Back to Journals » OncoTargets and Therapy » Volume 11
DACH 1 inhibits glioma invasion and tumor growth via the Wnt/catenin pathway
Authors Wang J , Zou Y, Wu X, Chen M, Zhang S, Lu X, Wang Q
Received 18 April 2018
Accepted for publication 9 July 2018
Published 17 September 2018 Volume 2018:11 Pages 5853—5863
DOI https://doi.org/10.2147/OTT.S168314
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Jing Wang,* Yan Zou,* Xuechao Wu, Mu Chen, Shuai Zhang, Xiaojie Lu, Qing Wang
Neurosurgery, The Affiliated Wuxi No 2 People’s Hospital, Nanjing Medical University, Wuxi, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Background/aim: Glioma is the most common and malignant nervous system tumor and is associated with high-grade malignancy and high recurrence. The mammalian Dachshund1 (DACH1) is a recognized anti-tumor site and has low expression in several malignant tumors, including glioma. We designed and conducted this study to further determine the mechanism of DACH1 in glioma.
Patients and methods: The data collected from specimens of patients with glioma from GSE16011 and REMBRANDT databases were analyzed. The effect of DACH1 on proliferation, migration, and invasion of U87 and U251 cell lines was analyzed in vitro. The symbol targets of the Wnt/β-catenin pathway were also evaluated through Western blot.
Results: DACH1 deficiency was found in glioma tissues, and the DACH1 level was negatively correlated with the tumor malignancy. DACH1 overexpression inhibited the tumor proliferation, migration, and invasion. High expression of DACH1 also dampened the Wnt/β-catenin pathway, and the activation of the Wnt/β-catenin pathway partly led to the limited proliferation in glioma cells.
Conclusion: Downregulation of DACH1 was related to the malignancy and poor prognosis of patients with glioma, and DACH1 overexpression inhibited the tumor proliferation via the Wnt/β-catenin pathway. These findings might assist in the discovery of novel potential diagnostic and therapeutic targets for DACH1, thereby reducing the malignancy and recurrence of glioma.
Keywords: DACH1, glioma, Wnt/catenin pathway, invasion, tumor growth
Introduction
Glioma is the most common and malignant primary brain tumor in adults. The current standard of care consists of surgical resection followed by radiotherapy and chemotherapy. However, the median survival time for patients diagnosed with glioma is only 12–18 months, with only 3% of the patients surviving longer than 5 years. This disease is characterized by high-grade malignancy and a high recurrence, highlighting the urgent need to develop novel and effective predictive and therapeutic strategies for this devastating and uniformly fatal disease.
Dachshund1 (DACH1) is the human homolog of the Drosophila gene dac, which is a dominant inhibitor of ellipse and encodes a key member of the Retinal Determination Gene Network (RDGN). It was originally classified exclusively as the primary administrator of organismal determination in the Drosophila eye, but it is now known to carry out various different functions during tumorigenesis and metastasis.1–3 Several studies have identified DACH1 as a negative regulator of TGF-β and Wnt signaling to repress cancer cell migration and invasion. DACH1 inhibits cell cycle progression and oncogenic transformation and blocks paracrine signaling.4–6 DACH1 attenuates the transcriptional activity of FOXM1b by competitively binding to a homologic DNA sequence.7 In addition, DACH1 associates with the estrogen and androgen receptors (ER and AR) to regulate signal transduction and proliferation especially in breast and prostate cancer cells.8,9 DACH1 inhibits epithelial–mesenchymal transition (EMT) in breast cancer10,11 and blocks mammary tumor growth by downregulating Nanog and Sox2.12 DACH1 can directly associate with p53 and enhance its function in breast and lung cancers.13,14 Clinically, the reduced expression of DACH1 in breast and endometrial cancers correlates with tumor progression and poor differentiation and predicts a short survival.4,15,16 Another study revealed that the poor prognosis of patients with gastric cancer is correlated with a lower DACH1 level. Moreover, in prostate cancer, DACH1 expression is also decreased, and the re-overexpression of DACH1 inhibits the proliferation of prostate cancer cells in vitro.8 A recent study has also found that the overexpression of DACH1 reduces the growth of glioma cells both in vitro and in vivo by inhibiting tumor-initiating cells in glioma.18 Further research has illustrated that DACH1 decreases the tumor-initiating cells via inhibiting transcriptional activation of fibroblast growth factor 2 (FGF2/bFGF).17
Wnt/β-catenin signaling is a pivotal morphogenetic pathway and accordingly is associated with a host of physiological and pathophysiological processes, including embryonic patterning, cell proliferation, cell differentiation, angiogenesis, and cancer.18–20 Wnt signaling is initiated by the binding of Wnt ligands to their cognate receptors, which shuttles palmitoylated Wnts to the plasma membrane, where they are released by the cell to initiate autocrine or paracrine signaling. Wnts can induce different modes of cellular signaling, either mediated by β-catenin or independent of this protein. According to their dependence on β-catenin for inducing cellular effects, Wnts are classified into canonical (β-catenin-dependent) and non-canonical (β-catenin-independent) subgroups.21,22 Except for in several stem cell niches, canonical Wnt/β-catenin signaling is typically not active in adult tissues, despite the constitutive production of Wnt ligands. In this resting state, cytosolic β-catenin is continuously phosphorylated at Ser33, Ser37, Thr41, and Ser45 residues located in exon 3 by a multiprotein complex consisting of glycogen synthase kinase 3β (GSK-3β), casein kinase 1α, adenomatous polyposis coli (APC), and Axin. These phosphorylations cause β-catenin to be recognized and polyubiquitinated by β-transduction repeat containing protein, followed by the degradation of β-catenin in the proteasome.23,24 The overall effect is that very little free cytosolic β-catenin is available for nuclear signaling, and thus, Wnt-mediated gene transcription is absent under normal conditions. Following the binding of Wnt ligands to a complex consisting of the FZD receptor and coreceptors, which include low-density lipoprotein receptor-related protein 5/6, the scaffolding protein disheveled is recruited to the membrane, an event that in turn causes the accumulation of β-catenin in the cytoplasm and its subsequent translocation to the nucleus. In the nucleus, β-catenin binds transcription factors of the T-cell factor 4 (transcription factor 7, transcription factor 7-like 1, and transcription factor 7-like 2)/lymphoid enhancer-binding factor family, triggering the transcription of downstream Wnt target genes, including CYCLIND1, AXIN2, MYC, and RNF43 (which encodes zinc/ring finger protein 3, ZNRF3).25 Our study focused on the tumor inhibitory effect of DACH1 in glioma and revealed that the inhibitory function occurred via the Wnt/β-catenin pathway.
Patients and methods
Patients and samples
The data of patients with glioma were gathered from GSE16011 database (32 low-grade glioma tissues, 85 anaplastic glioma tissues, 159 glioma tissues, and eight normal brain tissues) and REMBRANDT database (99 low-grade glioma tissues, 84 anaplastic glioma tissues, 227 glioma tissues, and 21 normal brain tissues). Kaplan–Meier survival analysis was used to estimate the survival distributions. The long-rank test was used to assess the statistical significance between stratified survival groups with Graphpad Prism six statistical software.
Cell culture
The glioma cell lines U87 and U251 were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). All the cells were maintained in DMEM/F12 supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA) in a humidified atmosphere containing 5% CO2 at 37°C.
Reagents and antibodies
The following antibodies were used: anti-DACH1 antibody (ab223703), anti-GSK-3β antibody (ab93926), anti-p-GSK-3β antibody (ab131097), and anti-GAPDH antibody (ab8245) purchased from Abcam (Cambridge, MA, USA) and anti-β-catenin antibody (8480S) and anti-Cyclin D antibody (2922S) purchased from Cell Signal Technology (Danvers, MA, USA). The following fluorescent secondary antibodies were used: goat anti-rabbit IgG: Alexa Fluor 555 purchased from Thermo Fisher Scientific.
Plasmids and transfection
The DACH1 overexpression vector and the control PCDF vector were purchased from Abcam. DACH1-siRNA was purchased from Abcam. The identities of the constructs were confirmed by sequencing. The overexpression vectors and siRNA were transfected by using Lipofectamine 3000 (Thermo Fisher Scientific) reagent according to the manufacturer’s instructions.
Cell proliferation assays
We used the MTT method to determine cell proliferation. Herein, 3×103 cells were plated in 96-well plates per well. Upon analysis, 100 μL of medium containing 10 μL of 5 mg/mL MTT in PBS was added to each well, and the cells were incubated for another 4 hours at 37°C. The supernatant was then removed, and 150 μL of DMSO was added to each well. The absorbance of each sample was measured with a microplate reader at the wavelength of 570 nm. The surviving cells were measured every day for three consecutive days.
Migration and invasion assay
For the migration assay, cells (4×104 cells) were suspended in 300 μL of serum-free medium and seeded in the upper transwell chamber (8 μm pore size; BD Biosciences, San Jose, CA, USA). For the invasion assay, cells in serum-free medium were placed into the upper chamber of an insert coated with Matrigel (BD Biosciences). After incubation for 12 hours at 37°C, non-migrated or non-invaded cells on the upper membrane were removed with a cotton swab. Cells that had migrated or invaded through the membrane were stained with 0.1% crystal violet, and three fields were randomly selected for cell number counting.
Reverse transcription polymerase chain reaction (RT-PCR)
RNA was extracted from cultured cells or tissues using PrimeScript RT Reagent Kit (Takara [Beijing, China]). RT reactions were performed with the QuantiFast SYBR Green PCR Kit (Qiagen NV, Venlo, the Netherlands). The amplification protocol started with a 2-minute enzyme activation at 95°C, followed by 40 cycles of 95°C for 10 seconds, 60°C for 40 seconds. The melting curve analysis was used for the verification of the lack of non-specific products. Measurements were normalized for the expression of GAPDH.
Western blot analysis
Cell homogenates were lysed in radioimmunoprecipitation assay (RIPA) lysis buffer supplemented with 1% PMSF and 1% phosphatase inhibitor (Cell Signaling Technology). Total proteins were quantified with a BCA Protein Assay Kit (Beyotime, Nanjing, China) and subjected to 8% SDS-PAGE. The blots were blocked in bovine serum albumin (BSA) (5% w/v in PBS+0.1% Tween 20) for 1 hour at room temperature and immunostained with antibodies at 4°C overnight. Immunoreactive bands were visualized with chemiluminescent HRP substrate (EMD Millipore, Billerica, MA, USA) according to the manufacturer’s instructions. Data were normalized to those for β-actin.
Immunocytochemistry
U87 cells were plated at 1×104 cells/mL and cotransfected with DACH1 or WT for 24 hours. Then, the cells were fixed in 4% paraformaldehyde overnight and permeabilized with 0.1% Triton-X100 for 15 minutes at room temperature. The samples were blocked with 1% BSA in PBS for 1 hour at room temperature, incubated with primary antibody (β-catenin: 1:200; Cyclin D1: 1:200) overnight at 4°C, and then incubated with fluorescent secondary antibody for 2 hours at room temperature. Images were acquired with a Zeiss confocal microscope. Image analysis and algorithm generation were performed using the Image-Pro Plus 6.0 software (Media Cybernetics, Silver Spring, MD, USA). A total of 40–80 cells randomly selected from three or four replicated experiments were quantified.
Statistical analysis
Data were collected from at least three (n≥3) independent experiments. Two-tailed Student’s test or one-way ANOVA was performed using SPSS software (version 16, Armonk, New York, USA). Values are presented as mean±SEM. Significance of differences was accepted at P<0.05 (*P<0.05, **P<0.01, and ***P<0.001).
Results
DACH1 is downregulated and predicts a poor prognosis in glioma
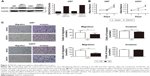
To confirm the expression level of DACH1 in glioma, the expression pattern of DACH1 was subsequently analyzed in the GSE16011 and REMBRANDT databases. In these two databases, the expression of DACH1 was downregulated in patients with glioma compared with that in low-grade glioma samples and normal brain tissues (Figure 1A and B).
Moreover, the expression level of DACH1 was coordinated with the glioma grade (one-way ANOVA P=0.0062 for GSE16011 and P<0.0001 for REMBRANDT, respectively). Furthermore, we compared the percentage survival of patients with glioma with high or low DACH1 expression and found that patients with high DACH1 expression survived significantly longer than patients with low DACH1 expression (Figure 1C and D). These results indicated that DACH1 may play an important role in glioma pathogenesis.
Overexpression of DACH1 inhibits glioma cell proliferation in vitro
In order to determine the precise mechanism of DACH1 in glioma pathogenesis, a DACH1 plasmid was constructed by cloning full-length DACH1 into the PCDNA3 vector, and the expression of DACH1 was verified by Western blotting and PCR (Figure 2A). The effects of DACH1 overexpression on cell proliferation and viability were first determined. A 3-day growth curve showed that the overexpression of DACH1 inhibited the proliferation of the U87 and U251 cell lines (Figure 2B). A CCK8 assay was performed to assess the role of DACH1 in U87 and U251 cell viability. The results of the CCK8 assay indicated that DACH1 significantly decreased the viability of U87 cells compared with that of control group after the transfection of DACH1 for 72 hours, but there were no significant differences at 24 hours or 48 hours (Figure S1A). However, in the U251 cell line, at all three time points, DACH1 inhibited the proliferation and cell viability (Figure S1B). Thus, DACH1 restricted the cell proliferation in both the U251 and U87 cell lines.
Overexpression of DACH1 inhibits the migration and invasion of glioma cell lines in vitro
To determine whether the overexpression of DACH1 plays a crucial role in the migration of a single cell, transwell assays were performed both in U87 and U251 cell lines. The assay results demonstrated that transfection with DACH1 resulted in lower migratory activities compared with that in the control. Compared to a reduction of 20% in the U87 cell line, the number of migrating cells decreased by more than 50% following the overexpression of DACH1 in the U251 cell line (Figure 2C). The inhibitory effect of DACH1 on invasion in the U87 cell line was lower compared to its effect on migration: a decrease of 10% by DACH1 compared to that by the vector. However, there was no significant difference in invasion due to DACH1 in the U251 cell line (Figure 2C). Thus, these results indicate that DACH1 facilitates migration in both U87 and U251 cells in vitro, but only facilitates invasion in U87 cells.
Mechanism by which DACH1 inhibits glioma proliferation
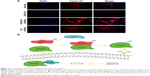
To determine the underlying mechanism by which DACH1 affects cell proliferation, the Wnt/β-catenin signaling pathway was analyzed in U87 cell line. Western blotting was used to measure the expression of the Wnt/β-catenin signaling pathway at the mRNA and protein level. RT-PCR indicates that overexpression of DACH1 decreases the mRNA expression of β-catenin and DACH1-siRNA reversed the downregulation of β-catenin (Figure 3A). Next, the protein expression of β-catenin was evaluated. The results demonstrated that the overexpression of DACH1 significantly reduced the level of β-catenin and that a higher expression of DACH1 was usually accompanied by a lower expression of β-catenin (Figure 3B). Cell cycle proteins downstream of the Wnt/β-catenin pathway might be responsible for the inhibition of proliferation in glioma. To address the underlying mechanisms, the protein levels of cell cycle elements were screened by Western blotting. When overexpressed, DACH1 significantly reduced the level of Cyclin D1 (Figure 3B). This result suggested that the decreased level of Cyclin D1 induced by DACH1 might play a key role in the negative regulation of proliferation and cell cycle progression in glioma. Thus, these findings indicate that DACH1 can suppress glioma cell growth by inhibiting Wnt signaling. Based on previous research, β-catenin is inhibited by phosphorylation of GSK-3β. Once p-GSK-3β binds to β-catenin, β-catenin is phosphorylated and restricts in the cytoplasm, inhibiting the transfer to nucleus. Thus, GSK-3β was examined via Western blotting following transfection. The results demonstrated that the ectopic expression of DACH1 significantly reduced the p-GSK-3β/GSK-3β ratio compared with those in the negative groups (Figure 3C). To confirm the effect of DACH1 on the Wnt/β-catenin signaling pathway, DACH1-siRNA was used to inhibit the DACH1 expression both in normal and overexpressing cell lines (Figure 3D). DACH1-siRNA upregulated the expression of β-catenin and Cyclin D1 (Figure 3E). Moreover, it upregulated the p-GSK/GSK ratio (Figure 3E). This result indicates that DACH1 upregulated the p-GSK-3β to inhibit β-catenin activation by restricting β-catenin in cytoplasm.
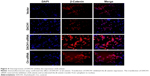
In order to further confirm these results, we performed an immunofluorescence assay to assess β-catenin and Cyclin D1 protein expression in U87 cell line. The results showed that the transfection of DACH1-plasmid decreased the fluorescence intensity of β-catenin in glioma cells (Figure 4). Meanwhile, DACH1 overexpression also decreased the Cyclin D1 protein expression (Figure 5A). The inhibition of DACH1 using DACH1-siRNA reversed the decrease in β-catenin and Cyclin D1, confirming the role of DACH1 in the inhibition of the Wnt/catenin pathway.
In conclusion, DACH1 promoted the proliferation and migration of U87 cells and activated the Wnt/β-catenin signaling pathway by directly regulating the corresponding target proteins (Figure 5B).
Discussion
The RDGN has been found to integrate multiple signaling pathways, and it is pivotal for the development of many organs and tissues such as eyes, muscles, ears, gonads, and the central nervous system.3 The dachshund gene, a component of the RDGN, encodes conserved, nuclear proteins that play a prominent role in controlling retinal cell fate determination and leg development in Drosophila. Dach/DACH, the mammalian homologs of the dachshund gene, were, respectively, isolated from the mouse and human genes. The loss of normal conserved RDGN gene is associated with tumorigenesis, especially in tumor initiation and progression.1–3 Specifically, DACH1 inhibits tumorigenesis as well as influences the malignant phenotype in carcinoma, including breast,4,10,14 retinal,5 lung,13 colorectal,26 and prostate cancers.8 Recent studies have revealed its opposing roles in breast cancer and prostate cancer via distinct mechanisms, demonstrating that the functions and regulatory mechanism of DACH1 in the progression of different tumors remain largely unclear.
Glioma is the most common central nervous system cancer, and it is characterized with high malignancy and recurrence. In this study, we compared the expression level of DACH1 in patients with malignant glioma from two databases. The expression of DACH1 was lower in glioma tissues and negatively correlated with the malignancy of glioma. These results indicated that DACH1 might function as an anti-glioma protein in glioma genesis. Thus, gain and loss of function studies were carried out to clarify the role of DACH1 in glioma. The ectopic expression of DACH1 inhibited the proliferation of glioma cells in vitro. Distant invasiveness is another malignant phenotype, which contributes to the high mortality of glioma, and we found that DACH1 attenuated the metastatic ability of glioma cell lines in vitro.27,28 However, the transfection of DACH1 could not inhibit the invasiveness of U251 cell lines, because U251 cell line exhibited cell-autonomous growth even with high DACH1 expression, there must be additional changes in oncogenes/tumor suppressor genes other than DACH1. All these results indicated that DACH1 functioned as a tumor suppressor in glioma, which agreed with the results of recent studies on DACH1 in breast and colon cancers.4,29
We then determined the possible mechanisms of the tumor inhibition effect of DACH1 in glioma. The Wnt/β-catenin signaling pathway has been demonstrated to play a critical role in modulating diverse processes, including cell proliferation, survival, differentiation, metastasis and polarity, specification of cell rate, self-renewal in stem cells, and maintenance of stem cell properties.19,30 Watson et al31 reported that canonical Wnt/β-catenin signaling was a novel genetic driver of Schwann cell tumor development and progression, and the downregulation of this pathway was sufficient to reduce the tumorigenic phenotype of human malignant peripheral nerve sheath tumors. Radulescu et al32 demonstrated that Wnt/β-catenin signaling pathway activation can act as an initiator in gastric neoplasia. Abnormal activation of Wnt/β-catenin signaling has been recognized as an important mechanism of glioma initiation and progression. Our results indicated that the ectopic expression of DACH1 inhibited canonical Wnt signaling activity in glioma and that the Wnt target functional gene Cyclin D1 was downregulated at the protein level. These results demonstrated that DACH1 suppressed glioma progression by inhibiting the Wnt/β-catenin pathway. We then investigated the possible mechanism by which DACH1 inhibits the Wnt/β-catenin pathway. Previous studies have suggested that the protein level of β-catenin is tightly controlled by the destruction complex composed of APC AXIN, ICAT, and GSK-3β33–36 through which β-catenin is finally phosphorylated by GSK-3β at Ser33 and Ser37, leading to its proteolytic degradation.20,30,31 We found that DACH1 reduced the protein level of β-catenin by reducing the phosphor-GSK-3β level. Accumulating lines of evidence indicate that GSK-3β mediated β-catenin phosphorylation is the key step in generating the β-TrCP-binding site for the subsequent degradation.32,37,38 Collectively, our data demonstrated that DACH1 exerted its inhibitory function by suppressing the Wnt/β-catenin pathway through promoting the phosphorylation degradation of β-catenin in a GSK-3β-dependent manner.
Conclusion
The results of this study indicate for the first time that DACH1 is a tumor suppressor in glioma that acts by inhibiting the Wnt/β-catenin pathway. These findings add to our current knowledge of the tumorigenesis process of glioma. The overexpression of DACH1 may inhibit glioma proliferation and distant invasiveness, possibly providing a new therapeutic site to restrict glioma genesis and recurrence.
Acknowledgments
All the authors declare that no support, financial or otherwise, has been received from any organization that may have an interest in the submitted work and that there are no other relationships or activities that could appear to have influenced the submitted work.
Disclosure
The authors report no conflicts of interest in this work.
References
Wu W, Ren Z, Li P, et al. Six1: a critical transcription factor in tumorigenesis. Int J Cancer. 2015;136(6):1245–1253. | ||
Jemc J, Rebay I. The eyes absent family of phosphotyrosine phosphatases: properties and roles in developmental regulation of transcription. Annu Rev Biochem. 2007;76:513–538. | ||
Popov VM, Wu K, Zhou J, et al. The Dachshund gene in development and hormone-responsive tumorigenesis. Trends Endocrinol Metab. 2010;21(1):41–49. | ||
Wu K, Li A, Rao M, et al. DACH1 is a cell fate determination factor that inhibits cyclin D1 and breast tumor growth. Mol Cell Biol. 2006;26(19):7116–7129. | ||
Chu Q, Han N, Yuan X, et al. DACH1 inhibits cyclin D1 expression, cellular proliferation and tumor growth of renal cancer cells. J Hematol Oncol. 2014;7:73. | ||
Wu K, Katiyar S, Li A, et al. Dachshund inhibits oncogene-induced breast cancer cellular migration and invasion through suppression of interleukin-8. Proc Natl Acad Sci U S A. 2008;105(19):6924–6929. | ||
Zhou J, Wang C, Wang Z, et al. Attenuation of Forkhead signaling by the retinal determination factor DACH1. Proc Natl Acad Sci U S A. 2010;107(15):6864–6869. | ||
Wu K, Katiyar S, Witkiewicz A, et al. The cell fate determination factor dachshund inhibits androgen receptor signaling and prostate cancer cellular growth. Cancer Res. 2009;69(8):3347–3355. | ||
Popov VM, Zhou J, Shirley LA, et al. The cell fate determination factor DACH1 is expressed in estrogen receptor-alpha-positive breast cancer and represses estrogen receptor-alpha signaling. Cancer Res. 2009;69(14):5752–5760. | ||
Wu K, Jiao X, Li Z, et al. Cell fate determination factor Dachshund reprograms breast cancer stem cell function. J Biol Chem. 2011;286(3):2132–2142. | ||
Wu K, Chen K, Wang C, et al. Cell fate factor DACH1 represses YB-1-mediated oncogenic transcription and translation. Cancer Res. 2014;74(3):829–839. | ||
Yamada Y, Arao T, Gotoda T, et al. Identification of prognostic biomarkers in gastric cancer using endoscopic biopsy samples. Cancer Sci. 2008;99(11):2193–2199. | ||
Chen K, Wu K, Cai S, et al. Dachshund binds p53 to block the growth of lung adenocarcinoma cells. Cancer Res. 2013;73(11):3262–3274. | ||
Chen K, Wu K, Gormley M, et al. Acetylation of the cell-fate factor dachshund determines p53 binding and signaling modules in breast cancer. Oncotarget. 2013;4(6):923–935. | ||
Powe DG, Dhondalay GK, Lemetre C, et al. DACH1: its role as a classifier of long term good prognosis in luminal breast cancer. PLoS One. 2014;9(1):e84428. | ||
Nan F, Lu Q, Zhou J, et al. Altered expression of DACH1 and cyclin D1 in endometrial cancer. Cancer Biol Ther. 2009;8(16):1534–1539. | ||
Watanabe A, Ogiwara H, Ehata S, et al. Homozygously deleted gene DACH1 regulates tumor-initiating activity of glioma cells. Proc Natl Acad Sci U S A. 2011;108(30):12384–12389. | ||
Qu B, Liu BR, du YJ, Yj D, et al. Wnt/β-catenin signaling pathway may regulate the expression of angiogenic growth factors in hepatocellular carcinoma. Oncol Lett. 2014;7(4):1175–1178. | ||
Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13(1):11–26. | ||
Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8(5):387–398. | ||
Yuzugullu H, Benhaj K, Ozturk N, et al. Canonical Wnt signaling is antagonized by noncanonical Wnt5a in hepatocellular carcinoma cells. Mol Cancer. 2009;8:90. | ||
van Amerongen R, Mikels A, Nusse R. Alternative Wnt signaling is initiated by distinct receptors. Sci Signal. 2008;1(35):re9. | ||
Dahmani R, Just PA, Perret C. The Wnt/β-catenin pathway as a therapeutic target in human hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2011;35(11):709–713. | ||
Hart M, Concordet JP, Lassot I, et al. The F-box protein beta-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr Biol. 1999;9(4):207–211. | ||
de Lau W, Peng WC, Gros P, Clevers H. The R-spondin/Lgr5/Rnf43 module: regulator of Wnt signal strength. Genes Dev. 2014;28(4):305–316. | ||
Yan W, Wu K, Herman JG, et al. Epigenetic regulation of DACH1, a novel Wnt signaling component in colorectal cancer. Epigenetics. 2013;8(12):1373–1383. | ||
Charafe-Jauffret E, Ginestier C, Iovino F, et al. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin Cancer Res. 2010;16(1):45–55. | ||
Mego M, Mani SA, Cristofanilli M. Molecular mechanisms of metastasis in breast cancer – clinical applications. Nat Rev Clin Oncol. 2010;7(12):693–701. | ||
Cao J, Yan XR, Liu T, et al. MicroRNA-552 promotes tumor cell proliferation and migration by directly targeting DACH1 via the Wnt/β-catenin signaling pathway in colorectal cancer. Oncol Lett. 2017;14(3):3795–3802. | ||
Jang GB, Kim JY, Cho SD, et al. Blockade of Wnt/β-catenin signaling suppresses breast cancer metastasis by inhibiting CSC-like phenotype. Sci Rep. 2015;5:12465. | ||
Watson AL, Rahrmann EP, Moriarity BS, et al. Canonical Wnt/β-catenin signaling drives human Schwann cell transformation, progression, and tumor maintenance. Cancer Discov. 2013;3(6):674–689. | ||
Radulescu S, Ridgway RA, Cordero J, et al. Acute WNT signalling activation perturbs differentiation within the adult stomach and rapidly leads to tumour formation. Oncogene. 2013;32(16):2048–2057. | ||
Li X, Zhu F, Jiang J, et al. Synergistic antitumor activity of withaferin A combined with oxaliplatin triggers reactive oxygen species-mediated inactivation of the PI3K/AKT pathway in human pancreatic cancer cells. Cancer Lett. 2015;357(1):219–230. | ||
Wang CZ, Zhang CF, Chen L, Anderson S, Lu F, Yuan CS. Colon cancer chemopreventive effects of baicalein, an active enteric microbiome metabolite from baicalin. Int J Oncol. 2015;47(5):1749–1758. | ||
Ganguly KK, Pal S, Moulik S, Chatterjee A. Integrins and metastasis. Cell Adh Migr. 2013;7(3):251–261. | ||
Herszényi L, Hritz I, Lakatos G, Varga MZ, Tulassay Z. The behavior of matrix metalloproteinases and their inhibitors in colorectal cancer. Int J Mol Sci. 2012;13(10):13240–13263. | ||
Li-Weber M. Targeting apoptosis pathways in cancer by Chinese medicine. Cancer Lett. 2013;332(2):304–312. | ||
Tian J, He H, Lei G. Wnt/β-catenin pathway in bone cancers. Tumour Biol. 2014;35(10):9439–9445. |
Supplementary material
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.