Back to Journals » International Journal of Nanomedicine » Volume 17
Cytotoxicity and Genotoxicity of Polystyrene Micro- and Nanoplastics with Different Size and Surface Modification in A549 Cells
Authors Shi X, Wang X, Huang R , Tang C, Hu C, Ning P, Wang F
Received 9 July 2022
Accepted for publication 16 September 2022
Published 24 September 2022 Volume 2022:17 Pages 4509—4523
DOI https://doi.org/10.2147/IJN.S381776
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Xiaorui Shi,1 Xinan Wang,1 Rong Huang,1 Chu Tang,1 Chong Hu,1 Pengbo Ning,1 Fu Wang1– 3
1Engineering Research Center of Molecular and Neuro Imaging, Ministry of Education, School of Life Science and Technology, Xidian University, Xi’an, 710071, People’s Republic of China; 2Xianyang Key Laboratory of Molecular Imaging and Drug Synthesis, School of Pharmacy, Shaanxi Institute of International Trade & Commerce, Xianyang, Shaanxi, 712046, People’s Republic of China; 3School of Basic Medical Sciences, Xi’an Jiaotong University, Xi’an, 710061, People’s Republic of China
Correspondence: Fu Wang; Pengbo Ning, Email [email protected]; [email protected]
Background: Micro- and nano-sized plastics (MPs and NPs) have become an environmental issue of global concern due to their small size, strong bio-permeability and high specific surface area. However, few studies have assessed the effect of polystyrene MPs and NPs on human lung cells. In this research, we evaluated the cytotoxicity and genotoxicity of polystyrene (PS) MPs and NPs with different sizes (2 μm and 80 nm) and surface modification (carboxy and amino functionalized polystyrene, pristine polystyrene) in A549 cells.
Methods: The zeta potential and hydrodynamic particle size of five types of PS plastic solutions were measured by dynamic light scattering, and their morphology and degree of aggregation were observed by scanning electron microscopy. After incubation of the PS plastics with A549 cells, the uptake and toxicity of the cells were assessed by fluorescence microscopy, laser scanning confocal microscopy, flow cytometry, MTT, micronucleus formation assay, and reactive oxygen species.
Results: The cytotoxicity and genotoxicity of A549 cells caused by nano-level PS is more serious than that of micro-level. Compared with unmodified PS-NPs, more surface-functionalized PS-NPs were found inside the cells, especially the accumulation of PS-NH2. Cell viability and the induction of micronuclei (MN) are appreciably impacted in a dose-dependent way. Compared with pristine PS-NPs, functionalized PS-NPs showed stronger cell viability inhibitory ability, and induced more MN scores.
Conclusion: This study shows that the intrinsic size properties and surface modification of PS plastics, the interaction between PS plastics and the receiving medium, intracellular accumulation are critical factors for evaluating the toxicological influences of PS plastics on humans.
Keywords: microplastics, nanoplastics, surface functionalization, cytotoxicity, genotoxicity, oxidative damage
Graphical Abstract:
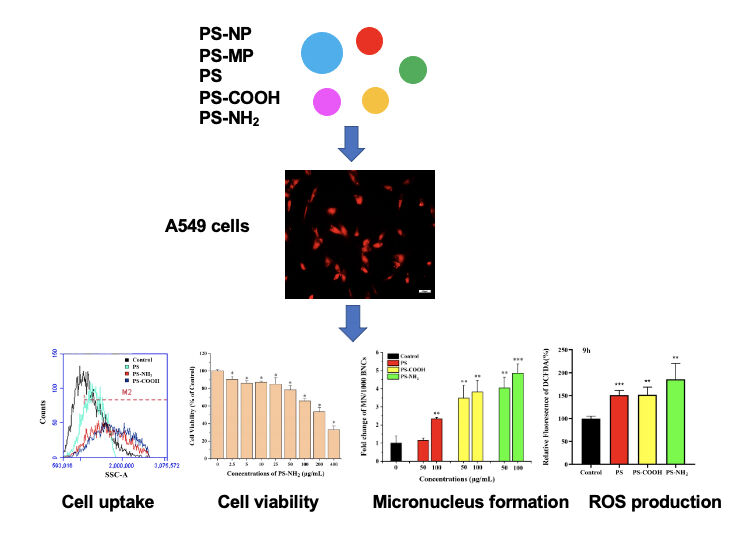
Synopsis
This study provides evidences for the behavior and effect of micro- and nanoplastics on human lung cells, and may contribute to a better understanding of their potential harm to environmental organisms, so as to avoid the risk of some diseases.
Introduction
Plastic, a high-molecular compound made of resin as the main component, can be made up of fillers, stabilizers, colorants, plasticizers and other additives through addition polymerization or polycondensation reaction. Common plastics include polypropylene (PP), polystyrene (PS) and polyethylene (PE).1 Since 1950, plastics have made a significant contribution to the development of society, with production of synthetic polymers increasing from an initial 1.7 million tons in 1959 to 280 million tons in 2016. With the increasing use of plastics worldwide, the current annual output has exceeded 380 million tons.2,3 However, most of the plastic waste has not been properly disposed of in time. Due to economic and social factors the global recycling rate of plastic remains low, creating a severe environmental pollution problem for the global. Meanwhile, own to the nature of material, increased in production, processing and low biodegradation rates, resulting in plastics that are hardly decompose and stay in the atmosphere for a long period of time. When exposed to environmental conditions like ultraviolet radiation, wave action, physical, chemical and biological processes, the bulk plastic materials are prone to aging and disintegration, leading to the formation of plastic debris in large quantities, ranging from a few meters to micro- or nanometers.4 Microplastics (MPs) refer to plastics with the size ranged from 1 μm to 5 mm, while nanoplastics (NPs) with size below 1 μm.5 As emerging pollutants, MPs and NPs are easily absorbed and have serious effects on biological functions due to their large specific surface area, small size and powerful biological permeability.6
Although it is generally considered that environmental MPs and NPs are mainly concentrated in water pollution, such as serious marine pollution, they are actually everywhere.7 For example, studies about terrestrial environments have found that microplastics can change the physical properties of the soil and be distributed and mobilized into the aquatic environment and air by wind.8,9 Small plastic fragments can easily escape from the landfill with the wind dispersal of debris into the air, giving rise to quite a number of air pollution incidents. Observational researches reported that inhalation of plastic particles or fibers, especially through occupational exposure, caused breathing difficulties for workers due to respiratory and interstitial inflammatory reactions.10 It has been confirmed in existing epidemiological studies that inhalation of particulate matter from air pollution, leads to a gradual increase in the morbidity and mortality of cardiovascular diseases and respiratory-related diseases in adults and children.11,12 Studies have found that microplastic fibers taken from human lung samples may be inextricably linked to lung-related malignant tumors.13 At present, microplastics have been found in human feces, colon specimens, placenta and even blood. It has been confirmed that microplastics in the feces of patients with inflammatory bowel disease are 1.5 folds higher than healthy people, and microplastics that entered the bloodstream will flow through the whole body with blood flow.14,15 Recent evidence indicated that humans constantly inhale and ingest microplastics by bottled water, food bags, cosmetics, and air exposure. However, whether these contaminants pose a substantial risk to human health is far from understood.16
As a common packaging thermoplastic, polystyrene (PS) is rarely recycled due to its light weight and low residual value. The resulting polystyrene micro- and nanoplastics (PS-MPs and PS-NPs) are also difficult biodegraded or photodegraded into the biogeochemical cycle. These plastics are now a major marine drift and cause damage to the digestive system and even the whole body of marine organisms that ingest them.17 Up to now, there are few studies on the impact of polystyrene microplastics and nanoplastics on terrestrial organisms, especially toxicological studies on the respiratory system. The A549 cell line is a stable cell line originally established by lung cancer tissue transplantation. Some substances can be spread through the alveoli, so it is very similar to human alveolar type II epithelial cell. It is often used as an ideal representative for in vitro studies of pharmacology, toxicology, lung tissue metabolism and lung injury.18,19
In this study, the effects of micro- (2 μm) and nano- (80 nm) plastics and different functionalized PS-NPs (PS, PS-NH2 and PS-COOH) on internalization, genotoxicity and cytotoxicity of human alveolar epithelial cells in vitro were investigated and compared. Using A549 cells as a surrogate model for simulated alveolar cells, and the cellular oxidative response was also evaluated. The evaluation of these aims was achieved by flow cytometry, laser confocal microimaging, MTT, micronucleus formation, and reactive oxygen species detection. The outcomes of our study could provide argument regarding the size and surface functionalization effects on the fate and behavior of PS plastics in alveolar epithelial cells, which might offer new opinion on the health risk assessment of PS-MPs and PS-NPs. Our results also could provide evidence for the behavior and effect of PS-MPs and PS-NPs in human lung cells, and may contribute to a better understanding of their potential harm to organisms, so as to avoid the risk of some diseases.
Materials and Methods
Polystyrene Plastics and Chemicals
Polystyrene micro- (PS-MPs) and nanoplastics (PS-NPs) with different diameters of 2 μm and 80 nm were purchased from Xi’an Ruixi Biological Technology Co., Ltd. Three types of red fluorescent polystyrene nanoplastics (PS-NPs) dispersions (excitation/emission wavelengths: 620 nm/680 nm) were obtained from the Tianjin Base Line Chrom Tech Research Centre (Tianjin, China). They were pristine polystyrene (PS), amino functionalized polystyrene (PS-NH2) and carboxy functionalized polystyrene (PS-COOH) nanoplastics with an average size of 80 nm. All micro- and nanoplastics mentioned were uniformly spherical. 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-Diphenyltetrazolium Bromide (MTT) (T100896) was purchased from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Reactive Oxygen Species (ROS) detection kit (The 6-carboxy-2’,7’ dichloro-dihydrofluorescein diacetate (DCFH-DA)) was obtained from Beyotime Biotechnology (Shanghai, China). Cytochalasin B (C6762) was purchased from Sigma (New Jersey, America).
Physicochemical Characterization of PS-MPs/NPs Dispersions
As a non-invasive method, dynamic light scattering (DLS) has been widely used to measure the shape, size and particle size distribution of suspended particles or polymers in solution.20 Five types of PS plastics were separately suspended in different media (double-distilled water (ddH2O), DMEM with serum or without) to get a final concentration of 50 μg/mL. Subsequently, the solutions were rapid ultrasound (40 kHz) for 5 minutes, the zeta potential and hydrodynamic particle size of them were measured via DLS using a Zetasizer Nano-ZS (Malvern Instruments, Malvern, UK). PS-MPs (2 μm) and PS-NPs (80 nm) separately suspended in ddH2O were sonicated for 5 minutes, a clean thin copper sheet was glued to the conductive adhesive, the solutions were dripped onto the copper sheet of a small amount by several times, waiting for the solvent to evaporate and dry completely, gold spraying was performed followed by scanning electron microscopy (SEM, Zeiss Sigma 300, Germany) to evaluate their morphology and degree of aggregation.
Cell Culture
Adenocarcinomic human alveolar basal epithelial cells (A549 cells) were purchased from the National Collection of Authenticated Cell Cultures (Shanghai, China), and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Hyclone, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, USA) and 100 U/mL penicillin/streptomycin (Hyclone, USA) at 37°C under a humidified atmosphere with 5% CO2.
Cellular Uptake of PS-MPs/NPs Detection
A549 cells were cultured and incubated with three kinds of fluorescent PS-NPs (pristine PS, PS-COOH and PS-NH2) (200 μg/mL) separately for 24 hours, fluorescence microscope (LEICA DMI 4000B, Leica, Germany) and laser scanning confocal microscope (FV3000, Olympus, Japan) were used to observe the uptake of the above PS-NPs by A549 cells visually.21 Based on the principle of flow cytometry, cell surface or internal structural characteristics like cellular granularity can be indicated by the side scattered (SSC) lights.22 Thus, flow cytometry was used to reflect the uptake of particles through the increase in cellular granularity. A549 cells were exposed to five kinds of PS-MPs/NPs of increasing concentrations (0, 5, 25, 100 μg/mL) separately for 24 hours. After treatment, cells were rinsed three times with Phosphate buffer saline (PBS, Gibco, USA), detached enzymatically, subsequently resuspended in PBS (100 μL). The cellular granularity and cellular fluorescence intensity were determined by flow cytometry (BD Biosciences, New Jersey, USA) outfitted with an air-cooled laser providing 15mW power at 488 nm to reflect the accumulation of corresponding PS-MPs/NPs in A549 cells. For all experiments, signals from 10,000 particles were collected and data were analyzed with BD Accuri C6 software (BD Biosciences, New Jersey, USA).
Cell Viability
The effects of PS-MPs/NPs on A549 cells viability was measured by the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) colorimetric assay.23 Working concentrations of five types of PS-MPs/NPs were compounded by diluting the original solutions (10 mg/mL) with DMEM (complete growth medium), then rapidly sonicated (40 kHz) for 10 minutes before use. Cells were cultured in 96-well plates and pre-cultured with 100 μL complete growth medium for 24 hours, treated with various concentrations of the above sonicated solutions (2.5, 5, 10, 25, 50, 100, 200 and 400 μg/mL) separately. After 24 hours incubation, the PS-MPS/NPs solutions were removed, cells were washed with PBS for three times, 100 μL of MTT working solution was added to each well, cells were incubated for 4 hours at 37 °C. MTT solution was replaced with 100 μL DMSO (MP Biomedicals, USA) for a further 30 mins incubation. The number of living cells was measured by the absorbance at 490 nm using a microplate reader (Molecular Devices, Sunnyvale, CA). Tests were performed in triplicate.
Assays for Detecting the Genotoxicity
To verify the genotoxicity caused by five kinds of PS-MPs/NPs, the micronucleus (MN) formation assay was employed here.24 Briefly, the A549 cells were separately incubated with different concentrations of PS-MPs/NPs (0, 50, 100 μg/mL) for 24 hours, washed with PBS three times, and re-seeded onto culture dishes (30mm diameter). The degree of micronuclei form was detected by the cytokinesis-block technique exploited by Michael and Alexander.25 The MN in the binucleated cells were distinguished by the criterion of Fenech using a fluorescence microscope (Olympus). At least 1000 binuclear cells were selected to evaluate the formation of micronuclei in each group, and the number of MN in the untreated group was normalized, the final result was showed as fold increase compared with the untreated group.
Reactive Oxygen Species Analysis
The production of intracellular reactive oxygen species (ROS) was measured by a ROS detection kit (DCFH-DA). Briefly, exponent tially growing A549 cells were seeded in 96-well plate at a density of 104 cells per well and continuously incubated for 24 hours. After individual incubation with 80 nm PS-NPs and 2 μm PS-MPs (100, 200 and 400 μg/mL) for 6 hours, or with three kinds of 80 nm fluorescent PS-NPs (pristine PS, PS-COOH and PS-NH2) of 100 μg/mL for 6, 9 and 24 hours (100 mM H2O2 treatment for 15 mins served as the positive control), all of the cells were washed thrice with D-Hank’s buffer added with 1% fetal bovine serum (FDBS), stained with CM-H2DCFDA ROS probe of 20 μM for 1 hour at 37 °C, washed again with icy test medium to wipe off spare dye. Fluorescence intensity was measured using a fluorescence spectrophotometer (GLOMAX® NAVIGATOR, Promega, USA) with an excitation/emission wavelength of 495/515 nm. The relative rise in ROS content were showed as fold increases related to control.
Statistical Analysis
All data were expressed as mean ± standard deviations (SDs). Using Origin 8.0 software for Student’s t-test and one-way analysis of variance (ANOVA) between the different groups. The statistically significant differences indicate a p-value of less than 0.05.
Result
Physicochemical Characterization of PS-MPs/NPs Aqueous Dispersions
The surrounding media of PS-MPs or PS-NPs affect the biological effects and bioavailability of particles to a large degree. We measured the zeta potential and hydrodynamic particle size of five kinds of PS plastics firstly. From Supplementary Tables S1 and S2, it was shown that all of the PS-MPs and PS-NPs suspended in different solutions were all negatively charged. The zeta potential values of 80 nm and 2 μm PS plastics in distilled water were −38.50±10.929mV and −31.23±1.415mV, respectively, indicating good dispersibility of both PS plastics. This result was confirmed by the SEM images (Figure S1). However, when dispersed in DMEM medium, especially in the FBS-containing medium, the zeta potential values of five PS-MPs and PS-NPs increased in varying degrees, together with the increase of PDI values, which indicated that the dispersion of five kinds of plastics had become particularly unstable. The average hydrodynamic size determined by cumulant analysis also confirmed the above outcome by showing that all plastics average size increased dramatically in DMEM medium (with or without FBS) when compared with the data from distilled water. It is worth noting that with the presence of fetal bovine serum in DMEM medium, the average size of all plastics decreased to a certain extent. For example, with the addition of FBS, the average size of 80 nm PS-MPs decreased from 203.0 ±3.959 to 123.9±1.852 nm, while 2 μm PS-MPs decreased from 3494±406.8 to 2423±424.1 nm.
Cellular Uptake of PS-MPs/NPs
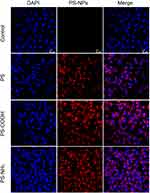
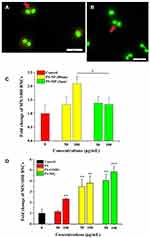
Flow cytometry analysis was performed to evaluate the internalization efficiency of PS-MPs and PS-NPs by A549 cells. The cellular granularity revealed the amounts of PS-MPs accumulated by cells. The intracellular localization and cellular amount always determine the biological responses of particles with low degradability and solubility (such as PS-NPs).26 In the present study, although the intracellular granularity of A549 cells exposed to 80 nm PS-NPs was slightly higher than control group (Figure 1A), both of the 80nm PS-NPs and 2 μm PS-MPs were not found to accumulate in the A549 cells by a dose-dependent manner (Figure 1B and C). As shown in Figures 2 and S2, laser scanning confocal microscope and fluorescence microscope were used to monitor the uptake of three types of red fluorescent PS-NPs by A549 cells. In comparison, regardless of the surface functionalization, widely distributed red fluorescence was uniformly observed when cells were co-incubated with PS, PS-NH2 and PS-COOH NPs respectively, which indicated that the three kinds of PS-NPs had indeed entered the A549 cells and localized intracellularly, particularly in the cytoplasm. Flow cytometry analysis was also used to study the internalization potency of these PS-NPs by A549 cells. The average fluorescence intensity and cellular granularity pointed out the number of polystyrene nanoplastics internalized by cells. As shown in Figure 1D–F, three PS-NPs with different modification were showed to accumulate in the A549 cells by a dose-dependent way. In addition, it has been proven that the biological behavior of NPs can be changed by their surface functionalization.27 In the present study, it was found that the average fluorescence intensity and intracellular granularity of A549 cells incubated with PS-NH2 or PS-COOH, showed obvious increase when compared with PS treated group (Figure 1G and H). In particular, the PS-NH2 treated cells showed the maximum increase in cellular fluorescence intensity (7.03×105), which was nearly twice that of the PS group (4.11×105). It was indicated that surface functionalization of polystyrene nanoplastics promoted the internalization process of A549 cells to them.
Cell Viability of PS-MPs/NPs on A549 Cells
As shown in Figure 3, in comparison, 80 nm PS-NPs (2.5 to 400 μg/mL) reduced the cell viability in a dose-dependent way. A 80 nm PS-NPs at a concentration of 25 μg/mL decreased the cell viability to 93.5 ± 0.93%, while at higher exposure doses of 50 to 400 μg/mL, the cell viability was reduced to 89.3 ± 2.91%, 85.9 ± 3.20%, 73.2± 3.00% and 55.0 ± 5.77%, respectively (Figure 3A). By contrast, there was no virtual difference on the cell viability between 2 μm PS-MPs treated group and the control under all concentrations, indicating that 2 μm PS-MPs had almost no cytotoxicity in A549 cells (Figure 3B). The results demonstrated that PS-NPs with smaller size (80 nm), triggered more cytotoxic effect than their larger ones. As shown in Figure 4, compared to the control, all three kinds of red fluorescent PS-NPs (2.5 to 400 μg/mL) decreased the cell viability via a dose-dependent manner. The cell viability of PS below 25 μg/mL had almost no effects. A 100 μg/mL PS decreased the cell viability to 83.9 ± 3.62%, while at higher exposure doses (200 to 400 μg/mL), the cell viability was greatly reduced to 71.4 ± 7.48% and 48.7 ± 9.59%, respectively (Figure 4A). Compared to the PS, PS-NH2 and PS-COOH demonstrated more serious cytotoxicity in A549 cells. When 50, 100 and 200 μg/mL PS-COOH were used to co-incubate with the cells, the cytotoxicity was 1.77, 1.67, and 1.33 folds higher than that of the same concentration of PS, respectively (Figure 4B). When 50, 100, 200 and 400 μg/mL PS-NH2 cells were exposed to the cells, the reduction in cells viability was 2.49, 2.11, 1.62 and 1.30 folds superior than that of same concentration of PS (Figure 4C). The results demonstrated that surface functionalized nanoplastics, especially the PS-NH2, seemed to trigger the most severe cytotoxic effect among three of the PS-NPs.
Effect of PS-MPs/NPs on A549 Cells Genotoxicity
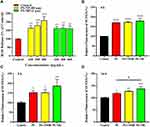
To furthermore research the effect of five kinds of PS plastics on the genotoxicity of A549 cells, the formation of micronuclei (MN) was determined. The average background value of MN proportion was 7 ± 2 per 103 binucleated cells (Figure 5A and B). As shown in Figure 5C, in comparison with the control group, treatment of cells with 80 nm PS-NPs and 2 μm PS-MPs at a dose of 50 μg/mL led to the increase in MN fractions by 1.33 ± 0.269 and 1.38 ± 0.243 folds, respectively. When cells were exposed to 100 μg/mL PS-NPs (80 nm), compared with the control the folds of MN fractions were statistically increased to 2.10 ± 0.178. It is indicated that the induction of micronuclei in cells treated with 80 nm PS-NPs was dramatically enhanced by a dose-dependent way. Compared the effects of above two kinds of PS plastics, it was shown that 80 nm PS-NPs at higher concentration induced more MN production in A549 cells, suggesting that 80 nm PS-NPs were more genotoxic to human A549 cells than 2 μm PS-MPs, which was in line with the cell viability results. To further study the role of surface modification of PS-NPs on the genotoxicity of A549 cells, the formation of micronuclei (MN) was also used. As shown in Figure 5D, in comparison to control, treatment of cells with 50 μg/mL PS, PS-NH2 and PS-COOH led to the increase in MN fractions by 1.14 ± 0.143, 3.48 ± 0.705 and 4.05 ± 0.577 folds, respectively. When cells were treated by PS, PS-NH2 and PS-COOH at 100 μg/mL, the folds of MN fractions were statistically increased to 2.33 ± 0.0825, 3.81 ± 0.644 and 4.86 ± 0.515, respectively. It is indicated that the induction of micronuclei in cells exposed to PS, PS-NH2 and PS-COOH was significantly enhanced through a dose-dependent way. Meanwhile, compared the roles of the three kinds of PS-NPs, it was shown that PS-NH2 and PS-COOH induced more MN production in A549 cells than PS. The above results demonstrated that surface functionalized nanoplastics, especially the PS-NH2, were more genotoxic to human A549 cells in comparison to the unfunctionalized PS, which was consistent with the conclusion of the cell viability experiment.
PS-MPs/NPs Exposure Induce Oxidative Stress
The reactive oxygen species (ROS) play a vital function in mediating the cellular oxidative damage and toxic response to many xenobiotic substrates. Therefore, the content of intracellular ROS was detected. A fluorescent probe particular to ROS revealed that individual incubation of cells with 80 nm PS-NPs and 2 μm PS-MPs produced different levels of ROS accumulation in A549 cells (Figure 6A). The increase in ROS content when cells were exposed to 80 nm PS-NPs at doses of 100, 200, 400 μg/mL for 6 hours were 1.64 ± 0.123, 1.79 ± 0.154 and 2.10 ± 0.159 folds higher than that of the control, respectively. In comparison, 2 μm PS-MPs at 100, 200 and 400 μg/mL increased the ROS production of A549 cells by around 1.60 folds, not showing the similar dose-dependent effect as 80 nm PS-MPs. These results also revealed that 80 nm PS-MPs triggered greater oxidative damage than 2 μm PS-MPs, which was in accordance with the conclusion of cell viability and genotoxicity results. A fluorescence probe specific for ROS revealed that exposure to cells with three kinds of differently surface modified PS-NPs produced different levels of ROS accumulation in A549 cells, also showing a time-dependent manner. The increase in ROS content when cells were exposed to PS, PS-COOH and PS-NH2 for 6 hours were 1.70, 1.72 and 1.79 folds upper than the control (Figure 6B). However, the degree of increase in ROS content was reduced with time extension (Figure 6C and D), indicating that enhancement of ROS production was negatively related to the treating time. Above all, our results revealed that at a higher incubation dose of 100 μg/mL, PS-NH2 invited the most serious oxidative damage compare with PS or PS-COOH, which was in accordance with the cell viability and genotoxicity results. What is more, ROS amount could have been generated in a relatively short period of time, but would decrease along with the time prolongation.
Discussion
The transport, fate, and behavior of PS-MPs or PS-NPs are key factors influencing their bioavailability/uptake/toxicity consequence. This has been confirmed by previous studies that it is not only dependent on the intrinsic physicochemical properties and surface modifications of PS-MPs/PS-NPs (eg hydrophobicity, charge, functional groups and so on),28,29 but also on the interactions between PS-MPs/PS-NPs and different substances present in the receiving medium.30 Based on this, the size distribution and zeta potential of five kinds of PS plastics suspended in different solutions were measured. The zeta potential in ddH2O showed that all of the PS plastics were negatively charged, together with the low polydispersity index (PDI), indicating that PS plastic particles tended to repel each other and that aggregation would be prevented. Indeed, both PS-MPs and PS-NPs, especially 80 nm PS-NPs, only aggregated slightly into larger complexes in ddH2O, exhibiting relatively good monodispersity. This is consistent with the findings of Sun et al who found that microplastics were more stable in freshwater due to the structural layer forces and strong Brownian motion, but preferentially aggregated while transport through eutrophication or brackish water.31 The dispersion of PS-MPs or PS-NPs is dependent on numerous factors such as pH, ionic strength, the presence of organic substances, natural colloids, as well as particle size and surface chemistry.32– The aggregation and instability of PS-MPs and PS-NPs in DMEM media may be attributed to the screening effect of particle surface charges due to the high ionic strength existed in the media, and also by other compounds or proteins in the surroundings.35 Wang et al investigated the stability and aggregation of PS-NPs under different hydrochemical conditions and founded the rapid aggregate of PS-NPs in media with high ionic strength or high natural organic matter.36 Interestingly, we also found that the presence of FBS remarkably reduced the average size of five PS plastics to a certain extent in the cell culture medium. It is well known that in biological fluids, particles can adsorb proteins and other biomolecules on their surface, forming a so-called protein corona.37 The decrease in plastic particle size was probably a consequence of the electrostatic repulsion between the protein corona.38 In conclusion, when PS-MPs and PS-NPs characterization is performed in complex media, the media selection and related parameters should always be considered, since parameters like ionic strength, pH value, salt and the existence of other proteins play important roles in studying the plastic particle behavior and their potential toxicity.
The physicochemical properties of PS-MPs or PS-NPs and their large surface area provide convenience for their interaction with cell membranes, which subsequently leads to the internalization of PS-MPs or PS-NPs by the cells. The uptake process is closely related to the fate of internalized plastic particles, also responsible for their consequent cytotoxicity.39 Therefore, it is crucial to determine the routes of uptake and further intracellular trafficking as well as the fate of the PS-MPs and PS-NPs. In this study, only 80 nm PS-MPs were observed to slightly accumulate in A549 cells, suggesting that nano-sized PS-NPs were absorbed more easily than micro-sized PS-MPs in A549 cells. It was confirmed by Xu’s study that smaller particle size is more conducive to the uptake of NPs by cells.40 While 2 μm PS-MPs showed almost no intracellular accumulation due to their large size and severe aggregation. In addition, the difference in uptake between larger and smaller particles may be due to the opportunity of the particle contacts with the cell, since the particle number per unit volume of 80 nm PS-NPs is much higher than 2 μm PS-MPs.41 However, both of the PS plastics were not observed to accumulate in the A549 cells in a dose-dependent way. With the increasing of the concentrations, the intracellular accumulation of both PS plastics was not increased gradually, however, showed some decrease probably as a result of particle release process. A series of time points need to be further considered to study the internalization process of PS-MPs and PS-NPs. For further study, three kinds of functionalized PS-NPs were found to be taken up by A549 cells with different efficiencies. Cells showed significantly higher efficiencies in internalizing amino-modified and carboxy-modified polystyrene nanoparticles than the plain PS-NPs. This observation was consistent with the conclusion from He’s study that more intracellular accumulation of PS-NH2 and PS-COOH than PS in HepG2 cells.42 Surface functionalization may enhance the ionic interactions between the PS-NPs and the cell membrane.43 It was reported that the PS-NH2 beads combined the cells tightly due to electrostatic attraction, inducing higher accumulation and toxicity than other PS beads that contacted the cells loosely by the Van der Waals forces, acid-base interactions and electrostatic forces.44 The study of Kimiko et al suggested that the preference of cells to ingest charged particles could also be due to the greater softness of amine and carboxyl-functionalized particles compared to plain ones.45 Therefore, the surface functionalization facilitated the internalization process of PS-NPs by A549 cells in our study. Above all, it is suggested that the size and aggregation of PS-MPs and PS-NPs, their interaction with the cell membrane, and the different surface functionalization might affect the cellular uptake of PS-MPs and PS-NPs particles and even the consequent toxicity.
Uptake, localization and toxicity were the main assessed targets, however so far, the potential genotoxicity of PS-NPs has rarely been evaluated. Genotoxicity is a very important toxicological endpoint mainly used as a surrogate biomarker of carcinogenesis, and other genetic associated pathologies.46 Accordingly, genotoxicity should also be considered a piece of relevant information in the evaluation of the adverse effects associated with PS-MPs or PS-NPs exposure. Regarding the use of the MN assay (measuring chromosome breaks and/or chromosome loss) to detect the genotoxic potential of polystyrene micro- and nanoparticles, both the studies of Avio and Magni showed that exposure to PS resulted in negative MN induction in the hemocytes of marine mussels.47,48 Similarly, by evaluating the effects of polystyrene nanoparticles on human intestinal epithelial Caco-2 cells, Cortés’s study showed the absence of MN induction and intracellular presence of ROS after PS exposure, confirming that PS was unable to induce significant effects on DNA integrity.49 However, in the present study, exposure to all kinds of the 80 nm PS-NPs led to the increase in cytotoxicity and genotoxicity of A549 cells in a dose-dependent manner. The reasons for the results should probably due to the intrinsic properties of NPs, specific cell types and different experimental systems. It is worth noting that the surface functionalized nanoplastics, especially the PS-NH2 triggered more severe toxicity in human A549 cells in comparison to PS. A lot of researches have proved that surface-modified polystyrene nanoparticles can mediate cell death through an apoptotic mechanism, which is mediated by damage to the mitochondria.40 Since mitochondria are the main organelles that control the generation of ROS, destruction of mitochondria potential will trigger and increase ROS production.50 Excess ROS may cause oxidative damage to lipids, protein and DNA.51 It has been documented that the induction of ROS is closely related to the toxicity mechanism of inhaled MPs.52 In the present study, different levels of ROS accumulation were observed when A549 cells were exposed to 80 nm PS-NPs and 2 μm PS-MPs for 6 hours. Similarly, 80 nm PS-NPs triggered greater oxidative damage than 2 μm PS-MPs, corresponding with the conclusion of cell viability and genotoxicity results. Actually, PS-NPs with smaller size and larger surface area have been reported to induce excessive ROS production.53,54 What is more, different levels of ROS accumulation were also observed in a time-dependent manner when A549 cells were separately exposed to three kinds of functionalized PS, PS-COOH and PS-NH2. The production of intracellular ROS could be very fast, reaching the maximum by the timepoint of 6 h. PS-NH2 still triggered the greatest oxidative damage among all PS-NPs, which was in line with the cell viability and genotoxicity results. Therefore, our study indicates size- and surface modification-dependent effect of PS-MPs and PS-NPs on oxidative stress related toxic response of A549 cells.
In conclusion, our study suggests that the size and aggregation state, the surface modification of PS-MPs and PS-NPs, internalization efficiency and the cellular accumulation are key factors for evaluating the toxicological effects of PS-MPs and PS-NPs on alveolar epithelial cells. The enhanced cytotoxicity and genotoxicity might be due to the increased intracellular accumulation and oxidative stress as a result of mitochondrial damage. More in-depth research is needed to further elucidate the toxicity mechanisms.
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 32271512, 82103801), The Natural Science Basic Research Program of Shaanxi (Program No. 2022JC-56, 2020JM-208), The Science and Technology Project in Guangzhou, China (No. 202206010049), the Fundamental Research Funds for the Central Universities (JB211208), and Xianyang Key Laboratory of Molecular Imaging and Drug Synthesis Program (No. 2021QXNL-PT-0008).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Danso D, Chow J, Streit WR, Drake HL. Plastics: environmental and biotechnological perspectives on microbial degradation. Appl Environ Microbiol. 2019;85(19):19. doi:10.1128/AEM.01095-19
2. Jambeck JR, Geyer R, Wilcox C, et al. Marine pollution. Plastic waste inputs from land into the ocean. Science. 2015;347(6223):768–771. doi:10.1126/science.1260352
3. Geyer R, Jambeck JR, Law KL. Production, use, and fate of all plastics ever made. Sci Adv. 2017;3(7):e1700782. doi:10.1126/sciadv.1700782
4. Song YK, Hong SH, Jang M, Han GM, Jung SW, Shim WJ. Combined effects of UV exposure duration and mechanical abrasion on microplastic fragmentation by polymer type. Environ Sci Technol. 2017;51(8):4368–4376. doi:10.1021/acs.est.6b06155
5. Andrady AL. Microplastics in the marine environment. Mar Pollut Bull. 2011;62(8):1596–1605. doi:10.1016/j.marpolbul.2011.05.030
6. Ribeiro F, O’Brien JW, Galloway T, Thomas KV. Accumulation and fate of nano- and micro-plastics and associated contaminants in organisms. Trends Analyt Chem. 2019;111:139–147. doi:10.1016/j.trac.2018.12.010
7. Wu P, Huang J, Zheng Y, et al. Environmental occurrences, fate, and impacts of microplastics. Ecotoxicol Environ Saf. 2019;184:109612. doi:10.1016/j.ecoenv.2019.109612
8. Rillig MC. Microplastic in terrestrial ecosystems and the soil? Environ Sci Technol. 2012;46(12):6453–6454. doi:10.1021/es302011r
9. Schell T, Rico A, Vighi M. Occurrence, fate and fluxes of plastics and microplastics in terrestrial and freshwater ecosystems. Rev Environ Contam Toxicol. 2020;250:1–43.
10. Chen G, Feng Q, Wang J. Mini-review of microplastics in the atmosphere and their risks to humans. Sci Total Environ. 2020;703:135504. doi:10.1016/j.scitotenv.2019.135504
11. Salvi S. Health effects of ambient air pollution in children. Paediatr Respir Rev. 2007;8(4):275–280. doi:10.1016/j.prrv.2007.08.008
12. Wang Y, Huang J, Zhu F, Zhou S. Airborne microplastics: a review on the occurrence, migration and risks to humans. Bull Environ Contam Toxicol. 2021;107(4):657–664. doi:10.1007/s00128-021-03180-0
13. Turner MC, Krewski D, Diver WR, et al. Ambient air pollution and cancer mortality in the cancer prevention study II. Environ Health Perspect. 2017;125(8):087013. doi:10.1289/EHP1249
14. Leslie HA, van Velzen MJM, Brandsma SH, Vethaak AD, Garcia-Vallejo JJ, Lamoree MH. Discovery and quantification of plastic particle pollution in human blood. Environ Int. 2022;163:107199. doi:10.1016/j.envint.2022.107199
15. Yan Z, Liu Y, Zhang T, Zhang F, Ren H, Zhang Y. Analysis of microplastics in human feces reveals a correlation between fecal microplastics and inflammatory bowel disease status. Environ Sci Technol. 2022;56(1):414–421. doi:10.1021/acs.est.1c03924
16. Vethaak AD, Legler J. Microplastics and human health. Science. 2021;371(6530):672–674. doi:10.1126/science.abe5041
17. Gambardella C, Morgana S, Ferrando S, et al. Effects of polystyrene microbeads in marine planktonic crustaceans. Ecotoxicol Environ Saf. 2017;145:250–257. doi:10.1016/j.ecoenv.2017.07.036
18. Ohlinger K, Kolesnik T, Meindl C, et al. Air-liquid interface culture changes surface properties of A549 cells. Toxicol In Vitro. 2019;60:369–382. doi:10.1016/j.tiv.2019.06.014
19. Tsai KF, Shen CJ, Cheung CW, et al. Lipotoxicity in human lung alveolar type 2 A549 cells: mechanisms and protection by tannic acid. Chin J Physiol. 2021;64(6):289–297. doi:10.4103/cjp.cjp_68_21
20. Bihari P, Vippola M, Schultes S, et al. Optimized dispersion of nanoparticles for biological in vitro and in vivo studies. Part Fibre Toxicol. 2008;5:14. doi:10.1186/1743-8977-5-14
21. Li L, Luo Y, Peijnenburg W, Li R, Yang J, Zhou Q. Confocal measurement of microplastics uptake by plants. MethodsX. 2020;7:100750. doi:10.1016/j.mex.2019.11.023
22. Adan A, Alizada G, Kiraz Y, Baran Y, Nalbant A. Flow cytometry: basic principles and applications. Crit Rev Biotechnol. 2017;37(2):163–176. doi:10.3109/07388551.2015.1128876
23. Prabst K, Engelhardt H, Ringgeler S, Hubner H. Basic colorimetric proliferation assays: MTT, WST, and resazurin. Methods Mol Biol. 2017;1601:1–17.
24. Sommer S, Buraczewska I, Kruszewski M. micronucleus assay: the state of art, and future directions. Int J Mol Sci. 2020;21(4):1534. doi:10.3390/ijms21041534
25. Michael Fenech AAM, Morley AA. Cytokinesis-block micronucleus method in human lymphocytes: effect of in vivo ageing and low dose X-irradiation. Mutanon Res. 1986;161:193–198. doi:10.1016/0027-5107(86)90010-2
26. Xia T, Kovochich M, Liong M, et al. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano. 2008;2(10):2121–2134. doi:10.1021/nn800511k
27. Shen J, Kim HC, Su H, et al. Cyclodextrin and polyethylenimine functionalized mesoporous silica nanoparticles for delivery of siRNA cancer therapeutics. Theranostics. 2014;4(5):487–497. doi:10.7150/thno.8263
28. Dash BC, Rethore G, Monaghan M, Fitzgerald K, Gallagher W, Pandit A. The influence of size and charge of chitosan/polyglutamic acid hollow spheres on cellular internalization, viability and blood compatibility. Biomaterials. 2010;31(32):8188–8197. doi:10.1016/j.biomaterials.2010.07.067
29. Bergami E, Bocci E, Vannuccini ML, et al. Nano-sized polystyrene affects feeding, behavior and physiology of brine shrimp Artemia franciscana larvae. Ecotoxicol Environ Saf. 2016;123:18–25. doi:10.1016/j.ecoenv.2015.09.021
30. Sharma VK, Ma X, Guo B, Zhang K. Environmental factors-mediated behavior of microplastics and nanoplastics in water: a review. Chemosphere. 2021;271:129597. doi:10.1016/j.chemosphere.2021.129597
31. Sun H, Jiao R, Wang D. The difference of aggregation mechanism between microplastics and nanoplastics: role of Brownian motion and structural layer force. Environ Pollut. 2021;268(Pt B):115942. doi:10.1016/j.envpol.2020.115942
32. Della Torre C, Bergami E, Salvati A, et al. Accumulation and embryotoxicity of polystyrene nanoparticles at early stage of development of sea urchin embryos Paracentrotus lividus. Environ Sci Technol. 2014;48(20):12302–12311. doi:10.1021/es502569w
33. Li S, Liu H, Gao R, Abdurahman A, Dai J, Zeng F. Aggregation kinetics of microplastics in aquatic environment: complex roles of electrolytes, pH, and natural organic matter. Environ Pollut. 2018;237:126–132. doi:10.1016/j.envpol.2018.02.042
34. Yu S, Shen M, Li S, et al. Aggregation kinetics of different surface-modified polystyrene nanoparticles in monovalent and divalent electrolytes. Environ Pollut. 2019;255(Pt 2):113302. doi:10.1016/j.envpol.2019.113302
35. Shams M, Alam I, Chowdhury I. Aggregation and stability of nanoscale plastics in aquatic environment. Water Res. 2020;171:115401. doi:10.1016/j.watres.2019.115401
36. Wang J, Zhao X, Wu A, et al. Aggregation and stability of sulfate-modified polystyrene nanoplastics in synthetic and natural waters. Environ Pollut. 2021;268(Pt A):114240. doi:10.1016/j.envpol.2020.114240
37. Monopoli MP, Aberg C, Salvati A, Dawson KA. Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol. 2012;7(12):779–786. doi:10.1038/nnano.2012.207
38. Junaid M, Wang J. Interaction of nanoplastics with extracellular polymeric substances (EPS) in the aquatic environment: a special reference to eco-Corona formation and associated impacts. Water Res. 2021;201:117319. doi:10.1016/j.watres.2021.117319
39. Jeong CB, Kang HM, Lee YH, et al. Nanoplastic ingestion enhances toxicity of Persistent Organic Pollutants (POPs) in the monogonont Rotifer Brachionus koreanus via Multixenobiotic Resistance (MXR) disruption. Environ Sci Technol. 2018;52(19):11411–11418. doi:10.1021/acs.est.8b03211
40. Xu M, Halimu G, Zhang Q, et al. Internalization and toxicity: a preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci Total Environ. 2019;694:133794. doi:10.1016/j.scitotenv.2019.133794
41. Lu Y, Zhang Y, Deng Y, et al. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ Sci Technol. 2016;50(7):4054–4060. doi:10.1021/acs.est.6b00183
42. He Y, Li J, Chen J, et al. Cytotoxic effects of polystyrene nanoplastics with different surface functionalization on human HepG2 cells. Sci Total Environ. 2020;723:138180. doi:10.1016/j.scitotenv.2020.138180
43. Liu Z, Huang Y, Jiao Y, et al. Polystyrene nanoplastic induces ROS production and affects the MAPK-HIF-1/NFkB-mediated antioxidant system in Daphnia pulex. Aquat Toxicol. 2020;220:105420. doi:10.1016/j.aquatox.2020.105420
44. Kim S, Marion M, Jeong B-H, Hoek EMV. Crossflow membrane filtration of interacting nanoparticle suspensions. J Memb Sci. 2006;284(1–2):361–372. doi:10.1016/j.memsci.2006.08.008
45. Kimiko Makino NY, Higuchi K, Harada N, Ohshima H, Terada H, Terada H. Phagocytic uptake of polystyrene microspheres by alveolar macrophages: effects of the size and surface properties of the microspheres. Colloids Surf B. 2003;27:33–39. doi:10.1016/S0927-7765(02)00042-5
46. Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461(7267):1071–1078. doi:10.1038/nature08467
47. Avio CG, Gorbi S, Milan M, et al. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ Pollut. 2015;198:211–222. doi:10.1016/j.envpol.2014.12.021
48. Magni S, Gagne F, Andre C, et al. Evaluation of uptake and chronic toxicity of virgin polystyrene microbeads in freshwater zebra mussel Dreissena polymorpha (Mollusca: bivalvia). Sci Total Environ. 2018;631–632:778–788. doi:10.1016/j.scitotenv.2018.03.075
49. Cortés C, Domenech J, Salazar M, Pastor S, Marcos R, Hernández A. Nanoplastics as a potential environmental health factor: effects of polystyrene nanoparticles on human intestinal epithelial Caco-2 cells. Environ Sci. 2020;7(1):272–285.
50. Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta. 2006;1757(5–6):509–517. doi:10.1016/j.bbabio.2006.04.029
51. Buonocore G, Perrone S, Tataranno ML. Oxygen toxicity: chemistry and biology of reactive oxygen species. Semin Fetal Neonatal Med. 2010;15(4):186–190. doi:10.1016/j.siny.2010.04.003
52. Anbumani S, Kakkar P. Ecotoxicological effects of microplastics on biota: a review. Environ Sci Pollut Res Int. 2018;25(15):14373–14396. doi:10.1007/s11356-018-1999-x
53. Donaldson K, Tran CL. Inflammation caused by particles and fibers. Inhal Toxicol. 2002;14(1):5–27. doi:10.1080/089583701753338613
54. Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622–627. doi:10.1126/science.1114397
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.