Back to Journals » OncoTargets and Therapy » Volume 11
Cytotoxic effect of Drimia maritima bulb extract and induction of mitochondrial apoptotic signaling in human breast cancer cells, MCF-7 and MDA-MB-468
Authors Hamzeloo-Moghadam M, Aghaei M, Abdolmohammadi MH , Khalaj A, Fallahian F
Received 7 August 2018
Accepted for publication 5 October 2018
Published 31 October 2018 Volume 2018:11 Pages 7669—7677
DOI https://doi.org/10.2147/OTT.S182786
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Carlos E Vigil
Maryam Hamzeloo-Moghadam,1 Mahmoud Aghaei,2 Mohammad Hossein Abdolmohammadi,3 Amir Khalaj,1,4 Faranak Fallahian3
1Traditional Medicine and Materia Medica Research Center and Department of Traditional Pharmacy, School of Traditional Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran; 2Department of Clinical Biochemistry, School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences, Isfahan, Iran; 3Cellular and Molecular Research Center, Qom University of Medical Sciences, Qom, Iran; 4Food and Drug Laboratory Research Center, Food and Drug Organization, Ministry of Health and Medical Education, Tehran, Iran
Background: Drimia maritima (D. maritima) is a plant belonging to the family Asparagaceae, which has been used for the treatment of several ailments including cancer around the world. To our knowledge, there is no comprehensive study about the molecular mechanisms of anticancer activity of this plant, yet.
Materials and methods: In the current study, cell viability, apoptosis induction, ROS production, mitochondrial apoptotic pathway, and ER stress mediators have been evaluated in breast cancer cells, MCF7, and MDA-MB-468 treated with D. maritima.
Results: Significant cytotoxic effects were observed in MCF-7 and MDA-MB-468 cells after exposure to D. maritima. Apoptosis induction was determined using Annexin-V-FITC and propidium iodide staining. Furthermore, an increase of ROS, loss of mitochondrial membrane potential, the release of cytochrome c, activation of caspases, and elevation in the Bax/Bcl-2 ratio was determined. D. maritima dose-dependently increased the mRNA expression of ER stress markers such as CHOP, ATF-4, GADD34, and TRIB3 in MCF-7, and MDA-MB-468 cells.
Conclusion: These data suggest that D. maritima induces apoptosis in human breast cancer cells via the mitochondrial-mediated pathway. In addition, endoplasmic reticulum stress seems to be involved in D. maritima-induced cell death.
Keywords: Drimia maritima, ROS, apoptosis, mitochondria, breast cancer, ER stress
Introduction
Breast cancer is one of the most common cancer in females. Despite advances in medical treatments and appropriate therapies, the mortality in this malignancy is still high.1 One of the major concerns in conventional therapeutic approaches is the risk of undesired and severe side effects arising from the targeting of both normal and cancer cells.2 Natural products with anticancer therapeutic potential have gained great attention due to their favorable safety and efficacy profiles. The discovery of plant-derived substances with therapeutic potential usually starts from a pharmacological investigation of plant extracts as the source of secondary metabolites.3
The Endoplasmic Reticulum (ER) serves many functions including protein synthesis and folding. A great variety of stress signals such as hypoxia, oxidative stress, and Ca2+ overload can cause disturbance of ER homeostasis and subsequent induction of unfolded protein response (UPR). The UPR is an adaptive stress response pathway that is mediated by activation of three transmembrane proteins: inositol-requiring enzyme 1 (IRE1), PKR-like ER kinase (PERK), and activating transcription factor 6 (ATF6). However, under certain circumstances that UPR fails to restore ER homeostasis, apoptotic signaling pathways will be triggered via several different mechanisms.4–6 The process of programmed cell death, or apoptosis, is the most potent defense in multicellular organisms against cancer; therefore, finding natural agents targeting signaling apoptosis pathways may be an effective method for cancer therapy and prevention.
Drimia maritima (D. maritima), plant belonging to the family Asparagaceae have been applied from the ancient time for the treatment of different diseases including cough and respiratory ailments, cardiac failure, jaundice, skin problems, and gastric disorders.7 Furthermore, D. maritima has been used in Iranian Traditional Medicine (ITM) considering cancer and cancer-related disorders.8 Phytochemical analysis has revealed the presence of cardiac glycosides, phenolic compounds, phytosterols, and other phytochemical constituents in this plants.7–9 A literature survey indicates that no comprehensive studies on the anticancer activities of D. maritima have been provided yet. Accordingly, the present study attempts to evaluate the anticancer properties of D. maritima and its underlying molecular mechanisms in two human breast cancer cells, MCF-7 and MDA-MB-468.
Materials and methods
Reagents and chemicals
RPMI 1640, Fetal Bovine Serum (FBS), Trypsin-EDTA, Penicillin-Streptomycin, and MTT were obtained from Thermo Fisher Scientific (Waltham, MA, USA). AnnexinV-FITC apoptosis detection kit and Caspase-6 and -9 colorimetric assay kits were bought from Biovision (Mountain View, CA, USA). Fluorescent Reactive Oxygen Species detection kit was obtained from Marker Gene Technologies (Eugene, OR, USA). The antibodies against Bax, Bcl-2, and cytochrome c were bought from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). JC-1 was purchased from Enzo Life Sciences (Farmingdale, NY, USA).
Plant material and preparation of the extract
D. maritima bulbs were collected from Kohgiluyeh va Boyer Ahmad Province, Iran (2015). The scientific name was authenticated by Dr Hamid Moazzeni Zehan, Traditional Medicine and Materia Medica Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran. A voucher specimen (TMRC 3722) was kept for future reference. The quality control assessment of D. maritima was conducted according to British Pharmacopoeia in triplicate and acid-insoluble ash and ethanol value were examined.10
For preparing the methanol extract, 10 mg of powdered shade-dried D. maritima bulbs were macerated with methanol (1:10) three times. The solvent was refreshed every 24 hours and the filtrates were combined and evaporated to dryness under reduced pressure in a rotatory evaporator. The extract was then dissolved in dimethyl sulfoxide (DMSO) (Sigma), sterilized by filtration, and subsequently diluted to appropriate working concentration. The solvent was added to the control cultures in all experiments. The final concentration of DMSO was not more than 0.1%.
Cell lines and culture condition
The breast cancer cell lines, MCF-7, MB-MDA-468, and normal fibroblast cell line AGO1522 were purchased from National Cell Bank of Iran (NCBI). The cells were cultured in RPMI-1640 supplemented with 10% FBS, 100 U/mL of penicillin, and 100 μg/mL of streptomycin, and incubated at 37°C, 5% CO2, and 95% humidity.
Evaluation of cell viability by MTT assay
Cytotoxicity of D. maritima was determined by the MTT assay, as described previously.11 Cells were seeded in a 96-well plate at a concentration of 5×103 cells/well and incubated at 37°C overnight. Afterward, cells were treated with D. maritima methanolic extract (0.1–500 μg/mL). After 48 hours, 20 μL of 5 mg/mL MTT solution was added to each well and further incubated for 4 hours. Thereafter, the supernatant was gently replaced by 200 μL DMSO and the absorbance values were measured at 570 nm using a microplate reader.
Apoptosis assay by flow cytometry
Apoptosis could be detected by staining the cells with Annexin V-FITC and Propidium iodide (PI) solution followed by flow cytometry analysis.11 In brief, cells were seeded to a density of 5×105 in a six-well plate and treated with D. maritima (10, 100, and 500 μg/mL) for 48 hours. Then, cells were washed with cold PBS and re-suspended in the 1x binding buffer containing Annexin V-FITC and PI solution. The stained cells were examined by FACS Calibur flow cytometry (BD Biosciences, San Jose, CA, USA).
Quantitative real-time RT-PCR
The total RNA of the MCF-7 and MDA-MB-468 cells were extracted using Trizol reagent (Thermo Fisher Scientific) and then reverse transcribed into first-strand cDNA using Revert Aid M-MuLV Reverse Transcriptase (Fermentas, Germany). Real-time PCR (qPCR) of cDNA was performed using Applied Biosystems instrument (ABI 7500 Real-Time PCR System, USA). Relative expression levels of genes were normalized to GAPDH and relative quantification values were determined using the comparative 2−ΔΔCt analysis method.12 Quantitative RT-PCR was performed using specific primers, which are listed in Table 1.12
  | Table 1 Primer sequences used for quantitative RT-PCR |
Western blot analysis
After treatment with D. maritima extract, cells were washed with cold PBS and lysed with an appropriate lysis buffer, RIPA (20 mM Tris–HCl, 0.5% Nonidet P-40, 0.5 mM PMSF, 100 mM b-glycerol 3-phosphate, and 0.5% protease inhibitor cocktail). The protein concentration was determined using the Bradford protein assay. Then, SDS-denatured samples were separated on SDS-polyacrylamide gels and then transferred to a PVDF membrane. The membrane was incubated with PBST solution (5% non-fat dry milk in PBS containing 0.1% Tween-20) and then incubation with the monoclonal antibodies against Bax, Bcl-2 and cytochrome c was performed, overnight. After incubation with corresponding secondary antibodies, detection was carried out using Enhanced Chemiluminescence (ECL).11
Caspase activity assay
Colorimetric assay kits were used to detect the activities of caspase-6 and -9 in the MCF-7 and MDA-MB-468 cells.13 The assay is based on spectrophotometric detection of the chromophore p-nitroaniline (p-NA) after cleavage from the labeled substrate (LEHD-pNA for caspase-9 and VEID-pNA for caspase-6). In brief, after centrifugation of cell lysates, the supernatant was added to a supplied reaction buffer containing dithiothreitol, and LEHD-pNA and VEID-pNA as substrates and incubated for 1 hour in the dark. The amount of p-NA released was measured at 405 nm using a Microplate Reader (Bio-Rad Laboratories Inc., Hercules, CA, USA).
ROS assay
The MarkerGene™ Live Cell Fluorescent ROS Detection Kit (Marker Gene Technologies) was used to measure ROS generation in MCF-7 and MDA-MB-468 exposed to D. maritima.14 This kit uses a cell-permeable substrate (DCFH-DA) as a fluorogenic marker for ROS detection. Briefly, after incubation with DCFH-DA, cells were harvested and washed with HBSS and analyzed by a Synergy HT Multi-Mode Microplate Reader (BioTek Instruments, Winooski, VT, USA).
Determination of mitochondrial membrane potential (ΔΨm) using JC-1
Lipophilic cationic dye, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) was used to evaluate ΔΨm.11 JC-1, in healthy cells with high mitochondrial ΔΨm, can enter into mitochondria and form complexes emitting red fluorescence. During the loss of ΔΨm in apoptotic cells, JC-1 remains in monomeric form with green fluorescence emission. The ratio of red to green fluorescence provides a measure of ΔΨm. Briefly, after treatment with D. maritima, the culture medium of the cells was replaced with HEPES buffer containing JC-1 and after 30 minutes, the Red/green fluorescence was measured using a Synergy HT Multi-Mode Microplate Reader (BioTek Instruments).
Statistical analysis
Data were analyzed by ANOVA with Dunnett’s post-hoc test using SPSS. The IC50 value was determined using GraphPad Prism5 software. Differences were considered statistically significant at P<0.05.
Results
Quality control of the plant material
The quality of the plant material was determined according to British Pharmacopoeia, which demonstrated that D. maritima was of good quality and the results were within acceptable limits (Table 2).
  | Table 2 Results of quality control assessment of Drimia maritima |
D. maritima inhibited cell viability in breast cancer cells
We first characterized the cytotoxic effect of D. maritima on MCF-7 and MDA-MB-468 cells using MTT assay. D. maritima significantly inhibited the viability of both MCF-7 and MDA-MB-468 cells in a dose-dependent manner (Figure 1). The most inhibitory effect of D. maritima were about 80% and 76% in MCF-7 and MDA-MB-468 cells, respectively, after treatment with 500 μg/mL.
The effective doses of D. maritima that inhibited 50% of growth (IC50) of MCF-7 and MDA-MB-468 cells were 20.48±1.17 μg/mL and 25.74±2.05 μg/mL, respectively. Interestingly, the data reveal that D. maritima display significantly lower cytotoxicity against AGO1522, a normal human fibroblast cell, with the IC50 values of 43.5±1.73 μg/mL (Figure 1).
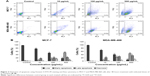
D. maritima induced apoptosis cell death in breast cancer cells
To explore whether the D. maritima anti-proliferative activity is related to the apoptosis induction, we used flow cytometry to measure the apoptosis rate. Following 48 hours of treatment with D. maritima (10, 100, and 500 μg/mL), cells were stained with Annexin V-FITC and PI and then analyzed by flow cytometry. The data of this experiment indicated that the rate of apoptosis (as the sum of early apoptotic cells and late apoptotic cells) increased in MCF-7 and MDA-MB-468 cells to 75.8% and 81.9%, respectively, after treatment with 500 μg/mL D. maritima (Figure 2A and B).
D. maritima induced ROS generation in breast cancer cells
Molecules of ROS in low doses are involved in cell cycle progression and proliferation of cancer cells. However, an increase in intracellular ROS can inhibit cancer cell growth and induce apoptosis through oxidative stress-mediated pathways.15 Our results showed that exposure to D. maritima significantly increased the intracellular ROS levels in MCF-7 and MDA-MB-468 cells in a dose-dependent manner (Figure 3).
Mitochondria is involved in the D. maritima-mediated apoptosis in breast cancer cells
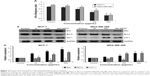
Mitochondrial dysfunction and subsequently decrease in the level of ΔΨm is considered as a central event in apoptosis.16 In order to determine the contribution of mitochondria in the apoptosis induction by D. maritima, cells were treated with different concentrations of D. maritima (1, 10, 100, and 500 μg/mL) for 48 hours and then incubated with JC-1 dye. Based on our results, as indicated in Figure 4A, D.maritima caused loss of ΔΨm in a dose-dependent manner in MCF-7 and MDA-MB-468 cells.
Bcl-2 family proteins have been considered as a main regulator of the mitochondrial apoptotic pathway. The Bcl-2-related proteins either inhibit (particularly Bcl-2) or promote apoptosis (particularly Bax), and the balance between these proteins determines the cellular fate.17 As shown in Figure 4B and C, expression of proapoptotic protein Bax was significantly increased, whereas the antiapoptotic Bcl-2 protein was decreased after treatment with D. maritima. It has been suggested that the increased Bax/Bcl-2 ratio can be recognized as a key factor for the apoptotic process by regulating the release of cytochrome c from mitochondria and activation of the intrinsic apoptotic pathway.18
Consistently, our results indicated that the treatment with D. maritima promoted the release of cytochrome c into the cytosol (Figure 4B and C). These results, together, suggest that D. maritima triggers mitochondria-mediated apoptotic pathway through the increase in Bax/Bcl-2 ratio and subsequent disturbance of mitochondrial outer membrane permeabilization (MOMP).
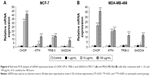
Involvement of caspase-6 and -9 in D. maritima-induced apoptosis
During the mitochondrial apoptotic pathway, the release of cytochrome c from mitochondria leads to activation of Apaf-1, which then cleaves procaspase-9 into the active form. Active caspase-9, as an initiating caspase, cleaves, and activates downstream executioner caspases such as caspase-3, -6, and -7. As shown in Figure 5A and B, D. maritima increased significantly the activities of caspase-6 and -9 in a dose-dependent manner in MCF-7 and MDA-MB-468 cells. MCF-7 cells do not express the caspase-3 protein due to a 47-base-pair deletion within exon 3 of the CASP-3 gene,19 and so we decided to measure the activity of caspase-6, as an executioner caspase in MCF-7 and MDA-MB-468 cells.
D. maritima seem to induce ER stress signaling pathway
To further elucidate the molecular mechanisms underlying D. maritima-induced apoptosis, we investigated the effects of D. maritima on UPR genes. The mRNA expression levels of ER stress-associated genes such as ATF4 (Activating Transcription Factor 4), CHOP (C/EBP Homologous Protein), GADD34 (Growth Arrest and DNA damage-inducible 34), and TRIB3 (tribbles-related protein 3) were evaluated by real-time PCR. ATF4 is a transcription factor regulating a variety of responses including antioxidant gene expression, amino acid synthesizing enzymes, and proapoptotic machinery.20 CHOP, whose induction strongly depends on ATF4, is considered as a central molecule in ER stress-induced apoptosis. Studied show that numerous target genes including GADD34 and TRIB3 are contributed in CHOP-mediated apoptosis.21,22
As shown in Figure 6A and B, after treatment with D. maritima, mRNA expression of CHOP, ATF-4, GADD34, and TRIB3 genes increased in a dose-dependent manner compared to untreated cells. In MCF-7 cells, after exposure to D. maritima 100 μg/mL, the expression levels of CHOP, ATF-4, GADD34, and TRIB3 genes showed an increase of 41±1.25, 12.88±3.65, 7.9±1.9, and 13.4±5.15, respectively. CHOP, ATF-4, GADD34, and TRIB3 genes showed an increase of 35.41±4.12, 16.5±2.39, 11.35±2.01, and 11.33±6.15, respectively, in MDA-MB-468 cells. These results suggest that UPR activation in response to D. maritima treatment could promote apoptosis through the CHOP-ATF-4 pathway in breast cancer cells.
Discussion
Targeting apoptotic death pathways has been considered as the most effective therapeutic strategy in all cancer types. In the current study, experiments using MTT assay revealed that methanolic extract of D. maritima, a traditionally used medicinal plant, exerts anti-proliferative activity against MCF-7 and MDA-MB-468 cells. Moreover, D. maritima-treated cells revealed a dose-dependent increase in the percentage of apoptotic cells. To investigate apoptosis induction, double staining with Annexin V-FITC and PI to measure membrane phosphatidylserine (PS) exposure was carried out. Exposure of PS on the external side of the plasma membrane is the key feature of the early apoptosis. Annexin V commonly is used to detect apoptotic cells because of its high binding affinity to PS. PI, a red fluorescent intercalating dye, was employed as a DNA stain to evaluate dead cells.
An active role for mitochondria in intrinsic apoptosis signaling pathway is well established. Several mitochondrial proteins, residing in the intermembrane space of mitochondria, in response to the variety of stimuli are released into the cytosol and activate apoptotic pathways.23 The BCl-2 family proteins have a key role in regulating the MOMP and release of mitochondrial proteins into the cytosol. It was shown that the increased Bax to Bcl-2 ratio could disrupt MOMP that could lead to decreased ΔΨm and release of apoptogenic proteins such as cytochrome c into the cytosol, where it triggered the execution of apoptosis by inducing caspases activation.23,24 Consistently, our results show that after treatment with D. maritima, the expression of Bax was significantly increased, whereas the Bcl-2 expression was downregulated. Furthermore, D. maritima treatment decreased ΔΨm and triggered the release of cytochrome c into the cytosol. The release of cytochrome c through its effects on Apaf-1 leads to the activation of initiator caspase-9. Active caspase-9 triggers the cleavage and subsequent activation of downstream executioner caspases such as caspase-3, -6, and -7.25 Our findings have shown a clear increase in the caspase-9 and -6 activity in MCF-7 and MDA-MB-468 cells after exposure to D. maritima. Therefore, we speculated that D. maritima could induce the mitochondrial pathway through the increase of Bax to Bcl-2 ratio, the release of cytochrome c, and caspase cascade activation.
Many anticancer agents exert their inhibitory effects through the generation of ROS and promotion of oxidative stress-induced cancer cell death.26 In our study, we found that the levels of intracellular ROS to be significantly elevated by D. maritima treatment in MCF-7 and MDA-MB-468 cells. Increased ROS levels also can induce ER stress response, which in the case of prolonged ER stress could initiate apoptotic death signals.27 During ER stress, the role of PERK–eIF2α–ATF4 branch of the UPR is essential in the upregulation of CHOP, one of the main molecule involved in the ER stress-mediated apoptosis. Numerous target genes including GADD34, TRIB3, and BCL-2 have been identified in the CHOP-induced apoptotic pathway.6 GADD34 promotes dephosphorylation of eIF2α which leads to the translational suppression and subsequent induction of apoptosis pathway.28,29 It has also been demonstrated that up-regulation of TRIB3, another target gene induced by CHOP, has a critical role in ER stress-induced cell death via the ATF4-CHOP pathway.30 It has been suggested that the most characterized mechanism of cell death induced by CHOP is the downregulation of Bcl-2 and upregulation of Bax proteins.31 In the present study, the effect of D. maritima on ER stress was investigated by focusing on the ATF4-CHOP pathway. We found that D. maritima dose-dependently increased the mRNA expression of ATF-4, CHOP, GADD34, and TRIB3 in MCF-7 and MDA-MB-468 cells. In addition, as mentioned earlier, the Western blot analysis indicated Bcl-2 upregulation and Bax downregulation in cells treated with D. maritima.
Conclusion
Collectively, our results support the hypothesis that the mitochondrial pathway and ER stress are involved in apoptosis induction by D. maritima in breast cancer cells. To the best of our knowledge, this is the first comprehensive study showing the promising cytotoxic effects of D. maritima against breast cancer cells. Consequently, D. maritima extract can be evaluated further for potential anticancer properties and isolation of bioactive phytochemicals.
Acknowledgments
The authors thank the Vice-Chancellor for supporting the research at the Qom University of Medical Sciences (Project number: 94613) and Vice-Chancellor for research at the Shahid Beheshti University of Medical Sciences (Project number: 93–151) financially.
Author contributions
Dr Hamzeloo-Moghadam contributed to interpretation of data and preparing the manuscript; Dr Aghaei participated in designing and performing experiments; Dr Abdolmohammadi participated in data analysis; Dr Khalaj conducted the preparation of D. maritima extracts; Dr Fallahian contributed to analysis and interpretation of data and preparing the manuscript. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflict of interest in this work.
References
Youlden DR, Cramb SM, Yip CH, Baade PD. Incidence and mortality of female breast cancer in the Asia-Pacific region. Cancer Biol Med. 2014;11(2):101–115. | ||
Head J, Johnston SR. New targets for therapy in breast cancer: farnesyltransferase inhibitors. Breast Cancer Res. 2004;6(6):262–268. | ||
Nobili S, Lippi D, Witort E, et al. Natural compounds for cancer treatment and prevention. Pharmacol Res. 2009;59(6):365–378. | ||
Urra H, Dufey E, Lisbona F, Rojas-Rivera D, Hetz C. When ER stress reaches a dead end. Biochim Biophys Acta. 2013;1833(12):3507–3517. | ||
Rozpedek W, Pytel D, Mucha B, Leszczyńska H, Diehl JA, Majsterek I. The role of the PERK/eIF2α/ATF4/CHOP signaling pathway in tumor progression during endoplasmic reticulum stress. Curr Mol Med. 2016;16(6):533–544. | ||
Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7(9):880–885. | ||
Bozorgi M, Amin G, Shekarchi M, Rahimi R. Traditional medical uses of Drimia species in terms of phytochemistry, pharmacology and toxicology. J Tradit Chin Med. 2017;37(1):124–139. | ||
Naghibi F, Khalaj A, Mosaddegh M, Malekmohamadi M, Hamzeloo-Moghadam M. Cytotoxic activity evaluation of some medicinal plants, selected from Iranian traditional medicine Pharmacopoeia to treat cancer and related disorders. J Ethnopharmacol. 2014;155(1):230–239. | ||
Bozcuk H, Ozdogan M, Aykurt O. Urginea maritima (L.) Baker (Liliaceae) extract induces more cytotoxicity than standard chemotherapeutics in the A549 non-small cell lung cancer (NSCLC) cell line. Turk J Med Sci. 2011;41:101–108. | ||
The British Pharmacopoeia Commission. British Pharmacopoeia 2013. London: Stationary Office; 2012. | ||
Fallahian F, Aghaei M, Abdolmohammadi MH, Hamzeloo-Moghadam M. Molecular mechanism of apoptosis induction by Gaillardin, a sesquiterpene lactone, in breast cancer cell lines: Gaillardin-induced apoptosis in breast cancer cell lines. Cell Biol Toxicol. 2015;31(6):295–305. | ||
Aghaei M, Ghanadian M, Sajjadi SE, Saghafian R, Keyvanloo Shahrestanaki M. Pimpinelol, a novel atypical Sesquiterpene lactone from Pimpinella haussknechtii fruits with evaluation of endoplasmic reticulum stress in breast cancer cells. Fitoterapia. 2018;129:198–202. | ||
Lam TG, Jeong YS, Kim SA, Ahn SG. New metformin derivative HL156A prevents oral cancer progression by inhibiting the insulin-like growth factor/AKT/mammalian target of rapamycin pathways. Cancer Sci. 2018;109(3):699–709. | ||
Reiniers MJ, van Golen RF, Bonnet S, et al. Preparation and Practical Applications of 2′,7′-Dichlorodihydrofluorescein in Redox Assays. Anal Chem. 2017;89(7):3853–3857. | ||
Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44(5):479–496. | ||
Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305(5684):626–629. | ||
Kuwana T, Newmeyer DD. Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr Opin Cell Biol. 2003;15(6):691–699. | ||
Kale J, Osterlund EJ, Andrews DW. BCL-2 family proteins: changing partners in the dance towards death. Cell Death Differ. 2018;25(1):65–80. | ||
Jänicke RU, Sprengart ML, Wati MR, Porter AG. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem. 1998;273(16):9357–9360. | ||
Ameri K, Harris AL. Activating transcription factor 4. Int J Biochem Cell Biol. 2008;40(1):14–21. | ||
Marciniak SJ, Yun CY, Oyadomari S, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18(24):3066–3077. | ||
Qian B, Wang H, Men X, et al. TRIB3 is implicated in glucotoxicity- and oestrogen receptor-stress-induced β-cell apoptosis. J Endocrinol. 2008;199(3):407–416. | ||
Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21(1):92–101. | ||
Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000;7(12):1166–1173. | ||
Pradelli LA, Bénéteau M, Ricci JE. Mitochondrial control of caspase-dependent and -independent cell death. Cell Mol Life Sci. 2010;67(10):1589–1597. | ||
Moeinifard M, Hassan ZM, Fallahian F, Hamzeloo-Moghadam M, Taghikhani M. Britannin induces apoptosis through AKT-FOXO1 pathway in human pancreatic cancer cells. Biomed Pharmacother. 2017;94:1101–1110. | ||
Banerjee A, Banerjee V, Czinn S, Blanchard T. Increased reactive oxygen species levels cause ER stress and cytotoxicity in andrographolide treated colon cancer cells. Oncotarget. 2017;8(16):26142–26153. | ||
Brush MH, Weiser DC, Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 targets protein phosphatase 1 alpha to the endoplasmic reticulum and promotes dephosphorylation of the alpha subunit of eukaryotic translation initiation factor 2. Mol Cell Biol. 2003;23(4):1292–1303. | ||
Adler HT, Chinery R, Wu DY, et al. Leukemic HRX fusion proteins inhibit GADD34-induced apoptosis and associate with the GADD34 and hSNF5/INI1 proteins. Mol Cell Biol. 1999;19(10):7050–7060. | ||
Bi SJ, Wang CY, Zhang J, Lv ZP, Li YX. Atorvastatin up-regulates TRIB3 independent of ATF4-CHOP pathway in atherosclerotic patients. Int J Clin Exp Med. 2015;8(11):21635–21640. | ||
Mccullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21(4):1249–1259. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.