Back to Journals » International Journal of Nanomedicine » Volume 12
Cytokine induction of sol–gel-derived TiO2 and SiO2 coatings on metallic substrates after implantation to rat femur
Authors Urbanski W, Marycz K, Krzak J, Pezowicz C, Dragan SF
Received 13 June 2016
Accepted for publication 13 September 2016
Published 28 February 2017 Volume 2017:12 Pages 1639—1645
DOI https://doi.org/10.2147/IJN.S114885
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Wiktor Urbanski,1 Krzysztof Marycz,2 Justyna Krzak,3 Celina Pezowicz,4 Szymon Feliks Dragan1
1Department of Orthopaedic Surgery and Traumatology, Wroclaw University Hospital, 2Electron Microscope Laboratory, Wroclaw University of Environmental and Life Sciences, 3Institute of Materials Science and Applied Mechanics, 4Division of Biomedical Engineering and Experimental Mechanics, Wroclaw University of Technology, Wroclaw, Poland
Abstract: Material surface is a key determinant of host response on implanted biomaterial. Therefore, modification of the implant surface may optimize implant–tissue reactions. Inflammatory reaction is inevitable after biomaterial implantation, but prolonged inflammation may lead to adverse reactions and subsequent implant failure. Proinflammatory activities of cytokines like interleukin (IL)-1, IL-6, and tumor necrosis factor-alpha (TNF-α) are attractive indicators of these processes and ultimately characterize biocompatibility. The objective of the study was to evaluate local cytokine production after implantation of stainless steel 316L (SS) and titanium alloy (Ti6Al4V) biomaterials coated with titanium dioxide (TiO2) and silica (SiO2) coatings prepared by sol–gel method. Biomaterials were implanted into rat femur and after 12 weeks, bones were harvested. Bone–implant tissue interface was evaluated; immunohistochemical staining was performed to identify IL-6, TNF-α, and Caspase-1. Histomorphometry (AxioVision Rel. 4.6.3 software) of tissue samples was performed in order to quantify the cytokine levels. Both the oxide coatings on SS and Ti6Al4V significantly reduced cytokine production. However, the lowest cytokine levels were observed in TiO2 groups. Cytokine content in uncoated groups was lower in Ti6Al4V than in SS, although coating of either metal reduced cytokine production to similar levels. Sol–gel TiO2 or SiO2 coatings reduced significantly the production of proinflammatory cytokines by local tissues, irrespective of the material used as a substrate, that is, either Ti6Al4V or SS. This suggests lower inflammatory response, which directly points out improvement of materials’ biocompatibility.
Keywords: bone implant, surface modification, sol–gel coatings, inflammation, biomaterial
Introduction
The use of metallic implants is a substantial part of the treatment in orthopedic surgery. Necessary condition of their clinical success is effective osteointegration – bonding between bone and the implant. Immediately after implantation, host tissues react on the biomaterial with acute inflammation, which, within a few days, transforms into chronic phase lasting for months or even years. The dynamics of the latter phase determine the final outcome – long-lasting osteointegration or extensive inflammation leading to implant loosening and subsequent clinical failure.1 Tissue reaction and inflammatory response on the biomaterial is determined by cell activity (monocytes and macrophages mainly). The activated macrophages not only secrete cytokines to recruit other cell types involved in inflammation, but are also responsible for healing of the implant site.1,2 Cytokines are not only known for their regulatory role in inflammatory reactions and bone healing, but also they determine the presence and intensity of the foreign body reaction, and as well as in case of prolonged activity, can negatively affect bone turnover.3 Therefore, interleukin (IL)-1, IL-6, and tumor necrosis factor-alpha (TNF-α), with their proinflammatory activity and contribution to osteolytic processes, are attractive indicators to assess the biologic function of biomaterials.1,2,4,5
Implant surface is one of the most important factors regulating the interaction between the biomaterial and the bone tissue.6–10 Therefore, techniques of surface modifications are extensively studied in order to improve the clinical performance of biomaterials. The ideal surface is biocompatible, osteoconductive, and osteoinductive, limits corrosion and particle release from the material, and is also mechanically stable with antimicrobial properties.11–13
In the present study, silica (SiO2) and titanium dioxide (TiO2) thin films for biomedical applications have been synthesized by nonaqueous sol–gel dip-coating method on stainless steel 316L (SS) and titanium alloy (Ti6Al4V) substrates. Previously, the authors conducted surface studies and in vitro tests of these biomaterials, and demonstrated the potential for applications in biologic environment.14–16
The aim of this study was to evaluate the inflammatory response on stainless steel 316L (SS) and Ti6Al4V biomaterials coated with TiO2 and SiO2 sol -gel layers implanted to rat femur.
Materials and methods
Stainless steel 316L (SS) and Ti6Al4V prim-shaped implants, 10 mm long with square base 1×1 mm, were prepared for the in vivo experiments. SiO2 and TiO2 thin films were synthesized by nonaqueous sol–gel dip-coating method. Sol–gel synthesis was based on the hydrolysis of alkoxide precursors at room temperature. Tetraethoxyorthosilicate (Sigma-Aldrich Co.) and diethoxydimethylsilane (Sigma-Aldrich Co.) in a molar ratio of 1.79 were used as SiO2 precursors. As titania precursor, titanium (IV) isopropoxide (Sigma-Aldrich Co.) was used. Directly before coating, the substrates were washed with acetone or dilute HCl, then with distilled water, and finally with alcohol. Water necessary for hydrolysis was derived as moisture from the atmosphere, according to the method described previously.17 Synthesis of dioxide coatings, as well as physicochemical assessment, surface analysis, and mechanical studies were conducted as previously published.15,16,18,19
Thirty-two male Wistar rats, with body weight approximately 300 g, aged 3–6 months were used for the experiment. All rats were kept in the same room under standard conditions (12 hours/12 hours of light/darkness period, room temperature 20.5°C±1°C) in separate cages with free access to water and rat chow, without any movement restrictions.
Surgical procedures were carried out under general anesthesia in aseptic conditions. To anesthetize the animals, a mixture of 1 mL ketamine hydrochloride (100 mg/mL) and 0.5 mL xylazine hydrochloride (20 mg/mL) added to 10 mL of 0.9% NaCl was prepared and injected intraperitoneally at a dose of 1 mL/100 mg body weight. The animal leg was shaved, washed in chlorhexidine solution, and positioned and clothed in sterile sheets on the operating table. A curved incision measuring 10–15 mm was made on the anterolateral knee surface; the joint capsule was dissected, incised, and then the patella subluxated, exposing the femur intercondylar fossa (Figure 1A). Femur’s medullary canal was opened through the fossa using a 1.2 mm drill and the specimen was positioned in the medullary canal (Figure 1B). Subsequently, the wound was closed and each layer separately sutured: the joint capsule, fascia, subcutaneous tissues, and skin. X-rays were taken just after surgery to confirm proper location of the implants (distal metaphyseal–diaphyseal; Figure 1C). The animals were sacrificed after 12 weeks by administering intraperitoneal injections of pentobarbital (Morbital) at a dose of 2 mL/kg body weight. Distal femurs were dissected and evaluation carried out.
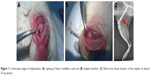  | Figure 1 Consecutive stages of implantation: (A) opening of femur medullary canal and (B) implant insertion. (C) Red arrow shows location of the implant on lateral X-ray picture. |
From each animal, three tissue samples were obtained. The samples consisted of 2–3 mm of trabecular bone adjacent to the implant, which originated from three different localizations (proximal, middle, and distal ends of the implant). The animals were randomized and divided into two control groups (uncoated SS 316L implants, n=4; uncoated Ti6Al4V, n=4) and four experimental groups (I. SS coated with SiO2, n=6; II. SS coated with TiO2, n=6; III. Ti6Al4V coated with SiO2, n=6; IV. SS coated with TiO2, n=6).
All procedures were conducted according to the guidelines for the care and treatment of laboratory animals (EU directive 2010/63/EU), and the study was approved by the local ethics board (The Second Local Bioethical Commission in Wroclaw, approval 86/2009).
With a microtome (Zeiss Microm HM 340E), the bones were cut into 3 μm thick sections, dehydrated in xylene and alcohol graded series, and placed on histological slides. After fixation and dehydration, the specimens were incubated in Tris/ethylenediaminetetraacetic acid buffer (pH =9.0) for 20 minutes to carry out heat-induced epitope retrieval. Endogenous peroxidase activity was blocked in 3% hydrogen peroxide for 5 minutes; then, the samples were briefly rinsed with Tris-buffered saline (TBS) (3×5 minutes). The tissue samples were incubated for 1 hour at room temperature with primary antisera raised against IL-6 (rat, dilution 1:400; Abcam), TNF receptor I (rat, dilution 1:1,000; Abcam), and Caspase-1 (rat, dilution 1:5; Abcam). After subsequent rinsing in TBS (3×5 minutes), the sections were incubated with secondary antibodies (EnVision Systems; Dako) for 1 hour at room temperature. Subsequently, the samples were counterstained with Mayer’s hematoxylin, dehydrated in alcohol and xylene series, mounted with permanent mounting medium, and finally covered with a glass coverslip.
Approximately 2 mm layers of tissues adjacent to the implants were evaluated. High-resolution images were taken under a light microscope (Carl Zeiss Axio Imager A1) at 320× magnification, and all images were processed with the same parameters and histomorphometry was performed. AxioVision Rel. 4.6.3 (Carl Zeiss) software was used to identify and mark the tissue containing high concentrations of cytokines and its total area was estimated in μm2. In all specimens, the same cutoff parameters were applied to detect high cytokine levels.
Statistical analysis of independent and dependent variables, with respect to the total area (μm2) of the tissue containing cytokines, was performed using two-way analysis of variance. Based on the results obtained from analysis of variance, two sample t-test was used to compare the individual differences between the mean values (μm2) of cytokine-containing areas of the control and experimental groups. Mann–Whitney U test or Wilcoxon test was applied for nonparametric analysis. To verify whether the two sample t-test can be used, normal distribution of the variables was checked with Shapiro–Wilk test and homogeneity of variance with Brown and Forsythe test. A P-value <0.05 was considered statistically significant.
Results
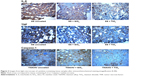
Uncoated SS implants induced much more intense cytokine production (IL-6, Caspase-1, TNF-α) than the SS implants coated with either TiO2 or SiO2. It was confirmed with a high significance obtained in statistical analysis (Table 1). Comparison between two tested coatings on SS revealed that TiO2 induced lower cytokine production than SiO2 (Figure 2); however, statistical significance was reached only for TNF-α with P=0.024 (Caspase-1 P=0.374, IL-6 P=0.059).
Coated Ti6Al4V with either TiO2 or SiO2 significantly decreased the cytokine content in comparison to uncoated Ti6Al4V, except that statistically insignificant result was obtained for Caspase-1 in Ti6Al4V + SiO2, P=0.129 (Figure 2; Table 1).
Comparison of cytokine content in the tissues surrounding coated titanium implants revealed more significant reduction of IL-6 and Caspase-1 in Ti6Al4V + TiO2. On the contrary, TNF-α was more abundant in Ti6Al4V + TiO2 than Ti6Al4V + SiO2. Differences, however, were small and insignificant statistically for IL-6 (P=0.0701) and TNF-α (P=0.1758), although for Caspase-1, they were more pronounced with a P=0.0021 (Figure 2).
The differences in cytokine content between uncoated SS and Ti6Al4V were very distinctive and smaller in Ti6Al4V (IL-6 P=0.008, Caspase-1 P=0.005, TNF-α P=0.015). Coating of either material declined the cytokine production; moreover, it brought them down to similar levels (Figures 2 and 3). The differences between SS- and Ti6Al4V-coated materials were highly insignificant, and it was proven in the statistical analysis for each cytokine.
Discussion
The extent of tissue reaction after material implantation is either host or implanted material dependent. Regarding biomaterial features, the most important are the material size, surface topology and chemistry, mechanical forces, and the release of degradation products from the implant.3,8–10,20,21 Hence, the interactions between the implant and bone are mostly determined by the biomaterial surface. Therefore, altering the surface and its features is a way to improve implant -tissue interaction.
In this study, the authors tested the biologic interactions of the implants coated with oxides (SiO2 and titania) obtained with sol–gel method. It was previously demonstrated that sol–gel-derived oxide films were bioactive in vitro and in vivo, and could induce bone attachment to the metallic materials, which confirmed their suitability as bone implants.22–26 The sol–gel method of SiO2- and titania-based coating synthesis is inexpensive and allows to control the film properties by changing the solution composition or deposition process details (eg, homogenous physicochemical structure, roughness, Young’s modulus, etc). It also affects the biomaterial’s surface features; topography, roughness, and wettability.27,28 With qualities appropriate for cell attachment, like high wettability and surface roughness, bioactivity of the tested sol–gel dioxides was proven and published previously.14–19
An act of implantation of a foreign material into bone always initiates certain cascade of reactions: hematoma, inflammation, and subsequently, either osteointegration or foreign body reaction and implant failure.1,3 Nevertheless, particles and debris from the material may induce high levels of proinflammatory cytokines, resulting in persisting inflammation. This may disturb osteointegration, induce FBR, or elicit osteolysis of previously integrated implant.2,29,30 The cytokines analyzed in the study – IL-6 and TNF-α – play an important role in osteolysis. Caspase-1 levels were also assessed, since its activity reflects IL-1 levels – Caspase-1 activates precursors of IL-1, another significant contributor to osteolytic process.31 It was demonstrated that TNF-α, IL-6, and IL-1 had adverse effects on osteoblastogenesis from mesenchymal stem cells and caused osteoclast-induced bone destruction.5,32–34 High concentrations of these proteins were observed in the tissues surrounding loosened endoprosthesis as well as in failed dental implants.30,35–38 Hence, local cytokine levels may be considered as an indicator of biomaterial compatibility and also its performance. Thus, creating biomaterial that results in the lowest possible tissue cytokine secretion might improve its clinical performance.
In this study, the authors observed substantial drop in cytokine levels after coating with Ti6Al4V and SS materials. More considerable decrease was noticed in SS-based materials than Ti6Al4V-based materials, because of good biocompatibility of titanium and its alloys and poor biocompatibility of SS. Coating of either material declined cytokine production and brought it down to similar levels (Figure 3), even enhancing the biocompatibility of coated SS above that of uncoated Ti6Al4V levels, particularly of SS + TiO2 (Figures 2 and 3). Thus, on modification of SS – an inexpensive metal with low biocompatibility, low corrosion resistance, containing allergenic ingredients (eg, nickel) but with excellent strength, we obtained a biomaterial with high biocompatibility, concomitantly maintaining its mechanical properties. Such a compound can be used for internal fixation of long bones as well as in the operative treatment of spinal deformity, since the rods used for correction and spine fixation in spinal surgery should be of high strength and stiffness.
To our knowledge, there is no report on alteration in cytokine levels on sol–gel TiO2 or SiO2 coatings, although reduction of inflammatory response after coating the implants with sol–gel oxide layers was reported by other authors.39–42 Data presented in this paper suggest better cytokine reduction of TiO2 coatings on Ti6Al4V and SS, which was consistent to other authors’ findings on inflammatory response. There are no reports of comparative analysis between these layers in the literature available. It seems that superiority of anti-inflammatory activity of TiO2 can be related to its additional antioxidant properties. According to the study of Contreras et al, titanium oxide reduces the level of reactive oxygen species (free radicals), both neutrophilic and of chemical origin.42 Another contribution may be the fact that SiO2 layer has inferior stability than TiO2, and it is a partially degradable material, releasing the particles to the environment.43
The strength of the study is that the analysis was conducted in bone tissue, an environment of the final implant, thus providing information concerned with the target tissue.
Lack of proper estimation of the concentration of the given cytokines in this study may be considered as a weakness; however, the method presented is simple and provides clear and reliable data. Common methods to assess the level of cytokine include either direct staining for substance concentration or molecular methods based on quantification of cytokine mRNA. To evaluate the protein concentration, exudative fluid is needed; but the methodology to obtain it is complicated and impedes assessing other parameters of implant integration. It was also demonstrated that the amount of mRNA was not always proportional to proinflammatory cytokine activity and was not equal to the observed inflammatory reaction.44
Conclusion
Sol–gel TiO2 or SiO2 coatings reduced significantly production of proinflammatory cytokines by the local tissues, irrespective of the material used as a substrate, that is, either Ti6Al4V or stainless steel 316L. This suggests lower inflammatory response, which directly points out improvement of materials’ biocompatibility. SS, an inexpensive metal popular in orthopedic and dental surgery, is known for possessing desired mechanical properties, but is of low biocompatibility. After oxide sol–gel coating, it is converted to a biocompatible biomaterial, which widens the range of its clinical applications.
Disclosure
The authors report no conflicts of interest in this work.
References
Lin TH, Tamaki Y, Pajarinen J, et al. Chronic inflammation in biomaterial-induced periprosthetic osteolysis: NF-κB as a therapeutic target. Acta Biomater. 2014;10(1):1–10. | ||
Gallo J, Raska M, Mrázek F, Petrek. Bone remodeling particle disease and individual susceptibility to periprosthetic osteolysis. Physiol Res. 2008;57(3):339–349. | ||
Chen SL, Jones JA, Xu YG, Low HY, Anderson JM, Leong KW. Characterization of topographical effects on macrophage behaviour in a foreign body response model. Biomaterials. 2010;31(13):3479–3491. | ||
Greenblatt MB, Shim JH. Osteoimmunology: a brief introduction. Immune Netw. 2013;13(4):111–115. | ||
Lacey DC, Simmons PJ, Graves SE, Hamilton JA. Proinflammatory cytokines inhibit osteogenic differentiation from stem cells: implications for bone repair during inflammation. Osteoarthritis Cartilage. 2009;17(6):735–742. | ||
Rani VV, Vinoth-Kumar L, Anitha VC, Manzoor K, Deepthy M, Shantikumar VN. Osteointegration of titanium implant is sensitive to specific nanostructure morphology. Acta Biomater 2012;8(5):1976–1989. | ||
Brown BN, Badylak SF. Expanded applications, shifting paradigms and an improved understanding of host–biomaterial interactions. Acta Biomater. 2013;9(2):4948–4955. | ||
Brodbeck WG, Nakayama Y, Matsuda T, Colton E, Ziats NP, Anderson JM. Biomaterial surface chemistry dictates adherent monocyte/macrophage cytokine expression in vitro. Cytokine. 2002;18(6):311–319. | ||
Xing S, Santerre JP, Labow RS, Boynton EL. Differential response to chemically altered polyethylene by activated mature human monocyte-derived macrophages. Biomaterials. 2002;23(17):3595–3602. | ||
Brodbeck WG, Voskerician G, Ziats NP, Nakayama Y, Matsuda T, Anderson JM. In vivo leukocyte cytokine mRNA responses to biomaterials are dependent on surface chemistry. J Biomed Mater Res A. 2003;64(2):320–329. | ||
Simchi A, Tamjid E, Pishbin F, Boccaccini AR. Recent progress in inorganic and composite coatings with bactericidal capability for orthopaedic applications. Nanomedicine. 2011;7(1):22–39. | ||
Chiriac AP, Nita LE, Neamtu I, Nistor MT. Sol-gel technique applied for biomaterials achievement. Recent Patents on Materials Science. 2011;4(3):224–237. | ||
Zhang BG, Myers DE, Wallace GG, Brandt M, Choong PF. Bioactive coatings for orthopaedic implants-recent trends in development of implant coatings. Int J Mol Sci. 2014;15(7):11878–11921. | ||
Urbanski W, Dragan S, Gebarowska E, et al. Preliminary evaluation of selected biologic properties of TiO2 and SiO2 layers on metallic substrates. Eng Biomaterials. 2010;13(96–98):129–133. | ||
Marycz K, Krzak-Ros J, Donesz-Sikorska A, Smieszek A. The morphology, proliferation rate, and population doubling time factor of adipose-derived mesenchymal stem cells cultured on to non-aqueous SiO2, TiO2, and hybrid sol-gel-derived oxide coatings. J Biomed Mater Res Part A. 2014;102(11):4017–4026. | ||
Marycz K, Krzak-Ros J, Urbanski W, Pezowicz C. In vitro and in vivo evaluation of sol-gel derived TiO2 coatings, based on a variety of precursors and synthesis conditions. J Nanomater. 2014;2014:14. | ||
Advincula MC, Petersen D, Rahemtulla F, Advincula R, Lemons JE. Surface analysis and biocorrosion properties of nanostructured surface sol–gel coatings on Ti6Al4V titanium alloy implants. J Biomed Mater Res Part B Appl Biomater. 2007;80(1):107–120. | ||
Tkaczyk M, Krzak-Ros J, Kaleta J. Evaluation of mechanical and physicochemical properties of protection coatings obtained by the sol-gel method. Mater Sci. 2012;48(3):323–331. | ||
Krzak-Ros J, Filipiak J, Pezowicz C, et al. The effect of substrate roughness on the surface structure of TiO2, SiO2 and doped thin films prepared by the sol–gel method. Acta Bioeng Biomech. 2009;11(2):21–29. | ||
Jones JA, Dadsetan M, Collier TO, et al. Macrophage behavior on surface-modified polyurethanes. J Biomater Sci Polym Ed. 2004;15(5):567–584. | ||
Refai AK, Textor M, Brunette DM, Waterfield JD. Effect of titanium surface topography on macrophage activation and secretion of proinflammatory cytokines and chemokines. J Biomed Mater Res A. 2004;70(2):194–205. | ||
Chai F, Ochsenbein A, Traisnel M, Busch R, Breme J, Hildebrand HF. Improving endothelial cell adhesion and proliferation on titanium by sol-gel derived oxide coating. J Biomed Mater Res A. 2010;92(2):754–765. | ||
Fathi MH, Doost Mohammadi A. Preparation and characterization of sol-gel bioactive glass coating for improvement of biocompatibility of human body implant. Mater Sci Eng A. 2008;474:128–133. | ||
Areva S, Paldan H, Peltola T, Narhi T, Jokinen M, Linden M. Use of sol-gel-derived titania coating for direct soft tissue attachment. J Biomed Mater Res Part A. 2004;70(2):169–178. | ||
Li P, de Groot K. Better bioactive ceramics through sol-gel process. J Sol-Gel Sci Technol. 1994;2:797–801. | ||
Li P, Ohtsuki C, Kokubo T, Nakanishi K, Soga N, de Groot K. A role of hydrated silica, titania, and alumina in forming biologically active bone-like apatite on an implant. J Biomed Mater Res. 1994;28(1):7–15. | ||
Lopez DA, Rosero-Navarro NC, Ballarre J, Duran A, Aparicio M, Cere S. Multilayer silica-methacrylate hybrid coatings prepared by sol-gel on stainless steel 316L: electrochemical evaluation. Surf Coat Technol. 2008;202(10):2194–2201. | ||
Orignac X, Vasconcelos HC, Du XM Almeida RM. Influence of solvent concentration on the microstructure of SiO2–TiO2 sol-gel films. J Sol-Gel Sci Technol. 1997;8(1):243–248. | ||
Revell PA. Biological causes of prosthesis joint failure. In: Revell PA, editor. Joint Replacement Technology. Cambridge, UK: Woodhead Publishing Limited and CRC Press LLC. 2008:298–369. | ||
Shanbhag A, Rubash HE, Jacobs JJ, editors. Joint Replacement and Bone Resorption. Pathology, Biomaterials, and Clinical Practice. London: Taylor & Francis Group, LLC; 2006. | ||
Sollberger G, Strittmatter GE, Garstkiewicz M, Sand J, Beer HD. Caspase-1: the inflammasome and beyond. Innate Immun. 2014;20(2):115–125. | ||
Kaji K, Katogi R, Azuma Y, Naito A, Inouc JI, Kudo A. Tumor necrosis factor alpha induced osteoclastogenesis requires tumor necrosis factor receptor – associated factor. J Bone Miner Res. 2001;16(9):1593–1599. | ||
Kotake S, Nanke Y. Effect of TNFα on osteoblastogenesis from mesenchymal stem cells. Biochim Biophys Acta. 2014;1840(3):1209–1213. | ||
Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest. 2005;115(12):282–290. | ||
Glant TT, Jacobs JJ, Molnar G, Shanbhag AS, Valyon M, Galante JO. Bone resorption activity of particulate-stimulated macrophages. J Bone Miner Res. 1993;8(9):1071–1079. | ||
Shanbhag AS, Jacobs JJ, Black J, Galante JO, Glant TT. Cellular mediators secreted by interfacial membranes obtained at revision total hip arthroplasty. J Arthroplasty. 1995;10(4):498–506. | ||
Holding CA, Findlay DM, Stamenkov R, et al. The correlation of RANK, RANKL and TNF alpha expression with bone loss volume and polyethylene wear debris around hip implants. Biomaterials. 2006;27(30):5212–5219. | ||
Ata-Ali J, Flichy-Fernández AJ, Alegre-Domingo T, Ata-Ali F, Palacio J, Peñarrocha-Diago. Clinical, microbiological, and immunological aspects of healthy versus peri-implantitis tissue in full arch reconstruction patients: a prospective cross-sectional study. BMC Oral Health. 2015;15:43. | ||
Rossi S, Tirri T, Paldan H, Kuntsi-Vaattovaara H, Tulamo R, Närhi T. Peri-implant tissue response to TiO2 surface modified implants. Clin Oral Impl Res. 2008;19(4):348–355. | ||
Erli HJ, Ruger M, Rago C, et al. The effect of surface modification of a porous TiO2/perlite composite on the ingrowth of bone tissue in vivo. Biomaterials. 2006;27(8):1270–1276. | ||
Kitsugi T, Nakamura T, Oka M, Yan W-Q, Goto T, Shibuya T. Bone bonding behavior of titanium and its alloys when coated with titanium oxide (TiO2) and titanium silicate (Ti5Si3). J Biomed Mater Res. 1996;32(2):149–156. | ||
Contreras R, Sahlin H, Frangos JA. Titanate biomaterials with enhanced antinflammatory properties. J Biomed Mater Res A. 2007;80(2):480–485. | ||
Kortesuo P, Ahola M, Karlson S, Kangasniemi I, Yli-Urpo A, Kiesvaara J. Silica xerogel as an implantable carrier for controlled drug delivery – evaluation of drug distribution and tissue effects after implantation. Biomaterials. 2000;21(2):193–198. | ||
Schutte RJ, Xie L, Klitzman B, Reichert WM. In vivo cytokine-associated responses to biomaterial. Biomaterials. 2009;30(2):160–168. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.