Back to Journals » Therapeutics and Clinical Risk Management » Volume 15
Current status of venous thromboembolism development during the perioperative period for colorectal cancer, its prevention with enoxaparin, and monitoring methods
Authors Aoki J, Sakamoto K, Takahashi R, Niwa K, Ishiyama S, Sugimoto K, Kamiyama H, Takahashi M, Kojima Y , Goto M , Tomiki Y , Iba T
Received 17 January 2019
Accepted for publication 8 May 2019
Published 21 June 2019 Volume 2019:15 Pages 791—802
DOI https://doi.org/10.2147/TCRM.S201954
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Jun Aoki,1 Kazuhiro Sakamoto,1 Rina Takahashi,1 Koichiro Niwa,1 Shun Ishiyama,1 Kiichi Sugimoto,1 Hirohiko Kamiyama,1 Makoto Takahashi,1 Yutaka Kojima,1 Michitoshi Goto,1 Yuichi Tomiki,1 Toshiaki Iba2
1Department of Coloproctological Surgery, Juntendo University, Faculty of Medicine, Tokyo, Japan; 2Department of Emergency and Disaster Medicine, Juntendo University, Faculty of Medicine, Tokyo, Japan
Background: There is a high incidence of venous thromboembolism (VTE) during the perioperative period for cancer. Therefore, there is an urgent need to elucidate the perioperative onset and appropriate prophylaxis for VTE.
Purpose: VTE during the perioperative period for colorectal cancer was evaluated by lower limb venous ultrasonic examinations (lower limb echo) under enoxaparin prophylaxis. We also examined the relationship between hemorrhagic adverse events and anti-Xa factor activity.
Patients and methods: Eighty-three subjects who underwent lower limb echo during the perioperative period for colorectal cancer were prospectively included. Enoxaparin was administered for 5 days, from day 1 to day 5 after surgery. Lower limb echo was performed before surgery and on day 5 after surgery. The activated partial thromboplastin time, D-dimer levels, and anti-Xa factor activity were measured before surgery and on days 1, 3, 5, 7, and 9 after surgery.
Results: VTEs before surgery were observed on lower limb echo for 16 patients (19.2%). Three patients (3.6%) had a new thrombus during the perioperative period. The preoperative D-dimer level was an independent prognostic factor for newly formed postoperative VTEs (p=0.0036; odds ratio, 19.37). Three patients (3.6%) had hemorrhagic events; however, there was no significant trend for anti-Xa factor activity.
Conclusion: VTE prevention using enoxaparin was relatively safe, and D-dimer measurements before surgery were useful for predicting perioperative VTE.
Keywords: postoperative complications, pulmonary thromboembolism, deep vein thrombosis, D-dimer, anti-Xa factor activity
Introduction
Approximately 24% of patients who undergo abdominal surgery in Japan develop deep vein thrombosis (DVT), including asymptomatic DVT.1 Furthermore, a similar frequency of DVT has been reported after colorectal cancer surgery.2 If DVT progresses and pulmonary thromboembolism (PE) develops, then the associated mortality rate can be as high as approximately 30%. The United States and Europe have started taking measures against the development of venous thromboembolisms (VTEs) during the early stages of the perioperative period, and the 2001 Guidelines of the American College of Chest Physicians (ACCP) has already recommended using low-molecular-weight heparin and synthetic anti-Xa factor inhibitors for their prevention.3 Based on the preventive guidelines for PE and DVT, Japan has also provided recommendations for countermeasures against VTE during the period after abdominal surgery.4 Because of the significance of enoxaparin for DVT,1 we have been administering enoxaparin prophylactically to patients after colorectal cancer surgery since 2012. Patients indicated for colorectal cancer surgery often have overlapping factors, such as chemotherapy, older age, and malignant tumors, which are risk factors for DVT. Therefore, some of the DVTs that have been recognized as postoperative VTE might have included asymptomatic DVTs that existed before surgery. To examine the efficacy of enoxaparin, it is necessary to screen patients before and after surgery to elucidate the onset of VTE. Lower limb echo is widely used for the screening of VTE, and D-dimer measurements are expected to be simpler and more objective for screening.5–8
Although enoxaparin for the prevention of VTE is considered medicine that does not require monitoring, it has been suggested that anti-Xa factor activity measurements are useful for assessing the risk of hemorrhagic adverse events.9 In the present study, we examined the efficacy of enoxaparin using DVT screening and lower limb echo before and after surgery for patients with indications for colorectal cancer surgery. Furthermore, we examined D-dimer measurements that were used for screening and anti-Xa factor activity levels, which were used as an indicator for hemorrhaging.
Methods
This prospective study included 83 subjects who underwent lower limb echo during the perioperative period for colorectal cancer at our department between February 2013 and April 2015. The following patients were excluded from this study: those with bleeding or complications that could lead to bleeding; those with thrombocytopenia (platelet count <10×104/μL); those with severe liver disorders; those with renal dysfunction (serum creatinine ≥1.5 mg/dL); those with hypersensitive reactions to heparin; those with a history of cerebral hemorrhage; those who underwent orthopedic, abdominal, or cardiovascular surgery within the past 3 months; those weighing <40 kg; those who underwent another form of anticoagulatory therapy (eg, heparin, oral anticoagulant) within the past 1 week; and those with a history of arterial thromboembolism.
Two 2,000-IU (at 9:00
 | Table 1 Administration schedule for enoxaparin and clinical examinations |
When a patient presented with symptoms indicative of VTE, such as dyspnea, chest pain, lower thigh edema, and the Homans sign, or when D-dimer levels were high, contrast computed tomography (CT) tests, which involved scanning over the chest to the lower extremities, were performed. Contrast CT tests were also performed for patients who presented with new asymptomatic DVT detected on lower limb echocardiography after surgery to screen for PE. Regarding hemorrhagic adverse events, in addition to monitoring by blood tests, the presence or absence of melena, drain properties, and wound conditions were assessed. Then, cases that were grade I or higher according to the Japan Clinical Oncology Group Postoperative Complication Criteria (Clavien-Dindo classification)10 were assigned to the bleeding group.
We compared the postoperative DVT group with the non-DVT group based on the results of lower limb echo. Furthermore, patients identified as having VTE after surgery but not before surgery were separately assigned to the new postoperative VTE group and then compared with other groups.
Age, sex, body mass index (BMI), cancer site, cancer stage, comorbidities (thrombotic disease, diabetes, dyslipidemia, hypertension), presence/absence of surgical history, preoperative D-dimer level, and presence/absence of preoperative DVT were the patient factors that were examined. Operative time, amount of blood loss, presence/absence of melena, and surgical approach (laparotomy or laparoscopy) were surgical factors that were examined. D-dimer levels on days 1, 3, 5, 7, and 9 after surgery, anti-Xa factor activity levels on days 3, 5, 7, and 9 after surgery, time to ambulation after surgery, and the presence/absence of postoperative complications (surgical site infection [SSI], anastomotic leakage, and ileus) were the postoperative endpoints that were examined. Colorectal cancer staging was determined according to the Union for International Cancer Control TNM classification of malignant tumors (eighth edition).11
The Mann–Whitney U test, Fisher’s exact test, and χ2 test were used for the univariate analysis, whereas a logistic regression analysis was used for the multivariate analysis. Each result of the analyses was considered statistically significant if p<0.05.
The present research was conducted according to a protocol approved by the Institutional Review Board of Juntendo University Hospital and complied with the Declaration of Helsinki. It is registered with the University Hospital Medical Information Network (UMIN ID: UMIN000009598). All patients provided written informed consent prior to study entry.
Results
Patient background
Backgrounds of all 83 patients are summarized in Table 2. The median age was 71 years (range, 32–89 years), and the case population consisted of 44 men and 39 women. The median BMI was 22.4 kg/m2 (range, 17.5–32.4 kg/m2). There were 34 cases of right colon cancer (7 cases in the cecum, 17 cases in the ascending colon, 10 cases in the transverse colon), 30 cases of left colon cancer (2 cases in the descending colon, 17 cases in the sigmoid colon, 11 cases in the rectosigmoid junction), and 21 cases of rectal cancer. There were two cases of multiple colorectal cancer. According to disease staging using the TNM classification, 29 cases were stage I, 33 were stage II, 18 were stage III, and three were stage IV. Two patients (2.4%) had a history of thrombotic diseases, 31 (49.4%) had hypertension, 8 (9.6%) had dyslipidemia, 9 (10.8%) had complications due to diabetes, and 27 (33.7%) had past surgical history. The median operative time and median blood loss were 250 mins (range, 138–547 mins) and 23.5 mL (range, 5–25.20 mL), respectively. Two patients (2.4%) received blood transfusions because of intraoperative blood loss. Laparotomy including conversion from laparoscopic surgery was performed for eight patients (9.6%). Regarding postoperative complications, 10 patients (12.0%) had SSI, 6 (7.2%) had anastomotic leakage, and 2 (2.4%) had ileus. All patients were able to ambulate within 3 days after surgery, and 75 (90.4%) were able to ambulate from day 1 after surgery. Enoxaparin administrations were discontinued for seven patients (8.4%), because three developed hemorrhagic adverse events, two started warfarin to treat PE, one experienced a drug-induced rash, and one refused to use enoxaparin.
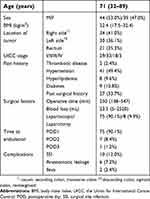 | Table 2 Patient background (n=83) |
DVT occurrence preoperatively and postoperatively
Lower limb echo performed before surgery identified solitary asymptomatic DVT in 16 patients (19.2%). These were localized to the soleal vein in 12 patients, to the posterior tibial vein in five patients, and to the popliteal vein (overlapped) in one patient. Four patients underwent contrast CT tests because of high D-dimer levels on day 1 after surgery, and one underwent CT because of the DVT newly discovered by lower limb echo on day 5. One patient presented with thrombosis in the right pulmonary artery and left posterior tibial vein. Although that patient had no subjective symptoms, the partial pressure of oxygen in arterial blood had decreased to pO2 65.3 mmHg. In another patient who underwent contrast CT, despite identifying a new thrombus formation in the right soleal vein, PE was not identified. These two patients had preoperative DVT detected by preoperative lower limb echo. No newly formed VTE was detected by the CT tests for the other three patients. The details of the five patients who underwent CT tests are provided in Table 3.
 | Table 3 Details of five patients who underwent CT testing |
Postoperative lower limb echo aided in identifying 15 cases (18.1%) of DVT. Of these 15 cases, 12 showed no changes before surgery compared to after surgery, and 2 had a new thrombosis detected on CT tests. One patient exhibited a new DVT after surgery despite not exhibiting DVT before surgery. For this patient, contrast CT tests were performed in addition to echo after surgery; however, PE was not identified. Thrombosis disappeared in 2 of 16 cases of solitary asymptomatic DVT before surgery (Figure 1). Therefore, there were three patients with newly formed thrombi after surgery who were assigned to the newly formed postoperative VTE group. Two of them were diagnosed by CT due to high D-dimer levels on day 1, which was before enoxaparin administration. The other single patient was diagnosed for the first time with lower limb echo screening performed on day 5 with no symptoms. Accordingly, only one patient (1.2%) developed VTE while receiving enoxaparin.
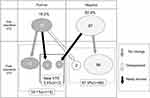 | Figure 1 Results of lower limb venous ultrasonography. Abbreviation: VTE, venous thromboembolism. |
Risk factors of postoperative VTE
The background factors of the 15 patients in the postoperative VTE group and 68 patients in the non-VTE group are shown in Table 4. A univariate analysis identified significant differences between age (p=0.030), presence/absence of DVT before surgery (p<0.001), and D-dimer levels before surgery (p<0.001) and on day 1 (p=0.016) and day 7 (p=0.0050) after surgery. No significant differences were found between sex, BMI, and D-dimer levels on day 3 and day 5 after surgery, anti-Xa factor activity level, APTT, cancer site, cancer stage, comorbidities, operative time, blood loss during surgery, presence/absence of blood transfusion, surgical approach, postoperative complications, and time to ambulation. The results of the multivariate analysis conducted for these factors indicated that the presence of preoperative DVT (p<0.001; OR, 462) was the only independent risk factor (Table 5).
 | Table 4 Risk factors for postoperative VTE (univariate analysis) |
 | Table 5 Risk factors for postoperative VTE (multivariate analysis) |
Risk factors for newly formed postoperative VTE
The background factors of three patients in the newly formed postoperative VTE group were compared with those of the other 80 patients (Table 6). A univariate analysis revealed significant differences between D-dimer levels before surgery (p=0.007) and on day 1 (p=0.0095), day 3 (p=0.011), and day 5 (p=0.0096) after surgery. However, there were no significant differences between age, sex, BMI, presence/absence of preoperative DVT, D-dimer levels after day 7, cancer site, cancer stage, comorbidities, operative time, blood loss during surgery, surgical approach, postoperative complications, and time to ambulation. The multivariate analysis indicated that the preoperative D-dimer level (p=0.0036; OR, 19.37) was the only independent risk factor (Table 7).
 | Table 6 Risk factors for newly formed postoperative DVT (univariate analysis) |
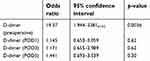 | Table 7 Risk factors for newly formed postoperative DVT (multivariate analysis) |
Hemorrhagic adverse events
Hemorrhagic adverse events were observed in three patients (3.6%), including two with anastomotic bleeding and one with wound bleeding. Each of these patients presented with grade I bleeding (according to the Clavien-Dindo classification) that was conservatively alleviated by pressure hemostasis or by discontinuation of enoxaparin. None required blood transfusion or repeated surgery. The trend for anti-Xa factor activity levels for each case is shown in Figure 2.
Of the patients with bleeding, one had anastomotic bleeding (case A) and exhibited a significantly high level of anti-Xa factor activity. A comparison of the anti-Xa factor activity levels and APTT levels of the bleeding and non-bleeding groups revealed no significant differences on any day after surgery (Table 8).
 | Table 8 Relationship between hemorrhagic adverse events and anti-Xa factor or APTT (univariate analysis) |
The relationship between the anti-Xa factor activity level and APTT level for the same patient measured at the same time was examined; no correlation was found between them (R2 =0.0027) (Figure 3).
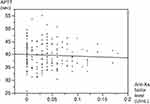 | Figure 3 Correlation between anti-Xa factor inhibitor levels and APTT. Abbreviation: APTT, activated partial thromboplastin time. |
Discussion
According to a review of necropsy cases, VTE is a leading cause of in-hospital postoperative mortality, with as many as 10% of in-hospital deaths attributable to pulmonary embolism.12 A study conducted in Japan indicated that lower limb echo screening identified soleal venous thrombosis in 8.5% of patients before general abdominal surgeries, including those for benign diseases.13 Gainsbury et al recently reported that 10% of patients undergoing major oncologic surgery had evidence of VTE preoperatively,14 suggesting that the presence of preoperative VTE in cancer patients is much higher than previously thought. In the present study, we identified DVT in 19.2% of patients awaiting colorectal cancer surgery. Another study conducted in Japan also used lower limb echo to screen patients awaiting colorectal cancer surgery and reported a DVT incidence of 18.4%, which was similar to our study.2 Patients who require cancer surgery often have medical histories that include chemotherapy and advanced age, which overlap with risk factors for VTE, such as malignant tumors. Therefore, asymptomatic DVT is highly likely to develop before surgery.
In our study, postoperative lower limb echo identified DVTs in 15 patients (18.1%). However, only three (3.6%) had new events identified after surgery. Only a few studies have evaluated DVT before and after surgery and examined the onset of thrombus formation. In one of those studies, lower limb echo before and after (on day 7) surgery found newly formed postoperative thrombosis in 7.9% of patients,2 which led us believe that many echo-recognizable DVTs have thrombi that existed preoperatively. Furthermore, in our study, two of three patients who had developed new thrombi showed high D-dimer levels on day 1 after surgery, indicating the need for emergency contrast CT scans that eventually facilitated the diagnosis of the condition. Preoperative lower limb echo was performed for all patients during the afternoon on the day before surgery. Therefore, it is highly likely that, for at least two of the three patients, the thrombus was formed during the periods during and/or immediately after surgery. Longer time under anesthesia and blood transfusion were reported as risk factors for VTE during surgery.15,16 However, no significant risk factors associated with events during surgery were identified in these two patients.
Although there is no concrete consensus regarding anticoagulatory therapy for asymptomatic DVT, the 2012 ACCP Guidelines (Antithrombotic Therapy and Prevention of Thrombosis, 9th ed.) validated the use of anticoagulatory therapy when thrombus activity is suggested,17 and the 2016 update of the same guidelines recommended 3 months of anticoagulatory therapy, even for distal solitary DVT, when surgery is required.18 Many preoperative asymptomatic DVTs tend to include stable thrombi. However, DVT screening before and after surgery would be effective for preventing symptomatic VTE and acute PE because newly formed postoperative DVT tends to advance. The PREVENT study in 2005, which used compression ultrasound to screen for asymptomatic DVT in patients with an acute medical condition requiring projected hospitalization, concluded that the high mortality rate for patients with asymptomatic proximal DVT underscored its clinical relevance and supported the idea of targeting asymptomatic proximal DVT for thromboprophylaxis.19 Similarly, the APEX study also used compression ultrasound to detect asymptomatic DVT for acutely ill, hospitalized patients; it concluded that asymptomatic DVT was associated with an approximately threefold increased risk of short-term all-cause mortality within the prior 77 days, and a positive linear trend was observed between the greater thrombus burden and mortality during the follow-up.20 Unfortunately, performing lower limb ultrasonography before and after surgery for all patients scheduled to undergo surgery would be time-consuming and expensive because of the low DVT frequency. Moreover, routine ultrasound screening is not recommended by current practice guidelines. When risk factors associated with preoperative VTEs are well defined, selective screening may be beneficial.
We focused on D-dimer levels because they can be easily tested. D-dimer levels are considered useful for excluding diagnoses because they have high negative predictive values for DVT and PE.21,22 Our examinations showed that the negative predictive value of D-dimer levels for diagnosing preoperative DVT was 100%, thereby demonstrating its usefulness.
It is necessary to set an appropriate postoperative cutoff value that is different from the regular reference values because postoperative D-dimer levels tend to be high in most cases due to their reflection of hypercoagulability attributable to surgical invasion.23 Previous reports of DVT and postoperative D-dimer levels provided no fixed view regarding the cutoff value or measurement timing,24–27 and reports pertaining to laparotomy are scarce. In our study, the preoperative D-dimer level was the only independent risk factor for newly formed postoperative VTE. This was in agreement with a previous study that showed that an elevated D-dimer level at baseline was independently associated with an increased risk of DVT.28 Therefore, it could be suggested that preoperative risk factors are highly related to postoperative thrombus formation.
A randomized controlled trial performed in Japan that used enoxaparin indicated that the incidence rates of postoperative VTE were 19.4% for the intermittent pneumatic compression group and 1.2% for the enoxaparin group, respectively.29 In the present study, only one patient (1.2%) had newly formed thrombi while receiving enoxaparin, thereby demonstrating a similar level of utility. In 2019, the AVERT study reported that the use of apixaban therapy for intermediate-risk to high-risk (Khorana score ≥2) ambulatory patients with cancer who were starting chemotherapy resulted in a significantly lower rate of VTE than placebo.30 Therefore, there is an urgent need to meet the unmet need for an extended duration of prophylaxis or anticoagulants with greater intensity for patients undergoing cancer surgery.
Enoxaparin, which is a low-molecular-weight heparin, exerts anti-Xa factor activity by binding to antithrombin. The antithrombin activity of enoxaparin is lower than that of unfractionated heparin because the ratio of macromolecular structures bound to thrombin is smaller than that of unfractionated heparin, and its anti-Xa-to-antithrombin activity ratio is thought to be 4.88.31 Therefore, it is difficult to monitor the anticoagulatory effects of enoxaparin through APTT.32 The ANNEXA-4 study indicated that 82% of patients with acute major bleeding associated with the use of a factor Xa inhibitor, and receiving andexanet alfa developed for the reversal of factor Xa inhibitors had excellent or good hemostatic efficacy at 12 hrs. However, the reduction in anti-Xa factor activity was not predictive of hemostatic efficacy overall.33 In our study, neither anti-Xa factor levels nor hemorrhagic adverse events were significant indicators of antithrombotic effects. Because of its significantly high anti-Xa factor activity levels, one case of anastomotic bleeding that we observed in our study might have been influenced by enoxaparin. However, surgical manipulation might have been a strong influence on anastomotic bleeding. Given the relatively small sample size of the present study, it is premature to conclude the usefulness of monitoring anti-Xa factor activity. Because the incidence of gastrointestinal bleeding after colorectal cancer surgery has been reported as between 1.8% and 2.2%,34,35 the three cases of hemorrhagic adverse events accounted for 3.6% of all cases, thereby indicating that enoxaparin only slightly increased the risk of bleeding. A previous study indicated that enoxaparin did not increase the risk of postoperative bleeding.29 Niwa et al reported that anticoagulant therapy with fondaparinux, which is also a low-molecular-weight heparin, increased the incidence of gastrointestinal bleeding after surgery and that anti-Xa factor activity measurements might be useful for predicting bleeding.36
There were some limitations to this study. First, it was a non-randomized, single-center study, and the sample size was relatively small. Second, detection of DVT by lower limb echo was limited. Third, thrombus formation and hemorrhagic adverse events are relatively infrequent with anticoagulant therapy involving enoxaparin. Therefore, it is necessary to accumulate further cases to demonstrate the effects of monitoring anti-Xa factor activity with enoxaparin.
Conclusion
The majority of DVTs in colorectal cancer patients have been present even before surgery. Preoperative D-dimer levels are useful for early detection of newly formed VTE after surgery. Prevention of VTE using enoxaparin is safe and without any significant adverse events. Further investigations are necessary to demonstrate the significance of anti-Xa factor activity to monitor the effects and adverse events of enoxaparin.
Acknowledgment
We thank all the staff of the Clinical Laboratory of the Department of Juntendo University Hospital for their cooperation in performing the coagulation-related tests for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sakon M, Maehara Y, Yoshikawa H, Akaza H. Incidence of venous thromboembolism following major abdominal surgery: a multi-center, prospective epidemiological study in Japan. J Thromb Haemost. 2006;4(3):581–586. doi:10.1111/j.1538-7836.2006.02117.x
2. Nakamura K, Fujita H, Watanabe T, Fushida Y, Fujimura T, Ota T. Incidence and risk factors of deep vein thrombosis during perioperative period of colorectal cancer surgery. J Abdom Emerg Med. 2013;33:1145–1152.
3. Geerts WH, Heit JA, Clagett GP, et al. Prevention of venous thromboembolism. Chest. 2001;119(1 Suppl):132S–175S.
4. Committee to Create Preventive Guidelines for Pulmonary Thromboembolism/Deep Vein Thrombosis (Venous Thromboembolism). Japanese Guideline for Prevention of Venous Thromboembolism. Tokyo: Medical Front International Ltd.; 2004.
5. Meyer G, Roy PM, Sors H, Sanchez O. Laboratory tests in the diagnosis of pulmonary embolism. Respiration. 2003;70(2):125–132. doi:10.1159/000070056
6. Nomura H, Wada H, Mizuno T, et al. Negative predictive value of D-dimer for diagnosis of venous thromboembolism. Int J Hematol. 2008;87(3):250–255. doi:10.1007/s12185-008-0047-x
7. Prisco D, Grifoni E. The role of D-dimer testing in patients with suspected venous thromboembolism. Semin Thromb Hemost. 2009;35(1):50–59. doi:10.1055/s-0029-1214148
8. Righini M, Perrier A, De Moerloose P, Bounameaux H. D-dimer for venous thromboembolism diagnosis: 20 years later. J Thromb Haemost. 2008;6(7):1059–1071. doi:10.1111/j.1538-7836.2008.02981.x
9. Makino S, Sugimura H, Suga N, et al. Establishment of a dosing monitoring method for preventive anticoagulatory therapy using low molecular weight heparin (enoxaparin). Heart. 2011;43:1021–1024.
10. Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. doi:10.1097/SLA.0b013e3181b13ca2
11. Union for international cancer control. TNM classification of malignant tumours.
12. Alikhan R, Peters F, Wilmott R, Cohen AT. Fatal pulmonary embolism in hospitalized patients: a necropsy review. J Clin Pathol. 2004;57(12):1254–1257. doi:10.1136/jcp.2003.013581
13. Kojima A. Deep vein thrombosis with central progression discovered by preoperative screening echo. Ann Vasc Dis. 2008;21:249–256.
14. Gainsbury ML, Erdrich J, Taubman D, et al. Prevalence and predictors of preoperative venous thromboembolism in asymptomatic patients undergoing major oncologic surgery. Ann Surg Oncol. 2018;25(6):1640–1645. doi:10.1245/s10434-018-6461-2
15. Agnelli G, Bolis G, Capussotti L, et al. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: the @RISTOS Project. Ann Surg. 2006;243(1):89–95. doi:10.1097/01.sla.0000193959.44677.48
16. Xenos S, Vargas HD, Davenport DL. Association of blood transfusion and venous thromboembolism after colorectal cancer resection. Thromb Res. 2012;129(5):568–572. doi:10.1016/j.thromres.2011.07.047
17. Holbrook A, Schulman S, Witt DM, et al. Evidence-based management of anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed.: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e152S–e184S. doi:10.1378/chest.11-2295
18. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease. Chest. 2016;149(2):315–352. doi:10.1016/j.chest.2015.11.026
19. Vaitkus PT, Leizorovicz A, Cohen AT, et al. Mortality rates and risk factors for asymptomatic deep vein thrombosis in medical patients. Thromb Haemost. 2005;93(1):76–79. doi:10.1160/TH04-09-0632
20. Kalayci A, Gibson CM, Chi G, et al. Asymptomatic deep vein thrombosis is associated with an increased risk of death: insights from the APEX Trial. Thromb Haemost. 2018;118(12):2046–2052. doi:10.1055/s-0038-1637745
21. Wada H, Matsumoto T, Yamashita Y. Diagnosis of fibrin related markers and thrombosis. J Clin Lab Med. 2014;58:955–960.
22. Linkins LA, Bates SM, Ginsberg JS, Kearon C. Use of different D-dimer levels to exclude venous thromboembolism depending on clinical pretest probability. Thromb Haemost. 2004;2(8):1256–1260. doi:10.1111/j.1538-7836.2004.00824.x
23. Kawai Y. Surgical invasion and coagulation system. Kessen to Junkan. 2008;16:176–181.
24. Jiang Y, Li J, Liu Y, Li YC, Zhang WG. Risk factors for deep vein thrombosis after orthopedic surgery and the diagnostic value of D-dimer. Ann Vasc Surg. 2015;29(4):675–681. doi:10.1016/j.avsg.2014.12.022
25. Hamidi S, Riazi M. Cutoff values of plasma D-dimer level in patients with diagnosis of the venous thromboembolism after elective spinal surgery. Asian Spine J. 2015;9(2):232–238. doi:10.4184/asj.2015.9.2.232
26. Maruhashi Y, Okamoto S, Mori Y, Nakase S. Significance of D-dimer measurements for venous thromboembolism in the lower limb in the perioperative period. J Japan Soc Fracture Rep. 2008;30:558–561.
27. Kobayashi T, Ito H, Minami K, et al. A study of postoperative deep venous thrombosis (DVT) by venography and its relationship with D-dimer levels. J Musculoskeletal Syst. 2004;17:897–903.
28. Chi G, Goldhaber SZ, Hull RD, et al. Thrombus burden of deep vein thrombosis and its association with thromboprophylaxis and D-dimer measurement: insights from the APEX Trial. Thromb Haemost. 2017;117(12):2389–2395. doi:10.1160/TH17-08-0538
29. Sakon M, Kobayashi T, Shimizu T. Efficacy and safety of enoxaparin in Japanese patients undergoing curative abdominal or pelvic cancer surgery: results from a multicenter, randomized, open-label study. Thromb Res. 2010;125(3):65–70. doi:10.1016/j.thromres.2009.09.009
30. Carrier M, Abou-Nassar K, Malick R, et al. Apixaban to P prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380(8):711–719. doi:10.1056/NEJMoa1814468
31. Imoto K, Makita S. Pharmacological properties and clinical trial results of the anticoagulant enoxaparin sodium (Clexane® Subcutaneous Injection Kit 2000 IU). J Pharmacol Sci. 2009;133:91–98.
32. Suzuki A, Kaneko M, Kanno N, Yatomi Y. Evaluation of anti-Xa activity of various heparins by synthetic substrate method and thrombin generation test. Japan J Clin Pathol. 2013;61:567–575.
33. Connolly SJ, Crowther M, Eikelboom JW, et al. Full study report of Andexanet Alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019;380(14):1326–1335. doi:10.1056/NEJMoa1814051
34. Kawabe A, Kimura Y, Okamoto K, Higashi Y, Suzuki K, Shimoda H. Re-surgery/prevention/indication/surgical technique. Gastrointestinal anastomotic bleeding. J Surg. 2005;59:1429–1433.
35. Masuzawa T, Tominaga S, Miyagaki H, Fukuzaki T. A case in which Argon plasma coagulation method was effective for postoperative bleeding after functional end-to-end anastomosis. J Japan Soc Coloproctol. 2008;61:329–332. doi:10.3862/jcoloproctology.61.329
36. Niwa K, Sakamoto K, Ro H, et al. The usefulness of measuring anti-Xa activity for the prediction of bleeding adverse events during postsurgical fondaparinux anticoagulant therapy. Juntendo Med J. 2016;62:399–405. doi:10.14789/jmj.62.399
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

