Back to Journals » International Journal of Women's Health » Volume 13
Cyclosporine A to Treat Unexplained Recurrent Spontaneous Abortions: A Prospective, Randomized, Double-Blind, Placebo-Controlled, Single-Center Trial
Received 4 August 2021
Accepted for publication 18 November 2021
Published 10 December 2021 Volume 2021:13 Pages 1243—1250
DOI https://doi.org/10.2147/IJWH.S330921
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Nan Wang,1,2 Hongshan Ge,1 Shu Zhou1
1Department of Obstetrics and Gynecology, Taizhou People’s Hospital, Taizhou, Jiangsu Province, People’s Republic of China; 2Department of Graduate College, Dalian Medical University, Dalian, Liaoning Province, People’s Republic of China
Correspondence: Hongshan Ge Email [email protected]
Background: The World Health Organization (WHO) has defined Unexplained Recurrent Spontaneous Abortion (URSA) as three and more consecutive miscarriages before the 20th week of gestation. To date, empiric therapy for patients with unexplained recurrent pregnancy loss (URPL) is not precise. Studies have shown that URSAs are associated with Th1/Th2 and Th17/Treg immune imbalances at the maternal-fetal interface. The immunosuppressant cyclosporine A (CsA) is widely used in patients with organ transplantation or autoimmune diseases, and it has a good safety profile in pregnant women. However, high-quality evidence for CsA treatment of URSAs is lacking. Our purpose with this study is to evaluate the efficacy and safety of CsA for improving pregnancy outcomes in patients with URSAs and to explore the role of CsA in regulating the immune balance.
Methods/Design: We expect to officially initiate our study at the Taizhou People’s Hospital in March 2022. We defined the live birth rate as the primary outcome, and the secondary outcomes include the rates of successful pregnancy, miscarriage, pregnancy complications, and adverse pregnancy outcomes, and newborn birth weights. Patients who meet URSA eligibility criteria will be randomized in a 1:1 ratio into either a study group receiving CsA 2 weeks after fertilization or a control group receiving placebo at 2 weeks after fertilization (the women in both groups will receive the relevant treatment for 6 months). In addition, we will collect peripheral blood samples of the participants before and after the treatments, and we will isolate mononuclear cells and measure cytokine levels (IFN-γ, TNF-α, IL-2, IL-10, IL-6, IL-4) and Th1/Th2, Th17/Treg ratios.
Discussion: This is the first randomized controlled trial to evaluate the clinical and immunomodulatory effects of CsA on the pregnancy outcomes of women with URSA and our results will provide evidence to evaluate the use of CsA as a treatment for women with URSAs.
Keywords: cyclosporine A, live birth rate, recurrent abortion of unknown cause, Th1, Th2
Introduction
Pregnancy loss (PL), defined as a spontaneous miscarriage from conception to 20 weeks of gestation, is a very common occurrence and affects up to 1000–1500/10,000 pregnancies/year. About 1% of all cases are recurrent PLs (RPLs). Although known predisposing factors explain some cases.3,4 The recognized causes of RSA include paternal chromosomal abnormalities (mainly mutual or Robertson’s translocations), infectious diseases, endocrine factors (thyroid defects, diabetes, and polycystic ovary), uterine abnormalities, antiphospholipid antibody syndrome, or other autoimmune diseases.5 There still 50–60% of all cases remain unexplained which known as unexplained recurrent Spontaneous abortion (URSA).3,6 The World Health Organization (WHO) has defined Unexplained Recurrent Spontaneous Abortion (URSA) as three and more consecutive miscarriages before the 20th week of gestation.1 The limited understanding of the underlying pathophysiological mechanisms results in a lack of effective intervention options, and standard treatments remain unavailable for these couples.2 Thus, clinical management is either empirical or focuses primarily on providing supportive care.
The endometrium proliferates and differentiates to establish endometrial receptivity under the synergistic action of progesterone and estrogen. Embryo implantation can only take place during this limited period, known as the implantation window. Attachment of the embryo to the luminal epithelium in the receptive state activates the proliferation and differentiation of the subepithelial stromal cells surrounding the embryo, a process called decidualization. Before the development of the placenta, new blood vessels appear in the ephemeral decidual tissue to provide oxygen and nutrients to the embryo invading the mother’s womb. The mother’s immune system has to tolerate the hemiallogeneic fetus during each gestation step.7 For a successful pregnancy, complex mechanisms need to maintain the balance between immune tolerance and effector immunity, and any imbalances in the regulatory system will lead to adverse reproductive outcomes. The regulation of maternal CD4+ T cells in response to fetal antigens is considered an important component of maternal-fetal tolerance during pregnancy. Primary CD4+ T cells can differentiate into different subsets (T helper [Th1, Th2, Th17] or T regulatory cells [Treg]) when confronted with antigens on the surface of antigen-presenting cells or driven by cytokines.8 Proinflammatory immune responses are considered problematic during pregnancy. Th1 type cells that secrete mainly IL-2, interferon-γ, and tumor necrosis factor-A (TNF-A) and Th2 type cells that secrete mainly IL-4, IL-6, and IL-10 have been shown to play important roles during pregnancy. Th1 type cells are involved in cellular immunity and inflammation and can kill embryos; Th2 type cells are involved in cell proliferation, B cell antibody production, and allogeneic immune tolerance and can help protect and maintain pregnancies. The transformation of the maternal immune response to the Th2 type is essential for the maintenance of pregnancy.9 When the Thl/Th2 system is out of balance, maternal rejection of the fetal allograft can occur leading to RSAs.10,11 Th17 cells also play a key role in the induction of inflammation, and abnormal Th17 cell levels are associated with autoimmune diseases.12 Treg cells express anti-inflammatory cytokines (including IL-10 and transforming growth factor [TGF]-β1) that inhibit hyperactive immune responses.13 Studies have shown that the balance between Treg and Th17 cells is also important for the maintenance of normal pregnancies, and a shift in the Th17/Treg ratio to more abundant Th17 cells is thought to cause a number of pregnancy-related diseases, including RSAs, preeclampsia, and gestational diabetes mellitus.14–16
Cyclosporine A (CsA) was first isolated from metabolites of Trichoderma polysporum and Cladospora, and it was first used in 1978 to treat patients with kidney transplant due to its immunosuppressive effects. CsA can significantly inhibit autoimmune responses, and has become a first-line therapy in pregnant women with renal transplantation.17 CsA may play a dual role regulating the maternal-fetal immunity: it inhibits maternal immune rejection of embryonic antigens and promotes the growth, movement, and invasion of trophoblast cells, constituting an effective drug for the treatment of pregnancy diseases such as RSA.18 Studies in pregnant patients after organ transplantation have shown that long term conventional doses of prednisone and CsA (prescribed to pregnant women to prevent immune rejection) are safe for fetuses and newborns.17
Li et al19 found that low dose CsA induces maternal-fetal tolerance and trophoblast cell regulation that promotes proliferation, invasion and migration of villous trophoblast cells, helping implantation succeed. The main role of CsA in the cytoplasm is to form a special complex with cyclophilin that binds calcineurin phosphatase and prevents lymphocyte proliferation and the transcription of lymphocyte factors (TNF-α, IL-2 and IFN-γ) by inhibiting serine threonine protein phosphatase activity, leading to immunosuppression.20 Thus, CsA may improve pregnancy outcomes in women with RSA, primarily in those with elevated Th1 immune-response phenotypes. Azizi et al21 also proved that CsA treatment in women with RSA and an elevated TH1/TH2 ratio can significantly increase the live birth rate and improve the ratio. In addition, clinical studies have shown that CsA can also improve the embryo implantation and clinical pregnancy rates of patients undergoing in vitro fertilization and embryo transfer (IVF-ET).22 The study of Wang et al23 suggested that CsA reduces the proportion of Th17 cells and promotes Treg cell dominance in pregnant women with a history of RSA. CsA decreases the production of IL-17A, the main effector of Th17 cells, and up-regulates the expressions of TGF-β1, IL-10, CTLA 4 and TIM 3. This suggests that CsA acts as a trophoblast functional promoter restoring the Th17/Treg cell balance and inducing a Th2 bias during pregnancy. JH Fu24 used CsA for the treatment of patients with RSA and refractory immune disorders (aspirin, prednisone, heparin, husband leukocyte immunotherapy, and intravenous immunoglobulin therapy were all unsuccessful), and he used CsA before and after pregnancy, achieving good pregnancy outcomes. Ling et al22 used CsA to treat URSA, they found that the peripheral blood CD3 levels were higher and the CD8 levels lower in patients with successful pregnancy than in the others.
Guidelines for the standard treatment of URSA do not exist. CsA has been used to treat RSA, but no clinical trials on its efficacy and safety have been conducted. We designed this study to evaluate the effect of CsA treatment to improve successful pregnancy rates of patients with URSA, and to investigate the role of CsA in the regulation of immune balance by measuring changes in Th1/Th2 and Th17/Treg balances and related cytokine levels in the peripheral blood of women with URSA.
Methods
Design
This is a prospective, randomized, superior efficacy, two-arm, 1:1 placebo-controlled, double-blind, single-center trial to evaluate the safety and efficacy of CsA for improving pregnancy outcomes in patients with URSA and to investigate its immunomodulatory effects.
Participants
We will start recruiting patients in March 2022. Eligible patients at the Department of Obstetrics and Gynecology (Taizhou People’s Hospital in Taizhou City, Jiangsu Province, China) will include women who have experienced at least three miscarriages and who are trying to get pregnant again. The researchers will screen them according to the admission criteria. The China Registered Clinical Trial Ethics Review Committee reviewed and approved this study. Qualified patients who sign an informed consent will be included in the study. The expected recruitment time is 2 years and the participation time for each patient is approximately 2 years.
Inclusion criteria (prior to the study, the participants must meet all the following requirements to be included in the study):
1. Age between 18 and 39 years, spontaneous abortions ≥ 3 episodes.
2. Lack of genetic diseases and normal chromosome karyotypes in both prospective parents.
3. Non-smoking prospective parents.
4. Normal semen analysis results for their male partner.
5. Voluntary participation in the clinical study with signed informed consent.
Exclusion criteria (prior to the study, we will exclude any participants presenting any of these conditions).
1. Any infectious, endocrine, or anatomical abnormalities.
2. Acquired or inherited thrombophilia.
3. Presence of anti-phospholipid antibodies or other autoantibodies in women.
4. Positive TORCH or other infection test results.
5. Severe heart, liver, or renal insufficiency.
6. Individuals vaccinated within the three months prior to their inclusion in the study.
7. Individuals using other investigational drugs or having participated in other clinical studies within 1 month prior to study inclusion.
8. Individuals allergic to CsA or its ingredients.
9. Participation of an individual deemed inappropriate by the attending physician.
Randomization and Blindness
After signing the informed consent form, eligible patients with URSA will be randomly assigned into one of two groups group (in a 1:1 ratio). We will use the random number generator of SPSS to generate the grouping and keep the relevant records. Three nurses, who are otherwise not involved in this study, chose the code name AB for the experimental and control groups. The custodian of the random coding table will determine whether an individual is assigned to group A or group B. The group code for each participant will only be disclosed after patient recruitment. The groups represented by AB will not be disclosed until the statistical analysis is completed. The investigator will keep a record of these instructions and implement the assignments accordingly. Patients, investigators, and all medical and nursing professionals caring for patients will be blinded to the treatment group. Placebo and CsA medications will be provided in liquid form and will have the same appearance and packaging.
Intervention Methods
Human chorionic gonadotropin (β-HCG) will be measured 2 weeks after the fertilization period. Patients with positive β-HCG results will be given CsA (Xinshanming) 2 to 4 mg/Kg twice a day for 6 months to maintain their lowest blood CsA concentration between 80 and 150 mg/L (measured on the 15th and 30th day of treatment). Patients in the control group will receive a placebo treatment of the same appearance and volume over the same period of time.
The participants will be evaluated during the screening and preparation phases and then periodically throughout the trial. The screening phase will last four weeks. We will record age, height, weight, pregnancy history, medication use, allergies and previous medical history of the participants. The screening phase will be followed by a one-year pre-pregnancy phase, and if participants do not become pregnant within that year, their treatment and participation will be terminated. Once the pregnancy is confirmed, we will initiate pregnancy evaluation and set an indicator time at week zero. For the next 42 weeks, transvaginal ultrasonography will be performed every 2 weeks to observe fetal heart rate and embryo development, or to diagnose miscarriage. All the newborns will be examined by a pediatrician for congenital abnormalities. The gestational age of abortion will be calculated according to the length of the fetus and the size of the gestational sac obtained by ultrasound examination. The assessments will end once spontaneous abortion or delivery occurs.
Peripheral blood samples will be collected twice from all participants, the first one before the first CsA or placebo treatment and the second one after the last treatment. Laboratory tests will include routine blood and urine work, and liver and renal function tests. In addition, peripheral blood mononuclear cells (PBMCs) will be extracted by Ficoll Hypaque density gradient centrifugation, washed twice with PBS buffer, and then cultured. The supernatant of cultured cells will be extracted and the levels of cytokines (IFN-γ, TNF-α, IL-2, IL-10, IL-6, IL-4) will be determined by enzyme-linked immunosorbent assays. The ratios of Th1/Th2 and Th17/Treg in peripheral blood will be measured by flow cytometry. During clinical research, the original data that subjects need to collect are as Table 1.
 |
Table 1 Clinical Trial Flow Chart |
Main Outcome
The live birth rate for CsA treatment of URSA will be calculated as the percentage of pregnant women who deliver healthy babies from all pregnant women in this group.
Secondary Outcomes
We will calculate the rates of successful pregnancy (≥20 weeks after initial treatment dose), miscarriage, pregnancy complications (including preeclampsia, gestational diabetes mellitus, gestational hypertensive disorders, and bleeding and thrombosis), and adverse pregnancy outcomes (including premature birth, stillbirth, and congenital abnormalities) and the birth weight of newborns as the secondary outcomes.
Safety
After recruitment and before randomization, all participants will undergo routine blood, liver, and kidney function tests to identify and exclude patients with severe heart, liver, or kidney diseases. At the end of the study, we will re-examine the participants to identify any possible side effects of the intervention.
An adverse event (AE) is any adverse medical event that occurs in a person who consents to a biomedical study, regardless of whether the event is associated with the study or experimental drug covered by the study. Serious adverse events (SAEs) are identified by one of the following findings: death, life-threatening condition, severe or persistent incapacity or disability, hospitalization, extended length of stay, deformity or birth defect, and any potentially serious event. Any SAE or related event, regardless of its causal relationship to the trial treatment or study, must be reported to the Sponsor as soon as possible within 24 hours of its occurrence. The investigators will be responsible for recording and reporting all SAE events that occur during the study’s expected follow-up period (6 months after the women stop treatment and 6 months after the birth of a newborn) from the date of signing the informed consent.
Sample Size
We set the test efficacy (POWER) at 80%, and adopted a balanced design (1:1 study group: control group ratio) with the live birth rate as the primary endpoint and 0.05 as the bilateral statistical significance level. We used data from reference publications16,20 to calculate our sample size. The published live birth rate of the CsA treatment group was 8.5% [16, P1]; and, the live birth rate in the control group was 4.7 [1] (P2). We used the following formula to calculate our sample size: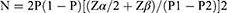
We initially estimated the sample size of each group at 53 cases assuming the following values: P1=0.8, P2=0.6, P = (P1+P2) /2, α = 0.05, and β =0.8. Considering factors such as withdrawal from the study, we estimated a number of 59 cases in each group to ensure significance. Finally, we assumed a 90% pregnancy rate and estimated the number of cases needed per group to be of 66 for a total of 132 cases.
Statistical Analysis
The statisticians who will perform these analyses will be blinded to the group distribution of participants. The efficacy analysis will be based on the intention to treat principle. All statistical tests will be conducted using bilateral tests, and a P value ≤0. 05 will be considered as statistically significant. All statistical analyses will be performed using the SPSS24.0 statistical software.
Descriptive Statistics
We will express measurement data as means, standard deviations, and confidence intervals and enumeration data as frequencies and corresponding percentages. Express qualitative data as ratio, proportion, rate, RR, OR.
We will apply t tests between the two groups, according to the numerical characteristics of the variables, to compare the baseline data of patients in the two groups (such as age, BMI, infertility time, previous abortion history, gestational age of previous abortion, etc.) and ensure the patients in the two groups have similar clinical backgrounds. We will use a Pearson χ2 test or Fisher’s exact probability method to infer the rates of live birth, clinical pregnancy, abortion, adverse pregnancy outcomes, and adverse reactions in the two groups of participants.
We will apply t/Wilcoxon rank sum tests between the two groups to compare the levels of Th1 and Th2 cytokines, and the Th1/Th2 and Th17/Treg ratios in the two groups of participants. We will use a paired t test to compare the differences in cytokine levels, and Th1/Th2 and Th17/Treg ratios in the each group before and after the treatments.
Finally, we will calculate the incidences of adverse events in the study and control groups using Pearson χ2 or Fisher’s exact test inferences.
Discussion
With this study we will compare live birth rates of patients with URSA treated with either cyclosporine A or placebo. We plan to enroll 132 women in the Taizhou People’s Hospital, beginning in March 2022.
After analyzing the data obtained from our single-center, randomized, double-blind, placebo-controlled trial, our results will provide class I evidence for a treatment strategy for women with URSA.
Clinicians need to rely on their experience to treat women with URSA because no standard guidelines exist. Empiric treatment options include suppression of maternal immunity (lymphocyte immunotherapy, hydroxychloroquine), anti-inflammatory aspirin, acetylsalicylic acid, anticoagulant low molecular weight heparin, Traditional Chinese medicine, and acupuncture. However, the ideal doses of these therapeutic measures such as that of low molecular weight heparin is unclear25 and the treatments may be expensive (intravenous immunoglobulin or assisted reproduction). For some treatments, only studies with small sample sizes have been conducted (see the evidence for hydroxychloroquine26 or that for acetylsalicylic acid).27 Therefore, investigating treatment options is important. Immune dysfunction is a reversible background factor affecting pregnancy outcomes in patients with URSA. We selected CsA, a first-line immunosuppressant for pregnancies complicated with kidney transplantation, to ensure the safety of the mother and child. CsA can pass through the placenta, where it inhibits the maternal rejection of embryos and promotes the proliferation, invasion, and migration of villous trophoblast cells by inducing the expression of proteins that regulate the function of HTR8/SVneo cells, thereby improving the embryo implantation rates.28 The evidence on the limited published data suggests a demonstrable clinical benefit of CsA for patients with URSA. However, studies on the therapeutic effect of URSA in pregnant women have been small and nonrandomized.22 Thus, a clinical benefit of CsA for the treatment of URSA has not been demonstrated by reliable methodological randomized controlled studies and large well-designed clinical trials to evaluate the efficacy of CsA in patients with URSA are still lacking. We hope to fill this gap with our study.
We designed this study hoping to improve pregnancy outcomes, while investigating the role of CsA as an immune balance regulator in women with URSA; our results should provide high-quality evidence to support treatment decisions for patients with URSA.
The inclusion of a placebo control group in this study may constitute a disadvantage due to possible withdrawals during the study. However, we plan to have a sufficient case base number, and we will recruit as many patients as possible by advertising the study. We will expand the number of patients as much as possible to ensure the minimum number of cases to achieve significance.
Ethical Approval
We chose the ethics committee of the China Clinical Trial Registry to conduct the ethical review of our study to ensure we followed higher registration standards. The China Registered clinical Trial Ethics Review Committee for research approved this study (Reference No. ChiECRCT20210257), which we will conduct following the tenets of the Declaration of Helsinki.
This study will be registered in the Chinese Clinical Trial Registry, the primary registration body of the International clinical trial registration Platform recognized by the World Health Organization, to ensure the uniqueness of this study. Clinical Trial Registration Number: ChiCTR2100046724
Patient and Public Involvement
We actively encourage the involvement of patients in research. We will collect information on the participants’ satisfaction during the study to improve future trials. We will encourage patients to tell their family and friends about the study and encourage them to advertise for participants. After the completion of the intervention, we will disclose the results of the study to the participants and thank them for their contributions. The participants have the right to discontinue their participation in the study at any time.
Acknowledgments
Funding from the National Natural Science Foundation of China (No. 81771586) and Natural Science Foundation of Jiangsu Province (No. BK20171317) are gratefully acknowledged. Thanks to Charlesworth for great edit.
Author Contributions
Hongshan Ge: Project administration, Validation, Review, Funding, study design; Nan Wang: Conceptualization, Methodology, Software, Analysis, Date, writing, execution, Review; Shu Zhou: Resources, Supervision, Review. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no conflicts of interest to declare.
References
1. Fainboim L, Belén S, González V, Fernández P. Evaluation of paternal lymphocyte immunotherapy and potential biomarker mixed lymphocyte reaction-blocking factor in an Argentinian cohort of women with unexplained recurrent spontaneous abortion and unexplained infertility. Am J Reprod Immunol. 2021;86(2):e13422. doi:10.1111/aji.13422
2. Li TY, Li R, Zeng L, et al. In vitro fertilization-embryo transfer in patients with unexplained recurrent pregnancy loss. Chin Med J. 2021;134(20):2421–2429. doi:10.1097/cm9.0000000000001657
3. Grandone E, Tiscia GL, Mastroianno M, et al. Findings from a multicentre, observational study on reproductive outcomes in women with unexplained recurrent pregnancy loss: the OTTILIA registry. Hum Reprod. 2021;36(8):2083–2090. doi:10.1093/humrep/deab153
4. Ahmadi M, Abdolmohammadi-Vahid S, Ghaebi M, et al. Effect of Intravenous immunoglobulin on Th1 and Th2 lymphocytes and improvement of pregnancy outcome in recurrent pregnancy loss (RPL). Biomed Pharmacother. 2017;92:1095–1102. doi:10.1016/j.biopha.2017.06.001
5. Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98(5):1103–1111. doi:10.1016/j.fertnstert.2012.06.048
6. Shahine L, Lathi R. Recurrent pregnancy loss: evaluation and treatment. Obstet Gynecol Clin North Am. 2015;42(1):117–134. doi:10.1016/j.ogc.2014.10.002
7. Rasmussen SA, Kelley CF, Horton JP, Jamieson DJ. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy: what obstetricians need to know. Obstet Gynecol. 2021;137(3):408–414. doi:10.1097/aog.0000000000004290
8. Zeng W, Liu Z, Liu X, et al. Distinct transcriptional and alternative splicing signatures of decidual CD4(+) T cells in early human pregnancy. Front Immunol. 2017;8:682. doi:10.3389/fimmu.2017.00682
9. Peng Y, Yin S, Wang M. Significance of the ratio interferon-γ/interleukin-4 in early diagnosis and immune mechanism of unexplained recurrent spontaneous abortion. Int J Gynaecol Obstet. 2021;154(1):39–43. doi:10.1002/ijgo.13494
10. Chen HY, Li OY, Pang LH, et al. Expression of FK506-binding protein 52 (FKBP52) in chorionic villi with early recurrent spontaneous abortion. J Matern Fetal Neonatal Med. 2015;28(10):1165–1169. doi:10.3109/14767058.2014.947572
11. Kwak-Kim JY, Chung-Bang HS, Ng SC, et al. Increased T helper 1 cytokine responses by circulating T cells are present in women with recurrent pregnancy losses and in infertile women with multiple implantation failures after IVF. Hum Reprod. 2003;18(4):767–773. doi:10.1093/humrep/deg156
12. Crome SQ, Wang AY, Levings MK. Translational mini-review series on Th17 cells: function and regulation of human T helper 17 cells in health and disease. Clin Exp Immunol. 2010;159(2):109–119. doi:10.1111/j.1365-2249.2009.04037.x
13. Jutel M, Akdis M, Budak F, et al. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol. 2003;33(5):1205–1214. doi:10.1002/eji.200322919
14. Hosseini A, Dolati S, Hashemi V, Abdollahpour-Alitappeh M, Yousefi M. Regulatory T and T helper 17 cells: their roles in preeclampsia. J Cell Physiol. 2018;233(9):6561–6573. doi:10.1002/jcp.26604
15. Muyayalo KP, Li ZH, Mor G, Liao AH. Modulatory effect of intravenous immunoglobulin on Th17/Treg cell balance in women with unexplained recurrent spontaneous abortion. Am J Reprod Immunol. 2018;80(4):e13018. doi:10.1111/aji.13018
16. Sheu A, Chan Y, Ferguson A, et al. A proinflammatory CD4(+) T cell phenotype in gestational diabetes mellitus. Diabetologia. 2018;61(7):1633–1643. doi:10.1007/s00125-018-4615-1
17. Zaffar N, Soete E, Gandhi S, Sayyar P, Van Mieghem T, D’Souza R. Pregnancy outcomes following single and repeat liver transplantation: an international 2-center cohort. Liver Transpl. 2018;24(6):769–778. doi:10.1002/lt.25071
18. Zhou WH, Dong L, Du MR, Zhu XY, Li DJ. Cyclosporin A improves murine pregnancy outcome in abortion-prone matings: involvement of CD80/86 and CD28/CTLA-4. Reproduction. 2008;135(3):385–395. doi:10.1530/rep-07-0063
19. Du MR, Dong L, Zhou WH, Yan FT, Li DJ. Cyclosporin a improves pregnancy outcome by promoting functions of trophoblasts and inducing maternal tolerance to the allogeneic fetus in abortion-prone matings in the mouse. Biol Reprod. 2007;76(5):906–914. doi:10.1095/biolreprod.106.056648
20. Archer TM, Stokes JV, Kummari E, et al. In vivo effects of aspirin and cyclosporine on regulatory T cells and T-cell cytokine production in healthy dogs. Vet Immunol Immunopathol. 2018;197:63–68. doi:10.1016/j.vetimm.2018.01.003
21. Azizi R, Ahmadi M, Danaii S, et al. Cyclosporine A improves pregnancy outcomes in women with recurrent pregnancy loss and elevated Th1/Th2 ratio. J Cell Physiol. 2019;234(10):19039–19047. doi:10.1002/jcp.28543
22. Ling Y, Huang Y, Chen C, Mao J, Zhang H. Low dose Cyclosporin A treatment increases live birth rate of unexplained recurrent abortion - initial cohort study. Clin Exp Obstet Gynecol. 2017;44(2):230–235.
23. Wang S, Li M, Sun F, et al. Th17/Treg-cell balance in the peripheral blood of pregnant females with a history of recurrent spontaneous abortion receiving progesterone or cyclosporine A. Exp Ther Med. 2021;21(1):37. doi:10.3892/etm.2020.9469
24. Fu JH. Analysis of the use of cyclosporin A to treat refractory immune recurrent spontaneous abortion. Clin Exp Obstet Gynecol. 2015;42(6):739–742.
25. Jiang F, Hu X, Jiang K, Pi H, He Q, Chen X. The role of low molecular weight heparin on recurrent pregnancy loss: a systematic review and meta-analysis. Taiwan J Obstet Gynecol. 2021;60(1):1–8. doi:10.1016/j.tjog.2020.11.001
26. Pasquier E, de Saint-martin L, Marhic G, et al. Hydroxychloroquine for prevention of recurrent miscarriage: study protocol for a multicentre randomised placebo-controlled trial BBQ study. BMJ Open. 2019;9(3):e025649. doi:10.1136/bmjopen-2018-025649
27. Blomqvist L, Hellgren M, Strandell A. Acetylsalicylic acid does not prevent first-trimester unexplained recurrent pregnancy loss: a randomized controlled trial. Acta Obstet Gynecol Scand. 2018;97(11):1365–1372. doi:10.1111/aogs.13420
28. Huang W, Lu W, Li Q, et al. Effects of cyclosporine A on proliferation, invasion and migration of HTR-8/SVneo human extravillous trophoblasts. Biochem Biophys Res Commun. 2020;533(4):645–650. doi:10.1016/j.bbrc.2020.09.072
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
