Back to Journals » Cancer Management and Research » Volume 11
Current Understanding of Circular RNAs in Gastric Cancer
Authors Tang X , Zhu J, Liu Y , Chen C, Liu T, Liu J
Received 14 July 2019
Accepted for publication 15 November 2019
Published 13 December 2019 Volume 2019:11 Pages 10509—10521
DOI https://doi.org/10.2147/CMAR.S223204
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Xueqiong Zhu
Xiaohuan Tang, Jiaming Zhu, Yuanda Liu, Chao Chen, Tianzhou Liu, Jingjing Liu
Department of Gastrointestinal Nutrition and Hernia Surgery, The Second Hospital of Jilin University, Jilin University, Changchun, Jilin, People’s Republic of China
Correspondence: Jingjing Liu
Department of Gastrointestinal Nutrition and Hernia Surgery, The Second Hospital of Jilin University, Jilin University, Changchun, Jilin 130041, People’s Republic of China
Tel +86-431-81136210
Email [email protected]
Abstract: Gastric cancer (GC) is the third most common cause of cancer-related death worldwide. Advanced diagnosis and high rates of relapse and metastasis are associated with the poor prognosis of this disease. GC has a complex etiopathogenesis of which the underlying mechanisms remain to be explored. Studies on circular RNAs (circRNAs), noncoding RNAs that may be potential targets in GC, have made substantial progress over the past few years. CircRNAs exert important effects on the onset and progression of GC. Hence, this article aims to summarize the findings of recent studies of circRNAs related to GC and to describe the underlying mechanisms and potential applications. The findings indicate that circRNAs participate in GC regulation, proliferation, invasion, and metastasis through regulating microRNAs, proteins, genes, and signaling pathways. In addition, dysregulated circRNAs may be used as novel diagnostic and prognostic biomarkers or therapeutic targets. This review is expected to facilitate a better understanding of GC, and it suggests novel circRNA-based methods to inhibit or prevent GC.
Keywords: gastric cancer, circular RNA, biomarkers, diagnosis, prognosis
Introduction
In 2018, gastric cancer (GC) was the fifth most commonly diagnosed malignancy and the third most common cause of cancer-related death worldwide.1 Although many mechanisms underlying the onset and progression of GC have been revealed over the past few years, delayed diagnosis and treatment are largely responsible for the high mortality rate among GC patients.2 Hence, novel biomarkers are crucial to improve early diagnosis and prognosis, and to identify effective therapeutic targets.
Over the past few decades, genetics-based GC studies have mainly concentrated on the exploration of protein-coding genes, as noncoding RNAs (ncRNAs) were largely regarded as the products of transcription errors.3 However, growing evidence has indicated that ncRNAs are involved in the regulation of cellular proliferation, invasion, migration, and apoptosis, as well as a remarkable variety of biological functions in tumorigenesis.4,5 Many studies have demonstrated that the expression profiles of some RNAs and proteins vary during cancer onset and progression, thus these RNAs might be useful as biomarkers for the diagnosis, treatment, and prognosis of GC.6–9 Non-coding RNAs (ncRNAs) are a class of non-protein-coding RNAs that are present in many cell types. Initially, ncRNAs were regarded as byproducts of genetic transcription. However, with the discovery of functional ncRNAs,10 many unique functional genomic products have been identified.11,12 In addition, ncRNAs have been reported to play crucial roles in the development of various diseases, including many cancers.13,14
There are three important types of ncRNAs: microRNAs (miRNAs), composed of ≥22 nucleotides; long ncRNAs (lncRNAs), composed of >200 nucleotides;15,16 and circRNAs, which are normally stable molecules characterized by a covalently closed loop structure of various lengths.17 In general, the regulatory mechanisms of circRNAs involve the targeting of mRNAs by miRNAs, whereas lncRNAs and circRNAs act as endogenous competitive RNAs or sponges of miRNAs, proteins, and genes, which influence the stability of binding partners.18 In addition, ncRNAs exert effects on various biological processes through many other mechanisms. For example, miRNAs can encode peptides, interact with non-Argonaute family proteins, activate Toll-like receptors, and upregulate protein expression.19
CircRNAs were first described approximately 40 years ago as unique ncRNAs with 3′ and 5′ ends that are covalently joined in a closed loop structure, leaving no free ends. The expression patterns of circRNAs have been described in many tumor types, including GC, but the characteristic loop structure was ignored because of a lack of understanding and was even regarded as a splicing error.20 With the development of RNA sequencing and bioinformatics technologies, a variety of circRNAs have been confirmed to have various functions in human cells.21 Many studies have reported the expression profiles of circRNAs in GC. For instance, Sui et al22 identified 1,285 differentially expressed circRNAs in GC, including 69 that are closely associated with miRNAs, as determined by microarray chip technology. CircRNAs are constitutively expressed in various cells and plasma, are tissue- and disease-specific, and have unique exon sequences, miRNA response elements (MREs), and protein-binding elements.23 These unique characteristics of circRNAs may have potential in the controlled regulation of cellular functions. CircRNAs play essential roles in the onset and progression of GC.24,25 For instance, Chen et al26 reported that circPVT1 can sponge miR-125 and consequently promote the proliferation of cancer cells; therefore, it may serve as a prognostic biomarker of GC. Many studies have provided novel information to improve our understanding of circRNAs in the pathogenesis of GC. However, current information has not yet been summarized in a review. Therefore, the aim of this article is to review the current understanding of the methods for the discovery of circRNAs and to clarify the underlying mechanisms and potential applications of circRNAs in GC.
CircRNAs in GC
CircRNAs are generally localized in the cytoplasm with lower abundances in the nucleus.23 Through various mechanisms, circRNAs can influence gene expression and transcription in many diseases, including GC. Substantial progress has been made in the application of circRNAs, especially as diagnostic and prognostic biomarkers, as well as therapeutic targets. The general attributes of circRNAs are summarized in Table 1.
 |
Table 1 Current Research on circRNAs Associated with Gastric Cancer |
Resources for Research on Target circRNAs
The discovery of novel circRNAs associated with GC has been the focus of many studies. Second-generation sequencing and bioinformatics technologies, the most common methods used in such studies, are crucial for the screening of circRNAs, as demonstrated by the recent characterizations of circPVT1 and circRNA_100269.26,27 Moreover, in a recent study, Josh et al28 detected the expression response of circRNAs in >2000 cancer samples through an exome capture RNA sequencing detection method that is more effective than previous methods. The authors established the most comprehensive multi-tumor circRNA database, MiOncoCirc, which provides circRNA expression data and enables the analysis of circRNA expression in different cancers, including 17 cancer cohorts. Of note, the prostate cancer data have been extensively studied, and some circRNAs in urine have been suggested to have potential as diagnostic or prognostic biomarkers. Numerous genes that are aberrantly expressed in many cancers have been identified by referencing studies of other diseases. For example, Pan et al validated the presence of circRNA ciRS-7 in GC by referring to a previous study of the brain.29 More recently, many databases have been created that are useful to predict the roles of various circRNAs. In fact, 15 of 47 relevant studies have reported referencing target circRNAs from databases (Table 2). The primary convenience of circRNA databases, such as CircBase and circ2Traits,30 is that they provide circRNA expression results. However, the identification and quantification of circRNAs can be complicated in some databases, such as that developed by the University of California, Santa Cruz. In mechanistic research, databases are indispensable to the discovery of potential miRNAs and protein targets in a timely and cost-effective manner. Some researchers have also used complementary sequences, as determined with sequencing technology, to predict potential miRNAs and protein targets. Hence, these methods are indispensable to the study of circRNAs.
 |
Table 2 Associated circRNA Databases |
The Underlying Mechanisms of circRNAs in GC
Accumulating evidence suggests that circRNAs play various functional roles in GC, although the underlying mechanisms remain unclear. Overall, the mechanisms of many direct binding targets have been identified, while many other potential mechanisms remain unknown. The targets of circRNAs include DNA, miRNAs, proteins, and ribosomes. CircRNAs can also affect GC progression by the epigenetic regulation of DNA-templated processes. Although some functional circRNAs have been implicated in the pathogenesis of GC, the underlying mechanisms remain to be explored. According to these findings, the mechanisms of circRNAs can be divided into two general groups: those with and those without known direct targets.
Direct Regulation of Specific Targets
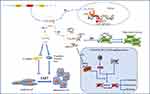
By directly targeting miRNAs, circRNAs can influence the traits of GC, the expression profiles of protein-coding miRNAs, and the regulation of related signaling pathways (Figure 1). In fact, the direct binding of circRNAs and proteins is known to affect the expression of target genes. Moreover, a recent report demonstrated that circRNAs can encode proteins by binding to the ribosome.
Modulation of miRNAs
Increasing evidence suggests that the most common mechanism of circRNAs in GC involves miRNA sponges. The sequences of circRNAs contain MREs that facilitate binding to miRNAs. Normally, the number of MREs, which are thought to be located within exon sequences, is closely related to circRNA length.31 Many studies have demonstrated that miRNAs can negatively regulate gene expression at the post-transcriptional level, mainly through mRNAs.32,33 For example, CircRNA_100269 inhibits the proliferation of GC cells by sponging miR-630.27 To our knowledge, an antisense sequence to the cerebellar degeneration-related protein 1 transcript (CDR1as) was the first circRNA reported to act as a miRNA sponge. CDR1as/ciRS-7 is the genome antisense strand to the human CDR1 locus (hence the name CDR1as), which targets miR-7 (hence the name ciRS-7–circular RNA sponge for miR-7). MiR-7 is a well-known tumor suppressor with 63 binding sites.21,34,35 Recently, ciRS-7 was reported to promote the development of GC by inhibiting miR-7-related functions.29 In addition, circPVT1,26 circular RNA_LARP4,36 circPSMC3,37 circNRIP1,38 circNF1,39 circ-SFMBT2,40 circFAT1(e2),41 circYAP1,42 circ-ZFR,43 circRNA_001569,44 hsa_circ_0000993,45 circPDSS1,46 circCOL6A3,47 hsa_circ_0008035,48 circDLST,49 circ-ERBB2,50 hsa_circ_0001368,51 circAKT3,52 circEIF4G3,53 circ-DCAF654 and circRNA004790555 also exert effects in GC by binding to miRNAs (Table 1). In general, upregulation of circRNAs in GC tissues exerts cancer-promoting effects, whereas downregulation inhibits the onset and development of GC. Notably, downregulated expression of hsa_circ_0000096 promotes tumorigenesis in GC.56 Although the underlying cause remains unclear, this anomalous finding indicates that other mechanisms are activated along with the sponge-like activities of miRNAs.
By regulating corresponding miRNAs, circRNAs can regulate the expression levels of downstream proteins. For example, Liu et al reported that circYAP1 upregulates p27 by sponging miR-367-5p.42 P27, also named KIP1, inhibits cyclin-dependent kinase (CDK), which influences cellular proliferation and apoptosis.57 The binding of miR-367-5p and p27 contributes to the inactivation of p27. When miR-367-5p is sponged by circYAP1, p27 is activated and subsequently promotes tumorigenesis in GC. In this case, circYAP1 acts as an endogenous competitive RNA that regulates the activation of p27. Huang et al found that circAKT3 inhibits the apoptosis of GC cells and promotes DNA damage repair in vivo and in vitro.52 CircAKT3 exerts its function by sponging miR-198 and upregulating its targeting PIK3R1 gene.52 ciRS-7,29 circPSMC3,37 circNRIP1,38 circNF1,39 circ-SFMBT2,40 circFAT1(e2),41 circ-ZFR,43 circRNA_001569,44 circPDSS1,46 circCOL6A3,47 hsa_circ_0008035,48 circDLST,49 circ-ERBB2,50 hsa_circ_0001368,51 circAKT3,52 circ-DONSON,58 and circ-SERPINE259 are also known to regulate the effects of various proteins after sponging corresponding miRNAs.
In addition, circRNAs can also influence the expression of proteins at the pre-transcriptional level. For example, circular RNA_LARP4 binds to miR-424, and the LATS1 gene is the target of miR-424, a miRNA that decreases the expression of LATS1 at the protein level. Regardless of the mechanism, circRNAs have important roles in the regulation of the expression profiles and activities of proteins.
Another important mechanism of circRNAs in cancer-related signaling occurs via the circRNA-miRNA-protein pathway. Researchers have identified many miRNA targets of known signaling pathways that are associated with circRNAs in GC. Proteins are key molecules for the regulation of various signaling pathways involved in the onset and progression of cancer, including GC,60,61 such as the phosphatase and tensin homolog (PTEN)/phosphatidylinositol 3‑kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) signaling pathway. After stimulation, PI3K activates Akt, which in turn is translocated to the nucleus, where it induces proliferation, metastasis, and metabolism in GC. mTOR complex 2 signaling activates other sites of Akt. PTEN negatively regulates the activation and recruitment of activated Akt.62 PTEN/PI3K/Akt/mTOR signaling is the most commonly studied pathway between circRNAs and GC. CircPSMC3 and circ-ZFR indirectly upregulate PTEN by sponging miRNAs.37,43 In the same way, circNRIP1 can increase the expression of Akt. However, miR-7, the target miRNA of ciRS-7, increases the expression of PTEN, while decreasing that of Akt and mTOR. CiRS-7 downregulates PTEN expression and upregulates that of Akt and mTOR.29 Liu et al reported that circ-ZFR inhibits tumor growth in GC via the p53 cascade, and p53 is a well-known tumor suppressor.43 CircOSBPL10 plays an oncogenic role in GC by activating Wnt/β-catenin signaling pathway.63 Given that the binding sites between miR-136-5p and WNT2, the expression level of WNT2 is upregulated because of overexpressed circOSBPL10, the molecular sponge of miR-136-5p. Thereby, WNT2 activates Wnt/β-catenin signaling pathway and promotes GC development.63 Besides that, circHIPK3 and circHECTD1 also promote GC development through upregulating Wnt/β-catenin pathway.64,65 Although associations among various signaling pathways and GC have been demonstrated, the involvement of other signaling cascades, such as the Notch signaling pathway, remains unclear.
Targeting Proteins
Regardless of the physiological or pathological conditions, proteins play unique roles in cells and organs. CircRNAs directly bind proteins and exert effects. For example, Y-box binding protein-1 (YBX1) can bind both DNA and RNA and consequently influence gene expression. In fact, studies have reported that YBX1 exerts an oncogenic function in GC. In the nucleus, circFAT1(e2) inhibits cell proliferation in GC by directly binding to YBX1.41 SOX4, a member of the SOX family, normally regulates cell biological processes through the high-mobility group domain, thereby mediating DNA binding. The deregulation of SOX expression plays an essential role in the onset and progression of cancer, and SOX4 usually exerts a carcinogenic effect.66 Circ-DONSON promotes the expression of SOX4 by recruiting the NURF complex to the promoter region of the SOX4 gene in the nucleus. The upregulated SOX4 then contributes to GC progression.58 Moreover, a database search revealed that EIF4A3 has potential binding sites for differentially expressed circRNAs in GC.
The Protein-Coding Ability of circRNAs Through Direct Binding to the Ribosome
CircPVRL3 has been reported to possess protein-coding abilities via protein-coding structures, open reading frames, internal ribosome entry sites, and m6A modification.67 CircPVRL3 is thought to directly bind the ribosome via internal ribosome entry sites and to subsequently inhibit translation. In addition, other mechanisms may exist that open the loop structure, thereby facilitating the conversion of circRNAs back to pre-mRNAs.23 Therefore, circRNAs appear to encode proteins after conversion to mRNAs. However, no study has reported an association between mRNAs and circRNAs. Because of the limited research results, further studies are needed to investigate such mechanisms, given that relationships are anticipated.
Indirect Regulation Without Accurate Targets
CircRNAs have also been reported to influence the expression of genes involved in epithelial-mesenchymal transition (EMT), although no direct binding target has been identified to date, as discussed in the following two sections.
Regulating Gene Transcription
Some studies have found that circRNAs regulate gene expression, but without known targets. For example, cyclin D1 and CDK6 are cycle-related proteins, and matrix metalloproteinase (MMP)-2 and MMP-9 are associated with migration. Hsa_circ_0000096 has been reported to positively regulate the protein levels of cyclin D1, CDK6, MMP-2, and MMP-9 in a dose-dependent manner.56 Overexpressed circFNDC3B decreases the expression of E-cadherin and increases CD44 expression, thus regulating the migration and invasion of GC cells.68 However, there is a lack of accurate information regarding the targets of many miRNAs and protein-coding genes.
EMT Related Mechanisms
The EMT process has increasingly been shown to have essential roles in various physiological and pathological processes in cancer.69 After adopting the traits of mesenchymal cells, epithelial cells become more flexible to migration and proliferation. The key proteins involved in the EMT process include E-cadherin, N-cadherin, vimentin, and snail. Of these, E-cadherin is a key molecule that ensures cell-cell contact, and decreased expression of E-cadherin is thought to be a key event in EMT.70 In GC, EMT is closely associated with invasion and metastasis. CircRNAs regulate the expression of these key proteins, thereby influencing the EMT process in GC (Figure 1). Through the upregulation of N-cadherin, vimentin, and snail, and the downregulation of E-cadherin, the circRNAs circFNDC3B, circRNA_0023642 and circ-104916 induce EMT and consequently promote invasion and metastasis in GC.68,71,72 Because no exact targets in the regulation of EMT have been identified, further studies are needed to clarify the underlying mechanisms. Besides that, circNHSL1 upregulates SIX1 expression level by targeting miR-1306-3p, and then SIX1 can increase Vimentin expression by binding to its promoter, thereby promote EMT process in GC cells.73
The Applications of circRNAs in GC
Because many circRNAs have crucial roles in GC, the exploitation of these molecules is promising in three main applications: diagnostic biomarkers, prognostic biomarkers, and therapeutic targets.
Promising Biomarkers for the Diagnosis of GC
According to cancer statistics, GC is fifth among cancers regarding incidence but third regarding mortality.1 Although substantial progress has been made in therapeutic strategies, the early stage diagnosis rate is too low to achieve timely treatment for some patients with advanced GC, thus resulting in the relatively low survival rate. Hence, the identification of promising biomarkers for early-stage diagnosis is a necessary strategy to improve survival of GC patients. Over the past few years, circRNAs have received extensive attention. The closed loop structure stabilizes circRNAs in tissues and plasma because of resistance to the enzymatic activities of exonucleases.74 Moreover, Shao et al have found that circRNAs exist in gastric juice.75 In addition, some circRNAs have been found to have better diagnostic power than carcinoembryonic antigen and carbohydrate antigen 19–9, which are currently used for the diagnosis of GC.30,75 As shown in Table 3, many circRNAs may be suitable as diagnostic biomarkers in GC.25,30,36,37,56,67,75–90 Among these circRNAs, hsa_circ_0001017 in the plasma and circPSMC3 in tissues have relatively better diagnostic values, with areas under the receiver operating characteristic curves of > 0.90. Combining two or more circRNAs may be a good method to improve diagnostic power. For instance, combining hsa_circ_0001017 and hsa_circ_0061276 in both plasma and tissues has resulted in a diagnostic power as high as an AUC of 0.966, a sensitivity of 0.955 and a specificity of 0.957.85 Related meta-analyses have indicated that circRNAs might be a good choice as a diagnostic biomarker for tumors, including GC.91,92 Although much progress has been made, further studies are needed, as current data are limited to tissues, even though collecting plasma samples for the diagnosis of early-stage GC would be easier.
 |
Table 3 CircRNAs Associated with GC Diagnosis |
Biomarkers for Prognosis
The incidence of GC has been consistent over the past several years, but the overall prognosis is poorer and survival times are shorter than those for other cancers.1 Surgical resection is the most effective treatment for GC, but relapse and metastasis severely affect postoperative prognosis. Recent studies have reported that circRNAs are closely associated with the clinicopathological features of GC and can serve as prognostic biomarkers. As shown in Table 4, many clinicopathological factors are related to circRNAs, especially the tumor-node-metastasis stage, which normally dominates the prognosis of GC patients. CircRNA_10026927 is associated with early relapse, whereas circPVT1,26 circLARP4,36 and circYAP142 have been recommended as prognostic biomarkers of survival in GC. Moreover, hsa_circ_0001895, hsa_circ_0000467, circNRIP1, circFAT1(e2), hsa_circ_0000993, circPDSS1, and ciRS-7 are associated with the prognosis of GC. Nonetheless, further information is needed to determine the prognostic usefulness of circRNAs for the treatment of GC.
 |
Table 4 Altered Expression of circRNAs Associated with the Clinicopathological Features of GC Patients |
Therapeutic Targets
Many patients with advanced and unresectable GC receive chemotherapy. Targeted therapy is an important treatment option for chemotherapy-resistant GC. In recent years, given the improved understanding of the pathogenesis of GC, many molecules and signaling pathways may be suitable for targeted therapies. Trastuzumab, which inhibits the activation of human epidermal growth factor receptor 2, was the first targeted therapy for GC shown to improve survival.93 In contrast, several other targeted therapies for GC have shown little benefit. At present, exploring the applications and indications of novel molecules is crucial for the continued development of targeted therapies for GC.94 Some circRNAs target important GC-related molecules and signaling pathways, and consequently regulate the expression patterns of corresponding genes. In addition, circRNAs also influence some important clinicopathological features and are closely associated with prognosis. The overexpression or knockdown of circRNAs not only allows for better understanding of the mechanisms underlying the onset and progression of GC, but also provides useful information for the design of targeted therapies to regulate important GC-related molecules, signaling pathways, and genes. For example, circ-PSMC3 inhibits the growth of GC cells in vivo, whereas circNRIP1 has the opposite effect. Overexpression of circ-PSMC3 or knockdown of circNRIP1 has been predicted to inhibit the progression of GC.37,38 Cisplatin-resistant GC tissues show elevated expression of circAKT3, and the level of circAKT3 is negatively associated with disease-free survival. The role of circAKT3 in cisplatin resistance of GC also emphasizes its potential as a therapeutic target to reverse drug resistance.52 Overexpression results in translocation of circFAT1(e2) to the nucleus, where it binds YBX1, a tumor promoter. In addition, circ-SFMBT2,40 circRNA_001569,44 circ-ZFR,43 circPDSS1,46 and ciRS-729 have been investigated for use in targeted therapies, thus indicating promising clinical applications for circRNAs in the treatment of GC. In addition, the expression of PVT1 RNA is positively associated with regulation of c-myc, a key oncogene.95 CircPVT1 is encoded by exon 3 of the PVT1 gene, and overexpression of circPVT1 upregulates the level of c-myc.26 However, some circRNAs respond to the level of the therapeutic target and thereby may potentially serve as prognostic indicators of targeted therapy.
In addition, some circRNAs, such as circ-KIAA1244,96 circLMTK2,97 circ-ARHGAP26,98 hsa_circ_0000520,99 hsa_circ_0047905,100 hsa_circ_0138960,100 hascircRNA7690-15,100 cir_ITCH,101 and circHIPK3101 are differentially expressed in GC, although little is known about the underlying mechanisms and potential applications.
Conclusion
The onset and progression of GC involves many steps and factors, but the mechanisms underlying the etiopathogenesis must be further explored. Databases have played essential roles in bioinformatics research on circRNAs and have resulted in substantial progress in the field. CircRNAs have been found to act as miRNA sponges regulating the expression of proteins that directly affect signaling pathways and induce the EMT process, thereby influencing proliferation, invasion, and metastasis in GC. Various corresponding applications are also emerging, such as diagnostic biomarkers, prognostic biomarkers, and therapeutic targets. Although circRNA research is still in its infancy, circRNAs offer promising applications in the diagnosis, treatment, and prognosis of cancers, including GC.
Acknowledgments
Xiaohuan Tang and Jiaming Zhu are co-first authors. This study was supported by grants from the Jilin Province Science and Technology Development Project (20180101169JC), Excellent Talent Fund Project of Jilin Province Science and Technology Department (20190103091JH), and The Second Hospital of Jilin University Project (KYPY2018-14).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.v68.6
2. Lin MT, Song HJ, Ding XY. Long non-coding RNAs involved in metastasis of gastric cancer. World J Gastroenterol. 2018;24(33):3724–3737. doi:10.3748/wjg.v24.i33.3724
3. Cech TR, Steitz JA. The noncoding RNA revolution-trashing old rules to forge new ones. Cell. 2014;157(1):77–94. doi:10.1016/j.cell.2014.03.008
4. Hu X, Feng Y, Zhang D, et al. A functional genomic approach identifies FAL1 as an oncogenic long noncoding RNA that associates with BMI1 and represses p21 expression in cancer. Cancer Cell. 2014;26(3):344–357. doi:10.1016/j.ccr.2014.07.009
5. Martens-Uzunova ES, Bottcher R, Croce CM, Jenster G, Visakorpi T, Calin GA. Long noncoding RNA in prostate, bladder, and kidney cancer. Eur Urol. 2014;65(6):1140–1151. doi:10.1016/j.eururo.2013.12.003
6. Puneet KHR, Kumari S, Tiwari S, Khanna A, Narayan G. Epigenetic mechanisms and events in gastric cancer-emerging novel biomarkers. Pathol Oncol Res. 2018;24(4):757–770. doi:10.1007/s12253-018-0410-z
7. Zong W, Feng W, Jiang Y, Ju S, Cui M, Jing R. Evaluating the diagnostic and prognostic value of serum long non-coding RNA CTC-497E21.4 in gastric cancer. Clin Chem Lab Med. 2019;57:1063–1072. doi:10.1515/cclm-2018-0929
8. Zhang C, Liang Y, Ma MH, Wu KZ, Dai DQ. KRT15, INHBA, MATN3, and AGT are aberrantly methylated and differentially expressed in gastric cancer and associated with prognosis. Pathol Res Pract. 2019;215(5):893–899.
9. Zhang M, Du X. Noncoding RNAs in gastric cancer: research progress and prospects. World J Gastroenterol. 2016;22(29):6610–6618. doi:10.3748/wjg.v22.i29.6610
10. Lee RC, Feinbaum RL, Ambros V, The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. doi:10.1016/0092-8674(93)90529-Y
11. Johnsson P, Lipovich L, Grander D, Morris KV. Evolutionary conservation of long non-coding RNAs; sequence, structure, function. Biochim Biophys Acta. 2014;1840(3):1063–1071. doi:10.1016/j.bbagen.2013.10.035
12. Bejerano G, Pheasant M, Makunin I, et al. Ultraconserved elements in the human genome. Science. 2004;304(5675):1321–1325. doi:10.1126/science.1098119
13. Adams BD, Parsons C, Walker L, Zhang WC, Slack FJ. Targeting noncoding RNAs in disease. J Clin Invest. 2017;127(3):761–771. doi:10.1172/JCI84424
14. Jafari N, Abediankenari S. MicroRNA-34 dysregulation in gastric cancer and gastric cancer stem cell. Tumour Biol. 2017;39(5):1010428317701652. doi:10.1177/1010428317701652
15. Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16(3):203–222. doi:10.1038/nrd.2016.246
16. Ma F, Liu X, Zhou S, et al. Long non-coding RNA FGF13-AS1 inhibits glycolysis and stemness properties of breast cancer cells through FGF13-AS1/IGF2BPs/Myc feedback loop. Cancer Lett. 2019;450:63–75.
17. Rong D, Sun H, Li Z, et al. An emerging function of circRNA-miRNAs-mRNA axis in human diseases. Oncotarget. 2017;8(42):73271–73281. doi:10.18632/oncotarget.v8i42
18. Anastasiadou E, Jacob LS, Slack FJ. Non-coding RNA networks in cancer. Nat Rev Cancer. 2018;18(1):5–18. doi:10.1038/nrc.2017.99
19. Dragomir MP, Knutsen E, Calin GA. SnapShot: unconventional miRNA functions. Cell. 2018;174(4):1038. (). doi:10.1016/j.cell.2018.07.040
20. Sanger HL, Klotz G, Riesner D, Gross HJ, Kleinschmidt AK. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci U S A. 1976;73(11):3852–3856. doi:10.1073/pnas.73.11.3852
21. Memczak S, Jens M, Elefsinioti A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495(7441):333–338. doi:10.1038/nature11928
22. Sui W, Shi Z, Xue W, et al. Circular RNA and gene expression profiles in gastric cancer based on microarray chip technology. Oncol Rep. 2017;37(3):1804–1814. doi:10.3892/or.2017.5415
23. Liu J, Liu T, Wang X, He A. Circles reshaping the RNA world: from waste to treasure. Mol Cancer. 2017;16(1):58. doi:10.1186/s12943-017-0630-y
24. Meng S, Zhou H, Feng Z, et al. CircRNA: functions and properties of a novel potential biomarker for cancer. Mol Cancer. 2017;16(1):94. doi:10.1186/s12943-017-0663-2
25. Li P, Chen S, Chen H, et al. Using circular RNA as a novel type of biomarker in the screening of gastric cancer. Clin Chim Acta. 2015;444:132–136. doi:10.1016/j.cca.2015.02.018
26. Chen J, Li Y, Zheng Q, et al. Circular RNA profile identifies circPVT1 as a proliferative factor and prognostic marker in gastric cancer. Cancer Lett. 2017;388:208–219. doi:10.1016/j.canlet.2016.12.006
27. Zhang Y, Liu H, Li W, et al. CircRNA_100269 is downregulated in gastric cancer and suppresses tumor cell growth by targeting miR-630. Aging (Albany NY). 2017;9(6):1585–1594. doi:10.18632/aging.v9i6
28. Vo JN, Cieslik M, Zhang Y, et al. The landscape of circular RNA in cancer. Cell. 2019;176(4):869–881. doi:10.1016/j.cell.2018.12.021
29. Pan H, Li T, Jiang Y, et al. Overexpression of circular RNA ciRS-7 abrogates the tumor suppressive effect of miR-7 on gastric cancer via PTEN/PI3K/AKT signaling pathway. J Cell Biochem. 2018;119(1):440–446. doi:10.1002/jcb.26201
30. Huang M, He YR, Liang LC, Huang Q, Zhu ZQ. Circular RNA hsa_circ_0000745 may serve as a diagnostic marker for gastric cancer. World J Gastroenterol. 2017;23(34):6330–6338. doi:10.3748/wjg.v23.i34.6330
31. Verduci L, Strano S, Yarden Y, Blandino G. The circRNA-microRNA code: emerging implications for cancer diagnosis and treatment. Mol Oncol. 2019. doi:10.1002/1878-0261.12468
32. Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20. doi:10.1016/j.cell.2004.12.035
33. Mohr AM, Mott JL. Overview of microRNA biology. Semin Liver Dis. 2015;35(1):3–11. doi:10.1055/s-00000069
34. Jiang L, Liu X, Chen Z, et al. MicroRNA-7 targets IGF1R (insulin-like growth factor 1 receptor) in tongue squamous cell carcinoma cells. Biochem J. 2010;432(1):199–205. doi:10.1042/BJ20100859
35. Kong D, Piao YS, Yamashita S, et al. Inflammation-induced repression of tumor suppressor miR-7 in gastric tumor cells. Oncogene. 2012;31(35):3949–3960. doi:10.1038/onc.2011.558
36. Zhang J, Liu H, Hou L, et al. Circular RNA_LARP4 inhibits cell proliferation and invasion of gastric cancer by sponging miR-424-5p and regulating LATS1 expression. Mol Cancer. 2017;16(1):151. doi:10.1186/s12943-017-0719-3
37. Rong D, Lu C, Zhang B, et al. CircPSMC3 suppresses the proliferation and metastasis of gastric cancer by acting as a competitive endogenous RNA through sponging miR-296-5p. Mol Cancer. 2019;18(1):25. doi:10.1186/s12943-019-0958-6
38. Zhang X, Wang S, Wang H, et al. Circular RNA circNRIP1 acts as a microRNA-149-5p sponge to promote gastric cancer progression via the AKT1/mTOR pathway. Mol Cancer. 2019;18(1):20. doi:10.1186/s12943-018-0935-5
39. Wang Z, Ma K, Pitts S, et al. Novel circular RNA NF1 acts as a molecular sponge, promoting gastric cancer by absorbing miR-16. Endocr Relat Cancer. 2018.
40. Sun H, Xi P, Sun Z, et al. Circ-SFMBT2 promotes the proliferation of gastric cancer cells through sponging miR-182-5p to enhance CREB1 expression. Cancer Manag Res. 2018;10:5725–5734. doi:10.2147/CMAR
41. Fang J, Hong H, Xue X, et al. A novel circular RNA, circFAT1(e2), inhibits gastric cancer progression by targeting miR-548g in the cytoplasm and interacting with YBX1 in the nucleus. Cancer Lett. 2019;442:222–232. doi:10.1016/j.canlet.2018.10.040
42. Liu H, Liu Y, Bian Z, et al. Circular RNA YAP1 inhibits the proliferation and invasion of gastric cancer cells by regulating the miR-367-5p/p27 (Kip1) axis. Mol Cancer. 2018;17(1):151.
43. Liu T, Liu S, Xu Y, et al. Circular RNA-ZFR inhibited cell proliferation and promoted apoptosis in gastric cancer by sponging miR-130a/miR-107 and modulating PTEN. Cancer Res Treat. 2018;50(4):1396–1417. doi:10.4143/crt.2017.537
44. Shen F, Liu P, Xu Z, et al. CircRNA_001569 promotes cell proliferation through absorbing miR-145 in gastric cancer. J Biochem. 2019;165(1):27–36. doi:10.1093/jb/mvy079
45. Zhong S, Wang J, Hou J, et al. Circular RNA hsa_circ_0000993 inhibits metastasis of gastric cancer cells. Epigenomics. 2018;10(10):1301–1313. doi:10.2217/epi-2017-0173
46. Ouyang Y, Li Y, Huang Y, et al. CircRNA circPDSS1 promotes the gastric cancer progression by sponging miR-186-5p and modulating NEK2. J Cell Physiol. 2018;234(7):10458–10469.
47. Sun X, Zhang X, Zhai H, Zhang D, Ma S. A circular RNA derived from COL6A3 functions as a ceRNA in gastric cancer development. Biochem Biophys Res Commun. 2019;515(1):16–23.
48. Huang S, Zhang X, Guan B, et al. A novel circular RNA hsa_circ_0008035 contributes to gastric cancer tumorigenesis through targeting the miR-375/YBX1 axis. Am J Transl Res. 2019;11(4):2455–2462.
49. Zhang J, Hou L, Liang R, et al. CircDLST promotes the tumorigenesis and metastasis of gastric cancer by sponging miR-502-5p and activating the NRAS/MEK1/ERK1/2 signaling. Mol Cancer. 2019;18(1):80. doi:10.1186/s12943-019-1015-1
50. Li X, He M, Guo J, Cao T. Upregulation of circular RNA circ-ERBB2 predicts unfavorable prognosis and facilitates the progression of gastric cancer via miR-503/CACUL1 and miR-637/MMP-19 signaling. Biochem Biophys Res Commun. 2019;511(4):926–930. doi:10.1016/j.bbrc.2019.03.010
51. Lu J, Zhang PY, Li P, et al. Circular RNA hsa_circ_0001368 suppresses the progression of gastric cancer by regulating miR-6506-5p/FOXO3 axis. Biochem Biophys Res Commun. 2019;512(1):29–33. doi:10.1016/j.bbrc.2019.02.111
52. Huang X, Li Z, Zhang Q, et al. Circular RNA AKT3 upregulates PIK3R1 to enhance cisplatin resistance in gastric cancer via miR-198 suppression. Mol Cancer. 2019;18(1):71. doi:10.1186/s12943-019-0969-3
53. Wang Q, Wang T, Hu Y, et al. Circ-EIF4G3 promotes the development of gastric cancer by sponging miR-335. Pathol Res Pract. 2019;215(9):152507. doi:10.1016/j.prp.2019.152507
54. Wu L, Liu D, Yang Y. Enhanced expression of circular RNA circ-DCAF6 predicts adverse prognosis and promotes cell progression via sponging miR-1231 and miR-1256 in gastric cancer. Exp Mol Pathol. 2019;110:104273. doi:10.1016/j.yexmp.2019.104273
55. Lai Z, Yang Y, Wang C, et al. Circular RNA 0047905 acts as a sponge for microRNA4516 and microRNA1227-5p, initiating gastric cancer progression. Cell Cycle. 2019;18(14):1560–1572. doi:10.1080/15384101.2019.1618122
56. Li P, Chen H, Chen S, et al. Circular RNA 0000096 affects cell growth and migration in gastric cancer. Br J Cancer. 2017;116(5):626–633. doi:10.1038/bjc.2016.451
57. Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8(4):253–267. doi:10.1038/nrc2347
58. Ding L, Zhao Y, Dang S, et al. Circular RNA circ-DONSON facilitates gastric cancer growth and invasion via NURF complex dependent activation of transcription factor SOX4. Mol Cancer. 2019;18(1):45. doi:10.1186/s12943-019-1006-2
59. Liu J, Song S, Lin S, et al. Circ-SERPINE2 promotes the development of gastric carcinoma by sponging miR-375 and modulating YWHAZ. Cell Prolif. 2019;52(4):e12648. doi:10.1111/cpr.2019.52.issue-4
60. Liu X, Yun F, Shi L, Li ZH, Luo NR, Jia YF. Roles of signaling pathways in the epithelial-mesenchymal transition in cancer. Asian Pac J Cancer Prev. 2015;16(15):6201–6206. doi:10.7314/APJCP.2015.16.15.6201
61. Fu Y, Li H, Hao X. The self-renewal signaling pathways utilized by gastric cancer stem cells. Tumour Biol. 2017;39(4):1010428317697577. doi:10.1177/1010428317697577
62. Hu M, Zhu S, Xiong S, Xue X, Zhou X. MicroRNAs and the PTEN/PI3K/Akt pathway in gastric cancer (Review). Oncol Rep. 2019;41(3):1439–1454. doi:10.3892/or.2019.6962
63. Wang S, Zhang X, Li Z, et al. Circular RNA profile identifies circOSBPL10 as an oncogenic factor and prognostic marker in gastric cancer. Oncogene. 2019;38:6985–7001. doi:10.1038/s41388-019-0933-0
64. Liu WG, Xu Q. Upregulation of circHIPK3 promotes the progression of gastric cancer via Wnt/beta-catenin pathway and indicates a poor prognosis. Eur Rev Med Pharmacol Sci. 2019;23(18):7905–7912. doi:10.26355/eurrev_201909_19004
65. Cai J, Chen Z, Wang J, et al. circHECTD1 facilitates glutaminolysis to promote gastric cancer progression by targeting miR-1256 and activating beta-catenin/c-Myc signaling. Cell Death Dis. 2019;10(8):576. doi:10.1038/s41419-019-1814-8
66. Grimm D, Bauer J, Wise P, et al. The role of SOX family members in solid tumours and metastasis. Semin Cancer Biol. 2019. doi:10.1016/j.semcancer.2019.03.004
67. Sun HD, Xu ZP, Sun ZQ, et al. Down-regulation of circPVRL3 promotes the proliferation and migration of gastric cancer cells. Sci Rep. 2018;8(1):10111. doi:10.1038/s41598-018-27837-9
68. Hong Y, Qin H, Li Y, et al. FNDC3B circular RNA promotes the migration and invasion of gastric cancer cells via the regulation of E-cadherin and CD44 expression. J Cell Physiol. 2019;234:19895–19910. doi:10.1002/jcp.v234.11
69. Chen T, You Y, Jiang H, Wang ZZ. Epithelial-mesenchymal transition (EMT): A biological process in the development, stem cell differentiation, and tumorigenesis. J Cell Physiol. 2017;232(12):3261–3272. doi:10.1002/jcp.v232.12
70. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi:10.1016/j.cell.2009.11.007
71. Zhou LH, Yang YC, Zhang RY, Wang P, Pang MH, Liang LQ. CircRNA_0023642 promotes migration and invasion of gastric cancer cells by regulating EMT. Eur Rev Med Pharmacol Sci. 2018;22(8):2297–2303. doi:10.26355/eurrev_201804_14818
72. Li J, Zhen L, Zhang Y, et al. Circ-104916 is downregulated in gastric cancer and suppresses migration and invasion of gastric cancer cells. Onco Targets Ther. 2017;10:3521–3529. doi:10.2147/OTT
73. Zhu Z, Rong Z, Luo Z, et al. Circular RNA circNHSL1 promotes gastric cancer progression through the miR-1306-3p/SIX1/vimentin axis. Mol Cancer. 2019;18(1):126. doi:10.1186/s12943-019-1054-7
74. Li Y, Zheng Q, Bao C, et al. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25(8):981–984. doi:10.1038/cr.2015.82
75. Shao Y, Li J, Lu R, et al. Global circular RNA expression profile of human gastric cancer and its clinical significance. Cancer Med. 2017;6(6):1173–1180. doi:10.1002/cam4.2017.6.issue-6
76. Tian M, Chen R, Li T, Xiao B. Reduced expression of circRNA hsa_circ_0003159 in gastric cancer and its clinical significance. J Clin Lab Anal. 2018;32(3):e22281. doi:10.1002/jcla.2018.32.issue-3
77. Rong D, Dong C, Fu K, Wang H, Tang W, Cao H. Upregulation of circ_0066444 promotes the proliferation, invasion, and migration of gastric cancer cells. Onco Targets Ther. 2018;11:2753–2761. doi:10.2147/OTT
78. Shao Y, Chen L, Lu R, et al. Decreased expression of hsa_circ_0001895 in human gastric cancer and its clinical significances. Tumour Biol. 2017;39(4):1010428317699125. doi:10.1177/1010428317699125
79. Lu R, Shao Y, Ye G, Xiao B, Guo J. Low expression of hsa_circ_0006633 in human gastric cancer and its clinical significances. Tumour Biol. 2017;39(6):1010428317704175. doi:10.1177/1010428317704175
80. Li WH, Song YC, Zhang H, et al. Decreased expression of Hsa_circ_00001649 in gastric cancer and its clinical significance. Dis Markers. 2017;2017:4587698. doi:10.1155/2017/4587698
81. Zhao Q, Chen S, Li T, Xiao B, Zhang X. Clinical values of circular RNA 0000181 in the screening of gastric cancer. J Clin Lab Anal. 2018;32(4):e22333. doi:10.1002/jcla.2018.32.issue-4
82. Wei J, Wei W, Xu H. et al. Circular RNA hsa_circRNA_102958 may serve as a diagnostic marker for gastric cancer. Cancer Biomark;2019. 1–7. doi:10.3233/CBM-182029
83. Xie Y, Shao Y, Sun W, et al. Downregulated expression of hsa_circ_0074362 in gastric cancer and its potential diagnostic values. Biomark Med. 2018;12(1):11–20. doi:10.2217/bmm-2017-0114
84. Shao YF, Yang YB, Lu RD, Xiao BX, Ye GL, Guo JM. Identification of tissue-specific circRNA hsa_circ_0000705 as an indicator for human gastric cancer. Int J Clin Exp Patho. 2017;10(3):3151–3156.
85. Li T, Shao Y, Fu L, et al. Plasma circular RNA profiling of patients with gastric cancer and their droplet digital RT-PCR detection. J Mol Med (Berl). 2018;96(1):85–96. doi:10.1007/s00109-017-1600-y
86. Chen S, Li T, Zhao Q, Xiao B, Guo J. Using circular RNA hsa_circ_0000190 as a new biomarker in the diagnosis of gastric cancer. Clin Chim Acta. 2017;466:167–171. doi:10.1016/j.cca.2017.01.025
87. Lu J, Zhang PY, Xie JW, et al. Hsa_circ_0000467 promotes cancer progression and serves as a diagnostic and prognostic biomarker for gastric cancer. J Clin Lab Anal. 2018;33:e22726.
88. Wang Y, Xu S, Chen Y, Zheng X, Li T, Guo J. Identification of hsa_circ_0005654 as a new early biomarker of gastric cancer. Cancer Biomark. 2019;1–8. doi:10.3233/CBM-190561
89. Kong S, Yang Q, Tang C, Wang T, Shen X, Ju S. Identification of hsa_circ_0001821 as a novel diagnostic biomarker in gastric cancer via comprehensive circular RNA profiling. Front Genet. 2019;10:878. doi:10.3389/fgene.2019.00878
90. Lu J, Zhang PY, Xie JW, et al. Circular RNA hsa_circ_0006848 related to ribosomal protein L6 acts as a novel biomarker for early gastric cancer. Dis Markers. 2019;2019:3863458. doi:10.1155/2019/3863458
91. Wang M, Yang Y, Xu J, Bai W, Ren X, Wu H. CircRNAs as biomarkers of cancer: a meta-analysis. BMC Cancer. 2018;18(1):303. doi:10.1186/s12885-018-4213-0
92. Chen Z, Zhang L, Han G, et al. A meta-analysis of the diagnostic accuracy of circular RNAs in digestive system malignancy. Cell Physiol Biochem. 2018;45(3):962–972. doi:10.1159/000487291
93. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–697. doi:10.1016/S0140-6736(10)61121-X
94. Yazici O, Sendur MA, Ozdemir N, Aksoy S. Targeted therapies in gastric cancer and future perspectives. World J Gastroenterol. 2016;22(2):471–489. doi:10.3748/wjg.v22.i2.471
95. Tseng YY, Moriarity BS, Gong W, et al. PVT1 dependence in cancer with MYC copy-number increase. Nature. 2014;512(7512):82–86. doi:10.1038/nature13311
96. Tang W, Fu K, Sun H, Rong D, Wang H, Cao H. CircRNA microarray profiling identifies a novel circulating biomarker for detection of gastric cancer. Mol Cancer. 2018;17(1):137. doi:10.1186/s12943-018-0888-8
97. He J, Chen J, Ma B, Jiang L, Zhao G. CircLMTK2 acts as a novel tumor suppressor in gastric cancer. Biosci Rep. 2019;39(5). doi:10.1042/BSR20190363
98. Wangxia LV, Fang Y, Liu Y, Zhao Y, Shi Z, Zhong H. Circular RNA ARHGAP26 is over-expressed and its downregulation inhibits cell proliferation and promotes cell apoptosis in gastric cancer cells. Saudi J Gastroenterol. 2019;25(2):119–125.
99. Sun H, Tang W, Rong D, et al. Hsa_circ_0000520, a potential new circular RNA biomarker, is involved in gastric carcinoma. Cancer Biomark. 2018;21(2):299–306. doi:10.3233/CBM-170379
100. Lai Z, Yang Y, Yan Y, et al. Analysis of co-expression networks for circular RNAs and mRNAs reveals that circular RNAs hsa_circ_0047905, hsa_circ_0138960 and has-circRNA7690-15 are candidate oncogenes in gastric cancer. Cell Cycle. 2017;16(23):2301–2311. doi:10.1080/15384101.2017.1380135
101. Ghasemi S, Emadi-Baygi M, Nikpour P. Down-regulation of circular RNA ITCH and circHIPK3 in gastric cancer tissues. Turk J Med Sci. 2019;49(2):687–695. doi:10.3906/sag-1806-50
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.