadherence in patients with COPD. Chest. 2005;128:3198–3204. doi:10.1378/chest.128.5.3198
104. Jung E, Pickard AS, Salmon JW, Bartle B, Lee TA. Medication adherence and persistence in the last year of life in COPD patients. Respir Med. 2009;103:525–534. doi:10.1016/j.rmed.2008.11.004
105. Krigsman K, Nilsson JL, Ring L. Refill adherence for patients with asthma and COPD: comparison of a pharmacy record database with manually collected repeat prescriptions. Pharmacoepidemiol Drug Saf. 2007;16:441–448. doi:10.1002/pds.1321
106. Rogliani P, Ora J, Puxeddu E, Matera MG, Cazzola M. Adherence to COPD treatment: myth and reality. Respir Med. 2017;129:117–123. doi:10.1016/j.rmed.2017.06.007
107. Bourbeau J, Bartlett SJ. Patient adherence in COPD. Thorax. 2008;63(9):831–838. doi:10.1136/thx.2007.086041
108. Vetrano DL, Bianchini E, Onder G, et al. Poor adherence to chronic obstructive pulmonary disease medications in primary care: role of age, disease burden and polypharmacy. Geriatr Gerontol Int. 2017;17(12):2500–2506. doi:10.1111/ggi.13115
109. Mueller S, Wilke T, Bechtel B, Punekar YS, Mitzner K, Virchow JC. Non-persistence and non-adherence to long-acting COPD medication therapy: a retrospective cohort study based on a large German claims dataset. Respir Med. 2017;122:1–11. doi:10.1016/j.rmed.2016.11.008
110. Melani AS, Bonavia M, Cilenti V, et al.; Gruppo Educazionale Associazione Italiana Pneumologi Ospedalieri. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105:930–938.
111. Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157:785–795. doi:10.7326/0003-4819-157-11-201212040-00538
112. Guest JF, Davie AM, Ruiz FJ, Greener MJ. Switching asthma patients to a once-daily inhaled steroid improves compliance and reduces healthcare costs. Prim Care Respir J. 2005;14:88–98. doi:10.1016/j.pcrj.2005.01.002
113. Price D, Robertson A, Bullen K, Rand C, Horne R, Staudinger H. Improved adherence with once-daily versus twice-daily dosing of mometasone furoate administered via a dry powder inhaler: a randomized open-label study. BMC Pulm Med. 2010;10:1. doi:10.1186/1471-2466-10-1
114. Storms W. Clinical trials: are these your patients? J Allergy Clin Immunol. 2003;112(5):S107–11. doi:10.1016/j.jaci.2003.09.019
115. Jonasson G, Carlsen KH, Mowinckel P. Asthma drug adherence in a long term clinical trial. Arch Dis Child. 2000;83:330–333. doi:10.1136/adc.83.4.330
116. Izquierdo JL, Paredero JM, Piedra R. Relevance of dosage in adherence to treatment with long-acting anticholinergics in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2016;10(11):289–293. doi:10.2147/COPD.S96948
117. Toy EL, Beaulieu NU, McHale JM, et al. Treatment of COPD: relationships between daily dosing frequency, adherence, resource use, and costs. Respir Med. 2011;105(3):435–441. doi:10.1016/j.rmed.2010.09.006
118. Price D, Lee AJ, Sims EJ, et al. Characteristics of patients preferring once-daily controller therapy for asthma and COPD: a retrospective cohort study. Prim Care Respir J. 2013;22(2):161–168. doi:10.4104/pcrj.2013.00017
119. Smith MC, Wrobel JP. Epidemiology and clinical impact of major comorbidities in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:871–888. doi:10.2147/COPD.S49621
120. Battaglia S, Basile M, Scichilone N, Bellia V. Prevalence of Co-morbidities and Severity of COPD. COPD. 2015;12:390–394. doi:10.3109/15412555.2014.974734
121. Scichilone N, Contino A, Figlioli GB, Paglino G, Bellia V. Patient perspectives in the management of asthma: improving patient outcomes through critical selection of treatment options. Patient Prefer Adherence. 2010;4:17–23. doi:10.2147/PPA.S5627
122. Scichilone N, Benfante A, Bocchino M, et al. Which factors affect the choice of the inhaler in chronic obstructive respiratory diseases? Pulm Pharmacol Ther. 2015;31:63–67. doi:10.1016/j.pupt.2015.02.006
123. Wieshammer S, Dreyhaupt J. Dry powder inhalers: which factors determine the frequency of handling errors? Respiration. 2008;75:18–25. doi:10.1159/000109374
124. Giraud V, Roche N. Misuse of corticosteroid metered-dose inhaler is associated with decreased asthma stability. Eur Respir J. 2002;19:246–251. doi:10.1183/09031936.02.00218402
125. van der Palen J, Klein JJ, van Herwaarden CL, Zielhuis GA, Seydel ER. Multiple inhalers confuse asthma patients. Eur Respir J. 1999;14:1034–1103. doi:10.1183/09031936.99.14510349
126. Dolovich MB, Ahrens RC, Hess DR, et al.; American College of Chest Physicians; American College of Asthma, Allergy, and Immunology. Device selection and outcomes of aerosol therapy: evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127:335–371.
127. Lavorini F, Pedersen S, Usmani OS; Aerosol Drug Management Improvement Team (ADMIT). Dilemmas, confusion, and misconceptions related to small airways directed therapy. Chest. 2017;151(6):1345–1355.
128. Han MK, Quibrera PM, Carretta EE, et al.; SPIROMICS investigators. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(8):619–626.
129. Hogg JC, Chu FS, Tan WC, et al. Survival after lung volume reduction in chronic obstructive pulmonary disease: insights from small airway pathology. Am J Respir Crit Care Med. 2007;176(5):454–459. doi:10.1164/rccm.200612-1772OC
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
