Back to Journals » Drug Design, Development and Therapy » Volume 15
Cytokine and Chemokine Profile Changes in Patients with Neovascular Age-Related Macular Degeneration After Intravitreal Ranibizumab Injection for Choroidal Neovascularization
Authors Sun T, Wei Q, Gao P, Zhang Y, Peng Q
Received 20 February 2021
Accepted for publication 28 April 2021
Published 9 June 2021 Volume 2021:15 Pages 2457—2467
DOI https://doi.org/10.2147/DDDT.S307657
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Tingting Sun, 1,* Qingquan Wei, 1,* Peng Gao, 1,* Yongjie Zhang, 2 Qing Peng 1
1Department of Ophthalmology, Shanghai Tenth People’s Hospital, Tongji University, Shanghai, 200072, People’s Republic of China; 2Department of Human Anatomy, Nanjing Medical University, Nanjing, Jiangsu Province, 211166, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qing Peng
Department of Ophthalmology, Shanghai Tenth People’s Hospital, Tongji University, Shanghai, 200072, People’s Republic of China
Email [email protected]
Yongjie Zhang
Department of Human Anatomy, Nanjing Medical University, Nanjing, Jiangsu, 211166, People’s Republic of China
Email [email protected]
Objective: To investigate the concentrations of cytokine and chemokines profiling in aqueous humor for choroidal neovascularization (CNV) due to neovascular age-related macular degeneration (nAMD) before and during Intravitreal injection of ranibizumab (IVR) and its relation with the disease’s active state.
Methods: The cytokine levels in aqueous humour were detected by the Bio-Plex® 200 System and the Bio-Plex™ Human Cytokine Standard 27-Plex, Group I. Aqueous humour samples of experimental group were collected from 19 patients diagnosed nAMD at baseline and at 1 month after IVR. Aqueous humour samples of control group were collected from 20 patients undergoing cataract surgery.
Results: Aqueous humor levels of basic fibroblast growth factor (basic FGF) and RANTES were significantly lower in nAMD patients than in the control group (P=0.044 and P< 0.001, respectively). Vascular endothelial growth factor-A (VEGF-A) was significantly higher in nAMD patients than in the control group (P < 0.001). The average Eotaxin levels were significantly higher in nAMD patients after IVR than before (P=0.03). Contrarily, the average VEGF-A levels were significantly lower in AMD patients after IVR than before (P < 0.001).
Conclusion: Angiogenic, growth factors and inflammatory are involved in the formation of neovascularization of AMD patients. IVR did not cause significant differences in any growth factors or inflammatory cytokines in nAMD patients with the exception of VEGF.
Keywords: choroidal neovascularization, CNV, neovascular age-related macular degeneration, nAMD, ranibizumab, cytokines
Introduction
Choroidal neovascularization (CNV) is a major cause of visual impairment in neovascular Age-related macular degeneration (nAMD) patients in developed countries.1–3 A variety of angiogenic, anti-angiogenic and inflammatory cytokines are involved in the occurrence and development of neovascularization. One of the most important molecules among the angiogenic cytokines is the vascular endothelial growth factor (VEGF). Fortunately, remarkable progress has been made in the treatment of nAMD with the introduction of anti-VEGF therapy, such as aflibercept, bevacizumab and ranibizumab.
Ranibizumab was first approved for the treatment of visual impairment from nAMD in 2006 which is a recombinant, humanized Fab fragment (48 kDa) with the Fc fragment removed from the parent molecule.4 It has exhibited high systemic safety, rapid systemic clearance and increased affinity for all isoforms of the VEGF-A molecule. Studies have shown that vision impairment can be improved and the leakage from CNV can be decreased by ranibizumab.5–8
Unfortunately, this treatment is not always effective. In addition, the concentration of many cytokines, especially VEGF, changed before and after anti-VEGF treatment.9 Inflammatory cytokines are postulated to play a role in the pathogenesis of nAMD.10–12 Therefore, it is immediately to know how the cytokines change in aqueous humor before and after intravitreal injection of ranibizumab (IVR). Studying cytokines in the aqueous humor of patients with nAMD would help us to understand the disease mechanisms and guide treatment strategies.
Materials and Methods
Setting and Design
This study was conducted in the Department of Ophthalmology, Shanghai Tenth People’s Hospital affiliated with Tongji University School of Medicine. This was a cross-sectional study comparing cytokine profiles of aqueous humor in 19 patients with nAMD and 20 cataract patients. The study was approved by the Research Ethics Committee of Shanghai Tenth People’s Hospital (ClinicalTrials.gov:ChiCTR2000036296) and abides by the principles of the Declaration of Helsinki. All patients who participated in the examinations and procedures signed informed consent.
Clinical Diagnosis of nAMD
All patients included in the study underwent a unified diagnosis by three retina specialists. Fundus imaging including color fundus photographs (CFP), optical coherence tomography (OCT), fundus fluorescein angiography (FFA) and indocyanine green angiography (ICGA) were performed in all AMD patients. FFA and ICGA showed early fluorescence staining and late fluorescence leakage in the macular area. Data on refractive error and intraocular pressure (IOP) were collected in two groups.
Inclusion Criteria
Several inclusion criteria are as follows: 1) subjects over 65 years of age who signed the informed consent and were willing to provide a sample of aqueous humor; 2) patients with active CNV secondary to AMD were required to undergo anti-VEGF therapy. Control subjects (>50 years of age) were patients undergoing cataract surgery without retinal disease and systemic immune disease.
Exclusion Criteria
Several exclusion criteria are as follows: 1) patients who have received various intraocular therapies in the past three months; 2) patients with active ocular or systemic infections; 3) patients with pathological myopia, polypoidal choroidal vasculopathy, diabetic retinopathy, or other intraocular diseases; 4) patients who were allergic to fluorescein sodium and indocyanine green; 5) patients with systemic immune diseases.
Intervention of IVR
Before nAMD patients entered the operating room, Alcaine eye drop from Alcon, Inc were applied twice. Subsequently, the patient was laid on the operating table 0.5% povidone-iodine (Shanghai Likang Co, Ltd.) was used for the local disinfection of the eye, followed by the disinfection of the eyelash with normal protection. Then, the eyelid opener was used for opening the eyes, and 0.05% povidone-iodine was used to rinse the conjunctival sac. After 60s, 2% Lidocaine was used to rinse the conjunctival sac, then, 0.05 mL of Lucentis (Novartis Pharma Schweiz, AG) was injected into the vitreous cavity by inserting a needle 4 mm behind the corneoscleral junction. After the needle was pulled out, hemostasis by compression was performed for 1–3 minutes.Following the operation, TobraDex ointment was applied to the conjunctival sac, and the operative eye was covered. The re-examination was performed at the next day.
Collection of Aqueous Humor
During the time of intravitreal injection, approximately 100 μL of aqueous humor was collected aseptically from patients using an insulin syringe by paracentesis of the anterior chamber of the corneal limbus. During cataract surgery, 100 μL of aqueous humor was also collected in a similar manner. After the collection, clinical samples were immediately transferred into pre-labeled sterile 1.5 mL Eppendorf tubes and stored in a −80°C freezer until final analysis. The time of 2nd and 3rd collection of aqueous humor was at the 2nd month and 3rd month. The method used was same as the first time. We added this expression into the manuscript.
Cytokine Profiling by Bio-Plex® 200 System
Aqueous humor samples were thawed on ice and centrifuged at 3000 rpm for 5 minutes. Aqueous humor samples were analyzed using a Bio-Plex® 200 System and Bio-Plex™ Human Cytokine Standard 27-Plex, Group I (Bio-Rad, Hercules, California, USA). The selection of cytokines is based on our previous research and related literature. 27 cytokines and chemokines were analyzed:, interleukin-1 beta (IL-1β), interleukin-1 receptor antagonist (IL-1ra), IL −2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15 and IL-17, platelet-derived growth factor bb (PDGFF-bb) Eotaxin, basic fibroblast growth factor (basic FGF), granulocyte colony-stimulating factor (G-CSF), interferon-gamma (IFN-γ), granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-gamma-induced protein 10 (IP-10), monocyte chemo-attractant protein 1 (MCP-1), macrophage inflammatory protein 1α and 1β (MIP-1α, MIP-1β), regulated upon activation normal T cell expressed and secreted (RANTES), tumour necrosis factor-alpha (TNF-α), and VEGF.
Statistical Analysis
All statistical analysis was performed using SPSS V.20.0 for Windows (SPSS, Chicago, Illinois, USA). Because of the small sample size, normality was determined by the Shapiro–Wilk test. If the sample conformed to a normal distribution, Student’s t-test was used to compare non-paired continuous variables. If not, the Friedman test was used to compare them. The comparison of determination values before and after administration was evaluated by One-way repeated-measures ANOVA. Spearman’s rank-order correlation coefficient or Pearson’s correlation coefficient was calculated to examine the relationships among the variables. P< 0.05 was showed to be statistically significant.
Results
Baseline Characteristics and Patient Demographics
Of the 19 cases with nAMD, the mean age was 71.15±7.92 years old (mean±SD) and the control group was 73±7.55 years old. The male and female ratios of the nAMD group and control group were 14:5 and 11:9 respectively. The baseline characteristics of patients are in Table 1.
 |
Table 1 Baseline Demographics of the nAMD Group and the Control Group |
Concentrations of Cytokines at Baseline
Of the 27 cytokines tested in each sample, 14 cytokines (IL-1ra, IL-5, IL-6, IL-8, Eotaxin, basic FGF, G-CSF, IFN-g, IP-10, MCP-1, MIP-1α, MIP-1β, RANTES, VEGF-A) had detection rates of more than 50% in the aqueous humor. Several cytokines including IL-1ra, IL-5, IL-6, IL-8, Eotaxin, G-CSF, IFN-g, IP-10, MCP-1, MIP-1α, MIP-1β had no significant differences in aqueous humor between the nAMD group and the control group. The concentrations of basic FGF and RANTES in aqueous humor were significantly lower in the AMD cohort than controls (p=0.044 and P<0.001, respectively; Figure 1). Concentrations of VEGF-A in aqueous humor were significantly higher in the AMD cohort than controls (P < 0.05; Figure 2). We noticed that basic FGF, RANTES, VEGF-A showed a 0.68-fold, 0.05-fold decrease and 3.01-fold increase in the AMD cohort compared to the controls respectively.For details see attached Table 2.
 |
Table 2 Mean Concentrations and Fold Change of Aqueous Humor Cytokines in nAMD Group and Control Group |
 |
Figure 2 Mean Concentrations of Aqueous Humor Cytokines (VEGF, IL-ra, IFN, IP-10, MCP-1) in the control group and nAMD patients group. Data shown are mean ± SE. *Indicates control vs nAMD: p<0.05. |
Changes in Fudus Imaging During Treatment
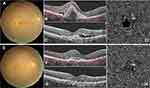
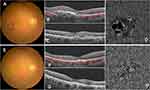
Subretinal hemorrhage and exudation were showed on colour photography and optical coherence tomography. Optical coherence tomography angiography showed choroidal new vessels before IVR. Subretinal hemorrhage and exudation were decreased on colour photography and optical coherence tomography. Optical coherence tomography angiography showing choroidal new vessels was shrinked after IVR (Figures 3 and 4).
Changes in Cytokine Levels During Treatment
The concentrations of the aqueous humor cytokine Eotaxin were significantly higher in the AMD cohort than pre-IVR patients.(P=0.03). Concentrations of aqueous humor cytokine VEGF-A were significantly lower in the AMD cohort than pre-IVR patients (P < 0.001). For details see attached Table 3.
 |
Table 3 Mean Concentrations of Aqueous Humor Cytokines in Cases Before and After Intravitreal Injection of Ranibizumab |
The expression level of IL-1ra was correlated with IP-10 and MIP-1β before IVR (p=0.05 and p=0.036, respectively). The expression level of IL-6 was correlated with IFN-g and MCP-1 before IVR (P=0.026 and P=0.041, respectively). The expression level of IL-8 was correlated with G-CSF, IFN-g, MCP-1, MIP-1α, MIP-1β and RANTES before IVR (all P<0.01). The expression level of Eotaxin was correlated with IFN-g, IP-10, and MCP-1 before IVR (P=0.014, P=0.001, and P=0.038, respectively). The expression level of G-CSF was correlated with MCP-1, MIP-1α and RANTES before IVR (P=0.045, P=0.028 and P<0.001, respectively). The expression level of IFN-g was correlated with IP-10, MCP-1, MIP-1α, and MIP-1β before IVR (P=0.002, P<0.001, P=0.013 and P=0.049, respectively). The expression level of MCP-1 was correlated with MIP-1α and MIP-1β before IVR (P=0.011 and P=0.038, respectively). The expression level of IP-10 was correlated with MCP-1 before IVR (P =0.014). The expression level of MIP-1α was correlated with MIP-1β before IVR (P < 0.001). The inner thickness of the retina was correlated with MIP-1α before IVR (P=0.009). For details see attached Table 4.
 |
Table 4 Correlations Between Aqueous Humour Factors Before IVR in nAMD Group |
The expressions of various cytokines were correlated in aqueous humor of nAMD patients after the 1st IVR. The expression level of IL-1ra and VEGF-A were correlated after 1st IVR (P=0.004). The expression level of IL-5 and RANTES were correlated after 1st IVR (P=0.021). The expression level of IL-6 and IL-8 as well as MIP-1α were correlated after 1st IVR (P=0.003 and p=0.01, respectively). The expression level of IL-8 was correlated with G-CSF, IFN-g, MCP-1, MIP-1α, MIP-1β, and RANTES after 1st IVR (P=0.014, P=0.012, P=0.019, P<0.001, P<0.001, P=0.016, respectively). The expression level of Eotaxin was correlated with IP-10 and VEGF-A after 1st IVR (P=0.04 and P=0.031, respectively). The expression level of G-CSF and MIP-1α was correlated after 1st IVR (P=0.006). The expression level of IFN-g and IP-10, MCP-1, MIP-1αas well as MIP-1β were correlated after 1st IVR (P=0.029, P<0.001, P=0.016 and P=0.015, respectively). The expression level of IP-10 and MCP-1 were correlated after 1st IVR (P=0.031). The expression level of MCP-1 and MIP-1α, as well as MIP-1β were correlated after 1st IVR (P=0.039 and P=0.031 respectively). The expression level of MIP-1α and MIP-1β were correlated after 1st IVR (P<0.001). For details see attached (Table 5).
 |
Table 5 Correlations Between Aqueous Humour Factors After IVR in nAMD Group |
Discussion
In this study, a large panel of cytokine profiles were evaluated in the aqueous humor of patients with nAMD.There were significant differences existed in the aqueous humor cytokine levels in patients with nAMD (basic FGF, RANTES, and VEGF-A) compared to control subjects. There were also significant differences existed in the aqueous humor cytokine levels in patients with nAMD (Eotaxin and VEGF-A) than pre-IVR patients.Our results support the theory that cytokine-mediated inflammation contributes to the pathogenesis of CNV.
Basic FGF, also termed Fibroblast growth factor-2 (FGF2), is a member of the FGF family that comprises nine members. It has a variety of functions in vivo and in vitro including stimulating hematopoiesis,13,14 wound healing, stimulating muscle cell growth and tissue repairing, and may play a key role in the various organ systems such as the eye and the skeleton.15,16 In the eye, basic FGF may play an important role in photoreceptor survival and may participate in photoreceptor signal transduction.16
A study revealed that injections of basic FGF into the eyes of Fischer 344 rats significantly delayed the progress of photoreceptor cell degeneration. They found that exogenous basic FGF might act as a survival-promoting factor in the aged retinas.17 In addition, another study showed that basic FGF stimulates photoreceptor differentiation in newborn rat retinal cells, increasing the expression level of opsin.18 In our study, the concentration of basic FGF was significantly lower in the nAMD group than the control group, which indicated that the function of photoreceptor cells was impaired. Contrarily, a recent study showed that serum basic FGF was significantly elevated in idiopathic choroidal neovascularization patients compared to the controls.19 The decrease of basic FGF locally and the increase of basic FGF in the whole blood circulation may be the result of the disturbance of cytokine homeostasis caused by nAMD locally. Other research found that IVR significantly reduced the level of basic FGF.20 However, our study did not find that the change in the concentration of basic FGF was significant before and after IVR. This may be due to the small sample size of the two groups, which is not enough to represent the population. In summary, basic FGF plays an indispensable role in the occurrence and development of nAMD.
Regulated on activation, normal T-cell expressed and secreted chemokine (RANTES), also known as CCL5, is a highly basic, 68 amino acid, pro-inflammatory chemokine that recruits various leukocytes, including granulocytes, monocytes, T cells as well as mast cells and dendritic cells.21,22 RANTES was the most potent and efficient arrest chemokine, which can be secreted by many cell types including macrophage, smooth muscle, platelet, endothelial cells, and activated T cell.23 A Norway study had found that RANTES was organized in filament-like structures on the endothelial cell surface.24 A present study reported that RANTES mediated critical interactions between amacrine cells, bipolar cells and RGC dendrites.They found that RANTES deficiency in Ccl5-/- mice resulted in the thinning of the inner retina, particularly the inner nuclear layer and inner plexiform layer.RANTES deficiency changed the pattern and timing of cell migration and apoptotic pruning during late-stage development.25 However, in our study, no correlation was found between the thickness of the inner retinal layer and RANTES before IVR. A report supported that RANTES were trophic modulators of the central nervous system’s development and function, extending far beyond the inflammatory contexts in which it was first characterized.26 In our study, the concentration of RANTES in the nAMD group was lower than those in the control group. This result suggested that patients with nAMD suffered from a thinning inner retina due to reduced RANTES in the eye.
Eotaxin is a member of the CC chemokine family, which is divided into three subfamilies, namely, CCL11/eotaxin-1, CCL24/eotaxin-2, and CCL26/eotaxin-3.CC chemokine receptor 3 (CCR3) interact with Eotaxin-1, eotaxin-2, and eotaxin-3. Type-2 helper T cells (Th2), eosinophils, and basophils expressed CCR3 on their cell surfaces.27 A study showed that CCR3 is specifically expressed in CNV endothelial cells in patients with AMD and that CCR3 blockade was more effective at reducing CNV than Anti-VEGF treatment, which is in clinical use at present.28 This result suggests that Eotaxin plays an indispensable role in angiogenesis. Some researchers have found that the concentration of Eotaxin decreased after the treatment of anti-VEGF injection, although Eotaxin had not elevated in AMD patients.29 Eotaxin in the nAMD group was 1.8 times higher than that in the control group, however, the two groups had no statistical difference. Contrarily, our study showed that Eotaxin increased significantly in nAMD patients after the first anti-VEGF treatment. However, the concentration of Eotaxin was reduced after the second anti-VEGF treatment. These two contradictory results may be due to the low expression of Eotaxin in the eye itself and the selective bias between the two selected samples. However, the two results are both significantly different, indicating that Eotaxin plays an important role in the occurrence and development of nAMD, independently of VEGF.
VEGF belongs to a family of proteins required for angiogenesis. There are many isoforms, including VEGF-A, VEGF-B, VEGF-C, VEGF-D and placental growth factor (PlGF), each of which plays a role in different angiogenesis environments such as embryonic and lymphatic angiogenesis.30,31 Among them, VEGF-A is the main regulator of angiogenesis. After selective shearing, four major subtypes of vascular endothelial growth factor-A with different lengths (121, 165, 189 and 206 amino acids) can be produced. The balance between different subtypes of vascular endothelial growth factor-A can regulate the growth and patterns of blood vessels.32 Other research found that the level of VEGF in the aqueous humor of patients with nAMD was significantly higher than that of controls.33 A report has demonstrated that the level of VEGF decreased significantly after the first injection of anti-VEGF drugs.34 The levels of cytokines were maintained after the second injection, which is parallel to changes in visual acuity and central macular thickness. Previous studies showed that the concentrations of VEGF did not correlate with those of other cytokines before IVR. In our study, it is worth noting that there was no significant correlation between VEGF-A and other cytokines before IVR, but there was a significant correlation between VEGF-A and IL-1ra as well as Eotaxin after IVR. The reason for this contradiction may be the inconsistency between the severity of the patients’ diseases in the two studies.
Several limitations of our research should be mentioned. Firstly, the number of subjects included in our study was relatively small. The true difference in cytokine levels between different groups may not be fully represented Secondly, the concentrations of intraocular cytokine levels were measured with aqueous humor but not vitreous humor. The vitreous is closer to the site of the lesion and more representative of the pathogenesis of the disease in the retina and choroid. However, it is more invasive to obtain vitreous humor and is not ethical in research.
Conclusion
In summary, our research showed that the levels of cytokines in the aqueous humor of nAMD patients were significantly different before and after IVR. Additionally, inflammation still plays an important role in the pathogenesis of nAMD. Understanding changes in cytokine profiles may aid in the discovery of biomarkers of diseases and identify new therapeutic targets in disease treatment.
Informed Consent
All participant details were de-identified and anonymous. We intend to share all of the individual participant data collected during the trial immediately after publication. All data generated or analysed during this study are included in this article.
Acknowledgments
This work was financially supported by Grants from the National Natural Science Foundation of China (81470025 and 81472081), and Shanghai Municipal Planning Commission of Science and Research Fund (No. SHDC2020CR5014 and No. ZY-ZWB-1001-CPJS10). Tingting Sun, Qingquan Wei, and Peng Gao are co-first authors for this study.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Kawasaki R, Yasuda M, Song SJ, et al. The prevalence of age-related macular degeneration in Asians: a systematic review and meta-analysis. Ophthalmology. 2010;117(5):921–927. doi:10.1016/j.ophtha.2009.10.007
2. Wong TY, Chakravarthy U, Klein R, et al. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology. 2008;115(1):116–126. doi:10.1016/j.ophtha.2007.03.008
3. Handa JT, Bowes Rickman C, Dick AD, et al. A systems biology approach towards understanding and treating non-neovascular age-related macular degeneration. Nat Commun. 2019;10(1):3347. doi:10.1038/s41467-019-11262-1
4. Ferrara N, Damico L, Shams N, Lowman H, Kim R. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006;26(8):859–870. doi:10.1097/01.iae.0000242842.14624.e7
5. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi:10.1056/NEJMoa054481
6. Heier JS, Antoszyk AN, Pavan PR, et al. Ranibizumab for treatment of neovascular age-related macular degeneration: a Phase I/II multicenter, controlled, multidose study. Ophthalmology. 2006;113(4):633–642. doi:10.1016/j.ophtha.2005.10.052
7. Gale RP, Pearce I, Eter N, et al. Anatomical and functional outcomes following switching from aflibercept to ranibizumab in neovascular age-related macular degeneration in Europe: SAFARI study. Br J Ophthalmol. 2020;104(4):493–499. doi:10.1136/bjophthalmol-2019-314251
8. Wada I, Oshima Y, Shiose S, et al. Five-year treatment outcomes following intravitreal ranibizumab injections for neovascular age-related macular degeneration in Japanese patients. Graefe’s Archive for Clinical and Experimental Ophthalmology = Albrecht Von Graefes Archiv Fur Klinische Und Experimentelle Ophthalmologie. 2019;257(7):1411–1418. German. doi:10.1007/s00417-019-04361-8
9. Agawa T, Usui Y, Wakabayashi Y, et al. Profile of intraocular immune mediators in patients with age-related macular degeneration and the effect of intravitreal bevacizumab injection. Retina. 2014;34(9):1811–1818. doi:10.1097/IAE.0000000000000157
10. Kauppinen A, Paterno JJ, Blasiak J, Salminen A, Kaarniranta K. Inflammation and its role in age-related macular degeneration. Cell Mol Life Sci. 2016;73(9):1765–1786. doi:10.1007/s00018-016-2147-8
11. Jabs DA, Van Natta ML, Trang G, et al. Association of age-related macular degeneration with mortality in patients with acquired immunodeficiency syndrome; role of systemic inflammation. Am J Ophthalmol. 2019;199:230–237. doi:10.1016/j.ajo.2018.12.002
12. Scotti F, Milani P, Setaccioli M, et al. Increased soluble urokinase plasminogen activator receptor (suPAR) levels in neovascular age-related macular degeneration: a role for inflammation in the pathogenesis of the disease? Graefe’s Archive for Clinical and Experimental Ophthalmology = Albrecht Von Graefes Archiv Fur Klinische Und Experimentelle Ophthalmologie. 2019;257(5):899–903. German. doi:10.1007/s00417-018-04230-w
13. Bikfalvi A, Han ZC. Angiogenic factors are hematopoietic growth factors and vice versa. Leukemia. 1994;8(3):523–529.
14. Allouche M, Bikfalvi A. The role of fibroblast growth factor-2 (FGF-2) in hematopoiesis. Prog Growth Factor Res. 1995;6(1):35–48. doi:10.1016/0955-2235(95)00041-0
15. Logan A, Frautschy SA, Baird A. Basic fibroblast growth factor and central nervous system injury. Ann N Y Acad Sci. 1991;638:474–476. doi:10.1111/j.1749-6632.1991.tb49073.x
16. Bikfalvi A, Klein S, Pintucci G, Rifkin DB. Biological roles of fibroblast growth factor-2. Endocr Rev. 1997;18(1):26–45. doi:10.1210/edrv.18.1.0292
17. Lin N, Fan W, Sheedlo HJ, Turner JE. Basic fibroblast growth factor treatment delays age-related photoreceptor degeneration in Fischer 344 rats. Exp Eye Res. 1997;64(2):239–248. doi:10.1006/exer.1996.0208
18. Desire L, Courtois Y, Jeanny JC. Suppression of fibroblast growth factors 1 and 2 by antisense oligonucleotides in embryonic chick retinal cells in vitro inhibits neuronal differentiation and survival. Exp Cell Res. 1998;241(1):210–221. doi:10.1006/excr.1998.4048
19. Guo S, Yin H, Zheng M, et al. Cytokine profiling reveals increased serum inflammatory cytokines in idiopathic choroidal neovascularization. BMC Ophthalmol. 2019;19(1):94. doi:10.1186/s12886-019-1101-6
20. Yin H, Fang X, Ma J, et al. Idiopathic choroidal neovascularization: intraocular inflammatory cytokines and the effect of intravitreal ranibizumab treatment. Sci Rep. 2016;6:31880. doi:10.1038/srep31880
21. Deshauer C, Morgan AM, Ryan EO, Handel TM, Prestegard JH, Wang X. Interactions of the chemokine CCL5/RANTES with medium-sized chondroitin sulfate ligands. Structure. 2015;23(6):1066–1077. doi:10.1016/j.str.2015.03.024
22. Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702. doi:10.1146/annurev-immunol-032713-120145
23. Baltus T, von Hundelshausen P, Mause SF, Buhre W, Rossaint R, Weber C. Differential and additive effects of platelet-derived chemokines on monocyte arrest on inflamed endothelium under flow conditions. J Leukoc Biol. 2005;78(2):435–441. doi:10.1189/jlb.0305141
24. Oynebraten I, Barois N, Bergeland T, Kuchler AM, Bakke O, Haraldsen G. Oligomerized, filamentous surface presentation of RANTES/CCL5 on vascular endothelial cells. Sci Rep. 2015;5:9261. doi:10.1038/srep09261
25. Duncan DS, Weiner RL, Weitlauf C, et al. Ccl5 mediates proper wiring of feedforward and lateral inhibition pathways in the inner retina. Front Neurosci. 2018;12:702. doi:10.3389/fnins.2018.00702
26. Adler MW, Geller EB, Chen X, Rogers TJ. Viewing chemokines as a third major system of communication in the brain. AAPS J. 2006;7(4):E865–70. doi:10.1208/aapsj070484
27. Shiraki Y, Shoji J, Inada N. Clinical usefulness of monitoring expression levels of CCL24 (Eotaxin-2) mRNA on the ocular surface in patients with vernal keratoconjunctivitis and atopic keratoconjunctivitis. J Ophthalmol. 2016;2016:3573142. doi:10.1155/2016/3573142
28. Takeda A, Baffi JZ, Kleinman ME, et al. CCR3 is a target for age-related macular degeneration diagnosis and therapy. Nature. 2009;460(7252):225–230. doi:10.1038/nature08151
29. Sakamoto S, Takahashi H, Tan X, et al. Changes in multiple cytokine concentrations in the aqueous humour of neovascular age-related macular degeneration after 2 months of ranibizumab therapy. Br J Ophthalmol. 2018;102(4):448–454. doi:10.1136/bjophthalmol-2017-310284
30. McColl BK, Stacker SA, Achen MG. Molecular regulation of the VEGF family – inducers of angiogenesis and lymphangiogenesis. APMIS. 2004;112(7–8):463–480. doi:10.1111/j.1600-0463.2004.apm11207-0807.x
31. Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8(6):464–478. doi:10.1038/nrm2183
32. Guyot M, Pages G. VEGF splicing and the role of VEGF splice variants: from physiological-pathological conditions to specific pre-mRNA splicing. Methods Mol Biol. 2015;1332:3–23. doi:10.1007/978-1-4939-2917-7_1
33. Tong JP, Chan WM, Liu DT, et al. Aqueous humor levels of vascular endothelial growth factor and pigment epithelium-derived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am J Ophthalmol. 2006;141(3):456–462. doi:10.1016/j.ajo.2005.10.012
34. Roh MI, Lim SJ, Ahn JM, Lim JB, Kwon OW. Concentration of cytokines in age-related macular degeneration after consecutive intravitreal bevacizumab injection. Graefe’s Archive for Clinical and Experimental Ophthalmology = Albrecht Von Graefes Archiv Fur Klinische Und Experimentelle Ophthalmologie. 2010;248(5):635–640. German. doi:10.1007/s00417-009-1254-8
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.