Back to Journals » Journal of Pain Research » Volume 12
Cytokine production capabilities of human primary monocyte-derived macrophages from patients with diabetes mellitus type 2 with and without diabetic peripheral neuropathy
Authors Alvarado-Vázquez PA, Grosick RL , Moracho-Vilrriales C, Ward E, Threatt T , Romero-Sandoval EA
Received 4 September 2018
Accepted for publication 15 November 2018
Published 19 December 2018 Volume 2019:12 Pages 69—81
DOI https://doi.org/10.2147/JPR.S186372
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael Schatman
Perla Abigail Alvarado-Vázquez,1 Rachel L Grosick,2 Carolina Moracho-Vilrriales,2 Eileen Ward,2 Tiffaney Threatt,2 Edgar Alfonso Romero-Sandoval3
1Department of Medical Biochemistry and Microbiology, Uppsala University, SE-751 23 Uppsala, Sweden; 2Department of Pharmacy Practice, Presbyterian College School of Pharmacy, Clinton, SC, USA; 3Department of Anesthesiology, Pain Mechanisms Laboratory, Wake Forest University School of Medicine, Winston-Salem, NC, USA
Introduction: Monocytes from patients with diabetes mellitus type 2 (DM2) are dysfunctional, persistently primed, and prone to a proinflammatory phenotype. This may alter the phenotype of their differentiation to macrophages and result in diabetic peripheral neuropathy (DPN), nerve damage, nerve sensitization, and chronic pain. We have previously demonstrated that CD163 is a molecule that promotes an anti-inflammatory cellular phenotype in human primary macrophages, but this has not been proven in macrophages from patients with DM2 or DPN. Thus, we hypothesize that macrophages from patients with DM2 or DPN display an altered proinflammatory functional phenotype related to cytokine production and that the induction of CD163 expression will promote a more homeostatic phenotype by reducing their proinflammatory responsiveness.
Patients and methods: We tested these hypotheses in vitro using blood monocyte-derived macrophages from healthy subjects and patients with DM2 with and without DPN. Cells were incubated in the presence or the absence of 5 µg/mL of lipopolysaccharide (LPS). The concentrations of interleukin-10, interleukin-6, tumor necrosis factor-alpha (TNF-α), TGF-β, and monocyte chemoattractant protein-1 (MCP-1) were measured using ELISA assays. Macrophages were transfected with an empty vector plasmid or a plasmid containing the CD163 gene using mannosylated polyethylenimine nanoparticles.
Results: Our results show that nonstimulated DM2 or DPN macrophages have a constitutive primed proinflammatory state and display a deficient production of proinflammatory cytokines upon a proinflammatory challenge when compared to healthy macrophages. CD163 induction produced an anti-inflammatory phenotype in the healthy control group, and this effect was partial in DM2 or DPN macrophages.
Conclusion: Our results suggest that diabetic macrophages adopt a complex phenotype that is only partially reversed by CD163 induction. Future experiments are focused on elucidating this differential responsiveness between healthy and diabetic macrophages.
Keywords: primary human macrophages, CD163, transfection, LPS
Introduction
Diabetes is a multifactorial disease of epidemic proportions, which in 2015 affected 415 million people worldwide.1 Diabetes is associated with persistent inflammation, characterized by a proinflammatory phenotype in monocytes/macrophages.2 This contributes to the development of several complications, including diabetic peripheral neuropathy (DPN) that can progress to a painful condition.3,4 Chronic pain due to DPN is very difficult to treat; therefore, this condition has a severe impact on the quality of life of these patients. Furthermore, patients with DPN have a higher risk of developing chronic wounds.5 Such ulcers precede more than 85% of all lower extremity amputations in patients with diabetes and are a large source of health care expenditures costing the USA between 9 and 13 billion dollars in 2014.6,7
The complexity of the mechanisms underlying this condition could be a reason of the scarcity of effective treatments for painful DPN or diabetic ulcers. However, it is clear that the immune capabilities of an individual could determine the development of DPN. For instance, diabetic patients display elevated systemic levels of proinflammatory cytokines, chemokines, and acute phase proteins.4,8 Accordingly, this systemic inflammatory response seems to be driven by a dysfunctional proinflammatory phenotype in macrophages that play a key role in the pathogenesis of painful DPN9 or diabetic wounds.10–12
The normal resolution of inflammation requires a transition from an M1 (proinflammatory) phenotype in macrophages to an M2 (or alternatively activated) phenotype. However, in diabetic conditions, macrophages are persistently primed in an M1-like phenotype and promote diabetic neuropathy by their excessive production of proteases, cytokines, and reactive oxygen species, which creates an oxidative stress environment that degrades myelin and impairs nerve regeneration.13,14 Interestingly, M1 macrophages alter the normal function of pancreatic cells, which leads to insulin resistance and further exacerbates the disease and its complications due to hyperglycemia.15
Even though circulating monocytes seem to have an altered functional phenotype16,17 in patients with diabetes, macrophages are the final cellular effectors in pathological conditions such as ulcers, nerve damage, or pancreatic cells. In fact, we have previously shown that exposing human macrophages to a hyperglycemic in vitro environment alters their inflammatory response.18
Therefore, this study is focused on studying macrophages in an attempt to restore their normal functions by reducing their persistent primed/inflammatory phenotype.
Previous studies in our laboratory have revealed the anti-inflammatory capabilities of CD163 in human primary cells using a cell-directed gene therapy approach. CD163 is a cell-surface receptor expressed on peripheral blood monocytes and resident tissue macrophages.19 CD163-expressing macrophages are found during the resolving phase of acute inflammation, in chronic inflammation, and in healing.20,21 Interestingly, CD163-positive macrophages are reduced in patients with diabetes.17,22,23 This reduced CD163 expression has been associated with the presence of complications in these patients.23 Hence, the induction of CD163 in macrophages in patients with DM2 could restore normal macrophage functions.
Based on this evidence, we hypothesize that the induction of CD163 expression in macrophages from patients with DM2 or DPN will restore their phenotype and their capabilities to produce cytokines. To test our hypothesis, we first aimed to determine major cytokine production in macrophages from healthy subjects and patients with DM2 with and without DPN under basal conditions, and after the addition of a proinflammatory challenge. Second, we induced CD163 overexpression in isolated macrophages using a polyethyleneimine (PEI) nanoparticle grafted with a mannose receptor ligand (mannosylated PEI [Man-PEI]) that selectively targets macrophages. We have previously demonstrated the efficacy of Man-PEI to induce CD163 expression in THP-1 and human primary macrophages.24 Third, we tested whether the induction of CD163 promotes an anti-inflammatory phenotype in DM2 or DPN macrophages.
Patients and methods
Patients and healthy subjects
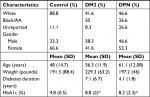
This study includes cells from 10 patients with a diagnosis of DM2 with no DPN, 15 patients with DPN, and eight healthy subjects. The demographic characteristics of the subjects are summarized in Table 1. Patients were recruited from the Presbyterian College School of Pharmacy (PCSP) Wellness Center, located in Laurens County, SC, USA, and control healthy volunteer subjects were recruited by word of mouth within the school and Laurens County community. Written informed consent was obtained from all participating subjects in accordance with the Declaration of Helsinki, and our protocol was approved by Presbyterian College International Review Board (IRB PC-201523).
Cell isolation and induction of monocyte-derived macrophages
Human monocytes were isolated from peripheral blood mononuclear cells (PBMC) of buffy coats obtained from all subjects using Ficoll-Hypaque solution (GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA) according to the manufacturer’s instruction. Then, monocytes were isolated from PBMC using Dynabeads untouched human monocyte kit (Thermo Fisher Scientific, Waltham, MA, USA). Primary human monocytes were cultured at 37°C under 5% CO2 in 24-well plates at a concentration of 2.5×105/mL in RPMI 1640 (supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin, and 1% sodium pyruvate) and M-CSF (100 ng/mL; eBioscience, San Diego, CA, USA) for 5–6 days to induce macrophage differentiation under normal glucose concentration (5 mM).
Cell transfection for CD163 induction
Macrophages from healthy controls and patients with DM2 or with DPN were transfected as previously described.24 Briefly, 0.5 µg of a plasmid-encoding CD163 gene (pCD163; Origene, Rockville, MD, USA) or an empty vector plasmid as the control (pEmpty, pCMV6-XL4 vector; Origene) was mixed with 1 µL of Man-PEI nanoparticle (Polyplus, New York, NY, USA) in a total volume of 100 µL of NaCl solution (150 mM). The solution was gently mixed and incubated at room temperature for 30 minutes. An N/P ratio of 5 was used to perform the transfection according to our previous work.24 Following the differentiation period, macrophages were incubated in the presence or the absence of lipopolysaccharide (LPS; Escherichia coli O111:B4, 5 µg/mL; Sigma-Aldrich Co., St Louis, MO, USA) for 48 hours. Cells or supernatants were collected at this time point for further analysis.
Immunocytochemistry
The membrane expression of CD163 was determined in LPS-stimulated macrophages, nonstimulated macrophages, and macrophages transfected with either pEmpty or pCD163 from healthy volunteers, diabetic patients, and patients with DPN.
Cells were collected after 48 hours in all the abovementioned conditions and plated on coverslips (12 mm diameter) precoated with fibronectin (Sigma-Aldrich Co.).
Then, the cells were fixed with 4% formaldehyde/PBS solution for 30 minutes at room temperature. The cells were washed with PBS and permeabilized with 0.25% Triton-X100 in PBS for 5 minutes (room temperature). Nonspecific binding was prevented by adding 0.5% FBS to the cells for 1 hour at room temperature. The cells were then incubated overnight at 4°C with a mouse antibody against human CD163 (1:150; Serotec, Raleigh, NC, USA). Then, the cells were rinsed and incubated for 1 hour at room temperature with the anti-mouse secondary antibody, which was raised in goat and conjugated to Alexa 555 (1:250; Thermo Fisher Scientific). The antibodies and the blocking solution were diluted in PBS that contained 0.25% Triton-X100. Coverslips containing cells were mounted on slides using an anti-fade medium (Vectashield; Vector Laboratories, Burlingame, CA, USA). Cell nuclei were visualized by adding DAPI to the mounting preparation (4′,6-diamidino-2-phenylindole dihydrochloride hydrate; Sigma-Aldrich Co.). Slides were examined at 40× using a Leica DMIL microscope and a Leica DFC345 FX Digital Camera (Leica Microsystems, Wetzlar, Germany). The fluorescence intensity of CD163 was quantified in each individual cell by a blinded experimenter. The mean background intensity was also calculated from areas outside of the cell perimeter. The average of intensity per cell was determined by subtracting the background using Sigma Scan Pro software (Systat Software Inc., San Jose, CA, USA).
ELISA
The concentrations of IL-6, monocyte chemoattractant protein-1 (MCP-1), TGF-β, IL-1β, tumor necrosis factor-alpha (TNF-α), and IL-10 were measured in cell-free supernatants using commercial ELISA kits (Ready-SET-Go! ELISA; eBioscience). Sensitivity of ELISA kits is as follows: 2 pg/mL for IL-6, 7 pg/mL for MCP-1, 8 pg/mL for TGF-β, 2.4 pg/mL for IL-1 β, 4 pg/mL, and 2 pg/mL for TNF-α and IL-10, respectively. These assays were performed in duplicates following the manufacturer’s instructions.
Statistics
All the statistical analyses were performed using GraphPad Prism 6.01 (GraphPad Software Inc., La Jolla, CA, USA). Two-tailed unpaired t-tests and two-way ANOVAs followed by Tukey’s post hoc were used as appropriate. A P value of 0.05 was considered to be statistically significant. For the cytokine studies using transfected cells, each value was normalized against their respective pEmpty control in at least three independent experiments, and we did statistical analysis and presented the data using the pooled values from these experiments.
Results
Cytokine profile in nonstimulated primary human macrophages
We cultured macrophages from healthy subjects (control group), patients with DM2, and patients with DPN and determined the levels of pro- and anti-inflammatory cytokines under basal nonstimulating conditions. When anti-inflammatory cytokines were measured, we found that macrophages from healthy subjects (control) produced detectable levels of IL-10, TGF-β, and sCD163 under basal conditions (Figure 1A–C). Macrophages from patients with DM2 produced similar levels of IL-10, TGF-β, and sCD163 to macrophages from control subjects (Figure 1A–C). Macrophages from patients with DPN produced significantly lower levels of IL-10 when compared to the control or the DM2 group (Figure 1A). Macrophages from patients with DPN produced similar concentrations of TGF-β (Figure 1B) and sCD163 (Figure 1C) to control and DM2 groups.
When proinflammatory cytokines were measured, we found that macrophages from healthy subjects (control) produced detectable levels of TNF-α, MCP-1, and IL-6 under basal conditions (Figure 1D–F). However, we did not detect IL-1β under these conditions in the control group or the DM2 and DPN groups. Macrophages from patients with DM2 produced similar levels of TNF-α and MCP-1 to macrophages from control subjects (Figure 1D and E). However, macrophages from patients with DM2 produced significantly higher levels of IL-6 when compared to the control group (Figure 1F). Macrophages from patients with DPN produced similar concentrations of TNF-α to the control and DM2 groups (Figure 1D). However, macrophages from the DPN group produced lower levels of MCP-1 than the control group, and this MCP-1 concentration was not significantly different from the DM2 group (Figure 1E). Macrophages from the DPN group produced similar levels of IL-6 to the control group, but these levels were significantly lower when compared to the high levels of IL-6 produced by macrophages from DM2 patients (Figure 1F).
Cytokine profile in LPS-stimulated primary human macrophages
We cultured macrophages from healthy subjects (control group), patients with DM2, and patients with DPN using LPS as inflammatory stimulus and determined the levels of pro- and anti-inflammatory cytokines under these conditions.
When anti-inflammatory cytokines were measured, we found that macrophages from healthy subjects (control) and patients with DM2 or DPN stimulated with LPS produced higher levels of IL-10, TGF-β, and sCD163 when compared to nonstimulated conditions (P<0.05; Table 2). Macrophages from the DM2 or DPN group produced similar concentrations of IL-10 (Figure 2A), TGF-β (Figure 2B), and sCD163 (Figure 2C) to macrophages from the control group under these inflammatory conditions. The concentration of these anti-inflammatory cytokines was similar in both diabetic and DPN groups.
When proinflammatory cytokines were measured, we found that macrophages from healthy patients (control) and patients with DM2 or DPN stimulated with LPS produced higher levels of TNF-α, MCP-1, IL-6, and IL-1β when compared to nonstimulated conditions (P<0.05; Table 3). Macrophages from the DM2 or DPN group produced lower levels of TNF-α, MCP-1, and IL-6 when compared to macrophages from the control group (Figure 2D–F). The levels of TNF-α, MCP-1, and IL-6 were similar in macrophages stimulated with LPS between the DM2 and DPN groups. Macrophages from the DM2 and DPN groups stimulated with LPS produced similar levels of IL-1β, and these levels were not different from the control group (Figure 2G).
Effects of CD163 overexpression on cytokine profile of LPS-stimulated primary human macrophages
Using Man-PEI nanoparticles we overexpressed CD163 in LPS-stimulated primary human macrophages from healthy subjects (control group), patients with DM2, and patients with DPN (Figure 3A–C, respectively).
When anti-inflammatory cytokines were measured, CD163-overexpressing macrophages (pCD163 group) from healthy control subjects produced similar levels of IL-10, TGF-β, and sCD163 (Figure 4A–C) when compared to the pEmpty group. However, CD163-overexpressing macrophages (pCD163 group) from patients with DM2 or DPN produced lower levels of IL-10 (Figure 4A) when compared to their respective pEmpty group. CD163-overexpressing macrophages (pCD163 group) from patients with DM2 or DPN produced similar levels of TGF-β and sCD163 (Figure 4B and C) when compared to their respective pEmpty group.
When proinflammatory cytokines were measured, CD163-overexpressing macrophages (pCD163 group) from healthy control subjects produced lower levels of TNF-α and IL-6 (Figure 4D and F) and similar levels of MCP-1 and IL-β (Figure 4E and G) when compared to the pEmpty group (Tables 4 and 5).
CD163-overexpressing macrophages (pCD163 group) from patients with DM2 produced lower levels of MCP-1 (Figure 4E) and no changes in the concentration of TNF-α, IL-6, and IL-β (Figure 4D, F and G) when compared to the pEmpty group.
CD163-overexpressing macrophages (pCD163 group) from patients with DPN produced lower levels of IL-6 (Figure 4F) and no changes in the concentration of TNF-α, MCP-1, and IL-β (Figure 4D, E and G) when compared to the pEmpty group (Tables 6 and 7).
Discussion
The major findings of our studies are as follows: 1) macrophages from diabetic patients displayed elevated levels of IL-6, indicative of a primed proinflammatory phenotype under basal conditions; 2) macrophages from DPN patients displayed a dysfunctional cytokine production repertoire under basal conditions, characterized by a lower production of IL-10 and MCP-1; 3) macrophages from patients with DM2 or DPN displayed a proinflammatory phenotype following LPS challenge; however, this phenotype shows a deficient cytokine production repertoire under inflammatory stimulation that affects mostly proinflammatory cytokines, such as TNF-α, MCP-1, and IL-6; and 4) the overexpression of CD163 in macrophages from patients with DM2 or DPN results in a differential and partial reduction of their proinflammatory phenotype. Our data indicate that the dysfunctional phenotype of diabetic macrophages regarding cytokine production differs from DM2 with or without DPN under nonstimulatory conditions; however, macrophages from both types of patients have a similar but dysfunctional capability to respond to a proinflammatory challenge. Surprisingly, this altered proinflammatory phenotype induced by LPS is differentially modulated by CD163 in DM2 with or without DPN. Even though these data demonstrate the complexity of the dysfunctional mechanisms of macrophages in diabetic conditions, the induction of CD163 could produce different therapeutic effects in either DM2 or DPN patients.
We have shown that culturing THP-1 macrophages under high glucose in vitro conditions induces a dysfunctional cellular phenotype following a proinflammatory challenge (LPS).18 Our current outcomes reflect the effects imprinted in monocyte phenotypes induced by long exposure to hyperglycemic conditions that persist upon differentiation to macrophages under normal glucose conditions. In consequence, our system captures phenotypic changes that arguably would not revert by the normalization of glycemic levels in patients with DM2. The clinical relevance of our findings is that DM2 patients with controlled glycemia might still be at the risk of developing complications derived from a proinflammatory priming phenotype of monocyte/macrophages, ie, following an infection, a cut, surgery, ulcer, or potentially the development of cardiovascular conditions or painful DNP as discussed subsequently.
Our findings indicate that the basal dysfunctional phenotype of macrophages from DM2 patients with no DPN is characterized by a higher production of IL-6 (with no changes in TNF-α, MCP-1, or major anti-inflammatory cytokines) when compared to healthy macrophages. Since our group populations differ in body mass index (BMI, healthy vs DM2 or DPN), this is a potential confounding factor in our studies. Macrophages infiltrate adipose tissue in a body mass-dependent manner,25 and these cells could be the source of high IL-6 serum levels observed in obese individuals.26 Curiously, high serum levels of MCP-1 are positively correlated with BMI (obesity) in patients with DM2 vs lean, healthy subjects,27 but we did not observe changes in MCP-1 in patients with DM2, which argues against a potential effect of BMI on our cytokine levels in these patient population. Similarly, we did not observe higher production of IL-6, TNF-α, MCP-1, or any major anti-inflammatory cytokine in patients with DPN, who also have a higher BMI that is comparable to patients with DM2 without DPN. These findings suggest that even though obesity or high BMI could influence the production of IL-6 (or other cytokines) in macrophages, DM2 and the presence or absence of DPN can also display changes in monocyte/macrophage phenotype under basal non-inflammatory conditions (perhaps due to the disease stage).
TNF-α has been shown to participate in the pathogenesis of insulin resistance and DM2,28,29 and high TNF-α expression levels in circulating macrophages have been associated with higher risk of developing painful diabetic neuropathy.9 Therefore, it was surprising that we did not observe differences in TNF-α production in DM2 or DPN macrophage groups when compared to macrophages from the healthy group. A potential explanation of this discrepancy is the inclusion of patients without painful DPN and/or the possibility that TNF-α has a more relevant role in the initiation of the development of DM2.
The relevance of our finding related to IL-6 in the context of diabetes is that high levels of IL-6 are positively related to insulin resistance in humans with obesity.26 Obesity and diabetes are associated with cardiovascular complications that initiate as atheromas. Interestingly, human atheromas or atherosclerotic lesions have high levels of IL-6 (and TNF-α).30–32 These vascular changes can promote a reduction in nutrients and oxygen supply to peripheral small neuronal fibers and therefore promote DPN and chronic pain in these patients. Interestingly, IL-6 production was not altered in DPN macrophages when compared to healthy cells, perhaps indicating that this cytokine is more relevant at previous DPN stages in patients with DM2 but not during neuropathic complications.
The level of dysfunction of macrophages from DPN patients involves both anti- and proinflammatory factors. The macrophage phenotype of patients with DPN was characterized by a reduced production of the proinflammatory chemokine MCP-1 and the anti-inflammatory cytokine, IL-10. MCP-1 is a strong chemoattractant for macrophages,33 and IL-10 is a potent molecule that induces resolution of the inflammatory process.34,35 These findings are in accordance with the higher risk (12-fold) of patients with DPN to develop chronic foot ulcers,36 which could be explained by the lack of a proper chemoattractant response (driven by MCP-1) and inadequate restraint signaling to acquire an M2 phenotype (driven by IL-10).
The dysfunctional phenotype observed in macrophages from these diabetic populations prompted us to examine their response capabilities upon a proinflammatory challenge using bacterial LPS. The responsiveness of macrophages to external stimulus in patients with diabetes is clinically relevant in the presence of open tissue damage, such as surgeries or foot ulcers, as LPS and microbacterial particles are present in patients with diabetic foot ulcers.37 Despite LPS inducing a higher production of all cytokines studied, we found that the magnitude of the production of proinflammatory cytokines (TNF-α, MCP1, and IL-6) was significantly lower in DM2 and DPN than in healthy macrophages. Interestingly, it has been reported that in a mouse with diabetes (rodent model), there is a reduced immune response (IL-6 and substance P), which is associated with a delayed wound healing process.38 On the other hand, an altered M1 proinflammatory phenotype seems to be sustained in time in diabetic chronic ulcers,10 and its restrained ability toward the acquisition of an M2 phenotype further complicates wound healing.39,40
We have previously shown that the induction of CD163 overexpression in primary macrophages from healthy humans stimulated with LPS promotes a transition from M1 to an alternative anti-inflammatory cellular phenotype. We have confirmed those findings in this study (reduction of TNF-α and IL-6). We anticipated observing similar effects in macrophages from our patient populations. Surprisingly, we found that these effects were only partially and differentially induced in macrophages from patients with DM2 (reduction only in IL-6) and DPN (reduction only in MCP-1), reflecting again the differential and complex phenotype that macrophages display in DM2 patients without neuropathy and in a more advanced stage of the disease with complications such as DPN.
The development of a cell-directed gene therapy based on the nanoparticle used in this study has been shown to be effective for gene induction and safe in HIV patients.41,42 Our approach to induce an alternative macrophage phenotype via CD163 induction to promote a more efficient resolution of inflammation and induction of wound healing would be suitable for patients with diabetes who have monocytes/macrophages in a persistent proinflammatory phenotype. This primed phenotype could predispose patients to have a suboptimal resolution of inflammation or wound healing following tissue damage such as foot ulcers. Thus, even though the induction of CD163 in macrophages from patients with DM2 or DPN was partial, a reduction of either MCP-1 or IL-6 may be beneficial during the healing process of chronic ulcers in these patients, where these cytokines should be reduced. Both MCP-1 and IL-6 serum levels are elevated in patients with chronic foot ulcers.43,44 In chronic stages in diabetic ulcers, the strong chemoattractant effects of MCP-133 could recruit more macrophages, which are persistently primed and dysfunctional, whereas IL-6 could promote the activation of naïve T cells.45,46 Therefore, these cytokines could perpetuate the inflammatory process in the local ulcer environment, preventing a proper wound healing.39,40 Thus, the local modulation of MCP-1 or IL-6 by CD163 induction in macrophages could promote a prowound healing environment.
One of the limitations of this study is that our in vitro setting might not completely mimic the complex systems found under diabetic conditions since different cell types contribute to the microenvironment in vivo. For instance, the effect of T cells on the regulation of the inflammatory environment during wound healing is well documented.47 In fact, in patients with diabetic complications such as foot ulceration48 or retinopathy,49 there is a dysregulation of effector and regulatory T cells. Whether the manipulation of the macrophage phenotype affects other cell types in vivo remains to be elucidated. However, our current studies are necessary to characterize our technique before exploring it in vivo and before moving it to clinical studies.
Patients with diabetic foot ulcers also present higher levels of TNF-α (and MCP-1 and IL-6) than patients with diabetes without foot ulcers.43,44 Interestingly, CD163 induction did not modify TNF-α in macrophages from either the DM2 or the DPN group. Whether this would impact the therapeutic potential of our approach remains to be elucidated.
Conclusion
Our data once again show that DM2 alters the phenotype of monocytes that persist after their differentiation to macrophages, and this phenotype is partially resistant to CD163 induction.
Acknowledgments
The authors would like to acknowledge Rita Allen Foundation and American Pain Society – Pain Scholar Award (EAR-S), NIH-NIGMS R15GM109333 (EAR-S), and Pharmacy Research Summer Internship (PAA-V, RLG, and CM-V) for funding.
Disclosure
The authors report no conflicts of interest in this work.
References
Ogurtsova LU, Guariguata L, Whiting D, Shaw J, da Rocha Fernandes JD. IDF Diabetes Atlas: estimates for 2015 and 2040. Diabetes Res Clin Pract Press. 2015;128:40–50. | ||
Barry JC, Shakibakho S, Durrer C, et al. Hyporesponsiveness to the anti-inflammatory action of interleukin-10 in type 2 diabetes. Sci Rep. 2016;6:21244. | ||
Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11(6):521–534. | ||
Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98–107. | ||
Juster-Switlyk K, Smith AG. Updates in diabetic peripheral neuropathy. F1000Res. 2016;5:738. | ||
Alvarsson A, Sandgren B, Wendel C, Alvarsson M, Brismar K. A retrospective analysis of amputation rates in diabetic patients: can lower extremity amputations be further prevented? Cardiovasc Diabetol. 2012;11:18. | ||
Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons NB. Burden of diabetic foot ulcers for medicare and private insurers. Diabetes Care. 2014;37(3):651–658. | ||
Spranger J, Kroke A, Möhlig M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52(3):812–817. | ||
Purwata TE. High TNF-alpha plasma levels and macrophages iNOS and TNF-alpha expression as risk factors for painful diabetic neuropathy. J Pain Res. 2011;4:169–175. | ||
Mirza RE, Fang MM, Ennis WJ, Koh TJ. Blocking interleukin-1β induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes. 2013;62(7):2579–2587. | ||
Mirza RE, Fang MM, Novak ML, et al. Macrophage PPARγ and impaired wound healing in type 2 diabetes. J Pathol. 2015;236(4):433–444. | ||
Mirza RE, Fang MM, Weinheimer-Haus EM, Ennis WJ, Koh TJ. Sustained inflammasome activity in macrophages impairs wound healing in type 2 diabetic humans and mice. Diabetes. 2014;63(3):1103–1114. | ||
Mcdonald DS, Cheng C, Martinez JA, Zochodne DW. Regenerative arrest of inflamed peripheral nerves: role of nitric oxide. Neuroreport. 2007;18(16):1635–1640. | ||
Conti G, Scarpini E, Baron P, et al. Macrophage infiltration and death in the nerve during the early phases of experimental diabetic neuropathy: a process concomitant with endoneurial induction of IL-1beta and p75NTR. J Neurol Sci. 2002;195(1):35–40. | ||
Eguchi K, Nagai R. Islet inflammation in type 2 diabetes and physiology. J Clin Invest. 2017;127(1):14–23. | ||
Dasu MR, Devaraj S, Park S, Jialal I. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care. 2010;33(4):861–868. | ||
Satoh N, Shimatsu A, Himeno A, et al. Unbalanced M1/M2 phenotype of peripheral blood monocytes in obese diabetic patients: effect of pioglitazone. Diabetes Care. 2010;33(1):e7. | ||
Grosick R, Alvarado-Vazquez PA, Messersmith A, Romero-Sandoval EA. High glucose induces a priming effect in macrophages and exacerbates the production of pro-inflammatory cytokines after a challenge. J Pain Res. 2018;11:1769–1778. | ||
Zwadlo G, Voegeli R, Schulze Osthoff K, Sorg C. A monoclonal antibody to a novel differentiation antigen on human macrophages associated with the down-regulatory phase of the inflammatory process. Exp Cell Biol. 1987;55(6):295–304. | ||
Philippidis P, Mason JC, Evans BJ, et al. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ Res. 2004;94(1):119–126. | ||
Verschure PJ, van Noorden CJ, Dijkstra CD. Macrophages and dendritic cells during the early stages of antigen-induced arthritis in rats: immunohistochemical analysis of cryostat sections of the whole knee joint. Scand J Immunol. 1989;29(3):371–381. | ||
Levy AP, Purushothaman KR, Levy NS, et al. Downregulation of the hemoglobin scavenger receptor in individuals with diabetes and the Hp 2-2 genotype: implications for the response to intraplaque hemorrhage and plaque vulnerability. Circ Res. 2007;101(1):106–110. | ||
Min D, Brooks B, Wong J, et al. Monocyte CD163 is altered in association with diabetic complications: possible protective role. J Leukoc Biol. 2016;100(6):1375–1383. | ||
Bernal L, Alvarado-Vázquez A, Ferreira DW, et al. Evaluation of a nanotechnology-based approach to induce gene-expression in human THP-1 macrophages under inflammatory conditions. Immunobiology. 2017;222(2):399–408. | ||
Weisberg SP, Mccann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. | ||
Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280(5):E745–E751. | ||
Janowska J, Chudek J, Olszanecka-Glinianowicz M, Semik-Grabarczyk E, Zahorska-Markiewicz B. Interdependencies among selected pro-inflammatory markers of endothelial dysfunction, C-peptide, anti-inflammatory interleukin-10 and glucose metabolism disturbance in obese women. Int J Med Sci. 2016;13(7):490–499. | ||
Ehses JA, Perren A, Eppler E, et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56(9):2356–2370. | ||
Moller DE. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2000;11(6):212–217. | ||
Barath P, Fishbein MC, Cao J, Berenson J, Helfant RH, Forrester JS. Detection and localization of tumor necrosis factor in human atheroma. Am J Cardiol. 1990;65(5):297–302. | ||
Seino Y, Ikeda U, Ikeda M, et al. Interleukin 6 gene transcripts are expressed in human atherosclerotic lesions. Cytokine. 1994;6(1):87–91. | ||
Rus HG, Vlaicu R, Niculescu F. Interleukin-6 and interleukin-8 protein and gene expression in human arterial atherosclerotic wall. Atherosclerosis. 1996;127(2):263–271. | ||
Wood S, Jayaraman V, Huelsmann EJ, et al. Pro-inflammatory chemokine CCL2 (MCP-1) promotes healing in diabetic wounds by restoring the macrophage response. PLoS One. 2014;9(3):e91574. | ||
Sabat R, Grütz G, Warszawska K, et al. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010;21(5):331–344. | ||
Siqueira Mietto B, Kroner A, Girolami EI, Santos-Nogueira E, Zhang J, David S. Role of IL-10 in resolution of inflammation and functional recovery after peripheral nerve injury. J Neurosci. 2015;35(50):16431–16442. | ||
Lavery LA, Armstrong DG, Vela SA, Quebedeaux TL, Fleischli JG. Practical criteria for screening patients at high risk for diabetic foot ulceration. Arch Intern Med. 1998;158(2):157–162. | ||
Trøstrup H, Holstein P, Christophersen L, et al. S100A8/A9 is an important host defence mediator in neuropathic foot ulcers in patients with type 2 diabetes mellitus. Arch Dermatol Res. 2016;308(5):347–355. | ||
Leal EC, Carvalho E, Tellechea A, et al. Substance P promotes wound healing in diabetes by modulating inflammation and macrophage phenotype. Am J Pathol. 2015;185(6):1638–1648. | ||
Driver VR, Yao M, Kantarci A, Gu G, Park N, Hasturk H. A prospective, randomized clinical study evaluating the effect of transdermal continuous oxygen therapy on biological processes and foot ulcer healing in persons with diabetes mellitus. Ostomy Wound Manage. 2013;59(11):19–26. | ||
Yao M, Hasturk H, Kantarci A, et al. A pilot study evaluating non-contact low-frequency ultrasound and underlying molecular mechanism on diabetic foot ulcers. Int Wound J. 2014;11(6):586–593. | ||
Rodriguez B, Asmuth DM, Matining RM, et al. Safety, tolerability, and immunogenicity of repeated doses of dermavir, a candidate therapeutic HIV vaccine, in HIV-infected patients receiving combination antiretroviral therapy: results of the ACTG 5176 trial. J Acquir Immune Defic Syndr. 2013;64(4):351–359. | ||
Lisziewicz J, Bakare N, Calarota SA, et al. Single DermaVir immunization: dose-dependent expansion of precursor/memory T cells against all HIV antigens in HIV-1 infected individuals. PLoS One. 2012;7(5):e35416. | ||
Tecilazich F, Dinh T, Pradhan-Nabzdyk L, et al. Role of endothelial progenitor cells and inflammatory cytokines in healing of diabetic foot ulcers. PLoS One. 2013;8(12):e83314. | ||
Weigelt C, Rose B, Poschen U, et al. Immune mediators in patients with acute diabetic foot syndrome. Diabetes Care. 2009;32(8):1491–1496. | ||
Ferreira RC, Rainbow DB, Rubio García A, et al. Human IL-6R hi TIGIT − CD4 + CD127 low CD25 + T cells display potent in vitro suppressive capacity and a distinct Th17 profile. Clinical Immunology. 2017;179:25–39. | ||
Xu E, Pereira MMA, Karakasilioti I, et al. Temporal and tissue-specific requirements for T-lymphocyte IL-6 signalling in obesity-associated inflammation and insulin resistance. Nat Commun. 2017;8:14803. | ||
Brockmann L, Giannou AD, Gagliani N, Huber S. Regulation of TH17 cells and associated cytokines in wound healing, tissue regeneration, and carcinogenesis. Int J Mol Sci. 2017;18(5):1033. | ||
Moura J, Rodrigues J, Gonçalves M, Amaral C, Lima M, Carvalho E. Impaired T-cell differentiation in diabetic foot ulceration. Cell Mol Immunol. 2017;14(9):758–769. | ||
Yang TT, Song SJ, Xue HB, Shi DF, Liu CM, Liu H. Regulatory T cells in the pathogenesis of type 2 diabetes mellitus retinopathy by miR-155. Eur Rev Med Pharmacol Sci. 2015;19(11):2010–2015. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.