Back to Journals » International Journal of Nanomedicine » Volume 18
Current Status and Prospect of Delivery Vehicle Based on Mesenchymal Stem Cell-Derived Exosomes in Liver Diseases
Authors Lu X, Guo H, Wei X, Lu D, Shu W, Song Y, Qiu N, Xu X
Received 15 January 2023
Accepted for publication 10 May 2023
Published 31 May 2023 Volume 2023:18 Pages 2873—2890
DOI https://doi.org/10.2147/IJN.S404925
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Phong A Tran
Xinfeng Lu,1,2,* Haijun Guo,2,3,* Xuyong Wei,2,3 Di Lu,2,3 Wenzhi Shu,2,4 Yisu Song,2,4 Nasha Qiu,1,2 Xiao Xu1– 4
1The Fourth School of Clinical Medicine, Zhejiang Chinese Medical University, Hangzhou, 310000, People’s Republic of China; 2Key Laboratory of Integrated Oncology and Intelligent Medicine of Zhejiang Province, Hangzhou, 310006, People’s Republic of China; 3Department of Hepatobiliary and Pancreatic Surgery, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, 310006, People’s Republic of China; 4Zhejiang University School of Medicine, Hangzhou, 310058, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiao Xu, Department of Hepatobiliary and Pancreatic Surgery, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, 310006, People’s Republic of China, Email [email protected] Nasha Qiu, The Fourth School of Clinical Medicine, Zhejiang Chinese Medical University, Key Laboratory of Integrated Oncology and Intelligent Medicine of Zhejiang Province, Hangzhou, 310006, People’s Republic of China, Email [email protected]
Abstract: With the improvement of the average life expectancy and increasing incidence of obesity, the burden of liver disease is increasing. Liver disease is a serious threat to human health. Currently, liver transplantation is the only effective treatment for end-stage liver disease. However, liver transplantation still faces unavoidable difficulties. Mesenchymal stem cells (MSCs) can be used as an alternative therapy for liver disease, especially liver cirrhosis, liver failure, and liver transplantation complications. However, MSCs may have potential tumorigenic effects. Exosomes derived from MSCs (MSC-Exos), as the important intercellular communication mode of MSCs, contain various proteins, nucleic acids, and DNA. MSC-Exos can be used as a delivery system to treat liver diseases through immune regulation, apoptosis inhibition, regeneration promotion, drug delivery, and other ways. Good histocompatibility and material exchangeability make MSC-Exos a new treatment for liver diseases. This review summarizes the latest research on MSC-Exos as delivery vehicles in different liver diseases, including liver injury, liver failure, liver fibrosis, hepatocellular carcinoma (HCC), and ischemia and reperfusion injury. In addition, we discuss the advantages, disadvantages, and clinical application prospects of MSC-Exos-based delivery vectors in the treatment of liver diseases.
Keywords: exosomes, mesenchymal stem cells, liver disease, nanocarriers
Introduction
The WHO identified that the world’s overweight and obesity rates have nearly tripled since 1975. By estimation, more than 1.9 billion (39%) adults (≥18 years) are overweight and 650 million (13%) individuals are obese.1 With the improvement of the average life expectancy and increasing incidence of obesity, the burden of various liver diseases, including non-alcoholic fatty cell liver disease (NAFLD), liver cirrhosis, and liver failure, is increasing.2 These high rates of morbidity pose a great threat to human health.3 Chronic liver disease and cirrhosis contribute to 2 million deaths per year globally, with a high burden of disability and treatment costs.4,5 The prevalence of alcoholic liver disease and NAFLD in the general adult population is approximately 7.4% and 20–33%, respectively. Hepatitis B virus (HBV) infection affects at least 2 billion people worldwide, and without radical treatment, all forms of chronic hepatitis eventually progress to end-stage disease. Hepatocellular carcinoma (HCC) was reported to be the leading cause of cancer death worldwide.6 Liver cancer is the fourth leading cause of cancer-related death worldwide and the second most lethal cancer with a 5-year survival rate of 18%.7,8 In 2030, the WHO estimates that more than 1 million patients will die from liver cancer.8 The liver disease accounts for about 2 million deaths worldwide every year,9 which poses a great threat to human health. Liver transplantation is the only effective treatment for many advanced liver diseases when other medical therapies have failed.10–12 However, we have to admit that the shortage of liver donors, high cost, postoperative complications, and other issues restrict the popularization of liver transplantation. Therefore, we hope to find effective alternative measures to cope with the occurrence and development of liver diseases.
MSC-Exos can inhibit the occurrence and development of liver injury, liver fibrosis, liver cancer, and other liver diseases through signal transduction, immune regulation, tissue regeneration promotion, drug delivery, and other pathways. However, at present, the mechanism of MSC-Exos in the treatment of liver disease has not yet been fully clarified. Preclinical studies are insufficient; therefore, the clinical application still faces several challenges. In this review, we summarize the roles and functions of MSC-Exo-based delivery systems in liver diseases, thereby enabling us to better understand the latest findings in the field and tackle accompanying clinical challenges.
Mesenchymal Stem Cells
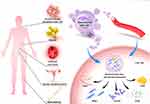
MSCs, a kind of stromal cells with self-renewal and multilineage differentiation abilities,13 were discovered in the 1960s and 1970s.14,15 MSCs can be isolated from various adult tissues, such as the bone marrow, umbilical cord, adipose, peripheral blood, liver, and tooth root.16 (Figure 1) Human-derived MSCs express relatively constant markers, including CD90, CD73, and CD105. However, the surface markers and characteristics of MSCs from different sources are slightly different.17 MSCs can be induced to differentiate into cells of the mesodermal lineage, such as adipocytes, skeletal cells, and muscle cells.18 Therefore, MSCs can promote tissue repair, proliferation, and regeneration. The regeneration of liver, kidney, heart, and pancreas tissues can be promoted under the action of MSCs.16,19–22 MSCs have been demonstrated to possess nutritional, anti-inflammatory, immunomodulatory, anti-apoptotic, and antibacterial properties.23 These characteristics provide MSCs with great advantages in the treatment of diseases. MSCs are effective in treating bone, brain, nerve, cardiovascular, and autoimmune diseases and promoting wound and soft tissue regeneration.23 In recent studies, researchers have found that MSCs have great potential in the treatment of liver diseases, including liver fibrosis, liver failure, liver cirrhosis, metabolic-associated fatty liver disease, and liver regeneration.24–28
Several studies have shown that mesenchymal stem cells are the most promising alternative therapy for the treatment of liver diseases, especially liver cirrhosis, liver failure, and complications of liver transplantation.29 However, case reports have suggested that MSC treatment may cause unexpected differentiation and unknown proliferative lesions,30 which may have potential tumorigenicity.
Exosomes
Extracellular vesicles (EVs) are cell-derived membrane vesicles that are secreted from almost all types of cells and play an important role in intercellular communication and regulation.31,32 EVs mainly include apoptotic bodies, microvesicles, and exosomes.33 Apoptotic cells can produce apoptotic bodies, and during this process, apoptotic cells can actively package their biomolecules into vesicles, so that drugs such as nucleic acids can be loaded into apoptotic bodies.34 However, there is still a considerable blank space in the study of the mechanism and function of apoptotic bodies.35 Microvesicles are derived from the direct budding of the plasma membrane in living cells and carry active components that can affect target cells and alter their behavior.36 Exosomes originate from the fusion of clathrin-coated vesicles, forming multivesicular endosomes by fusing with early endosomes, and eventually fusing with the cell membrane and shedding exosomes.37 EVs, typically between 50 nm and 500 nm in size, are important mechanisms of intercellular communication and are involved in a variety of physiological and pathological processes.38 EVs play a good role in the regulation of immunity, tumor progression, specific modulators of cell behaviors, and targeted delivery of drugs.38,39 Noncoding RNAs (ncRNAs) account for a small but important proportion of EV cargo, and MSC-EVs have been reported to contain ncRNAs related to various molecular mechanisms in liver diseases.37 Exosomes, as an important component of EVs, were first discovered in the 1980s.40,41 Almost all mammalian cells can secrete and absorb exosomes.42,43
Exosomes are small vesicles that are shed from the surface of the plasma membrane through outward budding with diameters ranging from 40 nm to 160 nm.44 According to the position statement from the International Society for Extracellular, at least two different techniques are generally used to characterize individual EVs. Exosomes, as one of the EVs, also follow this rule. Nanoparticle tracking analysis (NTA), dynamic light scattering, or resistive pulse sensing could be used to measure the concentration and size distribution of exosomes. Exosomes were visualized by transmission electron microscopy using the transmission electron microscope, atomic force microscopy, and Western blot analyses. The International Society for Extracellular also suggests that investigators report the amounts of 3 or more proteins in at least a semiquantitative manner in any exosome preparation.45
Since the first discovery of exosomes in the 1980s, research on exosomes has been advancing. Exosomes were confirmed to be formed from lipid bilayers derived from the plasma membrane. They have the same topology as cells and are rich in sugar conjugates, proteins, lipids, nucleic acids, and metabolites.46 The ExoCarta database (http://www.exocarta.org) lists thousands of proteins, RNA, and lipids that can all be biological cargoes delivered by exosome-based delivery vectors.47 Noncoding RNAs in exosomes have been shown to play a therapeutic role in a variety of liver diseases by inhibiting inflammatory response, alleviating oxidative stress in the liver, and inhibiting the activation and proliferation of hepatic stellate cells.48
Due to their good histocompatibility and material exchangeability, exosomes have become an important way of intercellular communication and regulating various physiological and pathological activities of the body. Exosomes have been proven to have many biological characteristics, including stability, histocompatibility, and good material exchange ability.49 Exosomes play an important role in many aspects, including disease development, immunity, cancer, and tissue regeneration through the intercellular vesicle transport pathway.46 Additionally, exosomes have the advantages of targeted delivery, low immunogenicity, and high repairability.50 Exosomes are naturally secreted by cells and have low immunogenicity, which can prevent immune rejection.51 The cell-free structure of exosomes helps prevent potential tumorigenic effects. Exosomes had been shown to have targeting properties, and exosomes from different cell sources affected biodistribution.52 The targeting properties of exosomes could be changed by exosome modification, so exosomes have the potential to become biological carriers for targeted drug delivery. In a mouse model of pulmonary metastases, exosomes released from macrophages are delivered through the airway and colocalize almost completely with cancer metastasis. Exosomes were shown to target cancer cells and effectively deliver paclitaxel (PTX, a chemotherapeutic agent).53 Modified exosomes could target the lesion region of the ischemic brain and effectively inhibit inflammation and apoptosis in this lesion region.54 Therefore, MSC-Exos have proven potential as drug delivery vehicles and may have the opportunity to treat liver diseases.
Mesenchymal Stem Cell-Derived Exosomes
EVs derived from MSCs are critical mediators of intercellular communication.55 EVs deliver materials from MSCs to effector cells,33 allowing MSCs to function.56 MSCs do not engraft and replace damaged tissues directly but exert therapeutic effects through secreted paracrine effectors by these cells. Therefore, the therapeutic effect of MSCs can be largely attributed to paracrine effectors, of which exosomes are considered to be critical.57 The biological functions of MSC-Exos are similar to those of their parental cells.58 Similar to general exosomes, MSC-Exos also contain a variety of biological substances, such as sugar conjugates, proteins, lipids, nucleic acids, and metabolites. Most exosomes expressed an evolutionarily conserved set of proteins, including the tetraspanin protein family (CD81, CD63, and CD9), heat shock proteins (HSP60, HSP70, and HSP90), ALIX, and tumor susceptibility gene 101 (TSG101).59 However, exosomes also express cell type-specific proteins, which correlate with their cellular origin. MSC-Exos expressed not only CD81 and CD9 but also mesenchymal stem cell surface markers such as CD44, CD73, and CD90.59 Among the different types of MSC-derived exosomes, half of the proteins were similar among all proteins.50
MSC-derived exosomes are derived from a wide range of sources (Figure 1), including bone marrow mesenchymal stem cell-derived exosomes (BMSC-Exos), adipose tissue-derived mesenchymal stem cell-derived exosomes (AMSC-Exos), human umbilical cord mesenchymal stem cell-derived exosomes (hUCMSC-Exos), exosomes derived from human menstrual blood-derived stem cells, and exosomes produced by human-induced pluripotent stem cell-derived mesenchymal stromal cells (hiPSC-MSC-Exos).
MSC-Exos, like MSCs, can help maintain tissue homeostasis and help tissues achieve optimal function.56 MSC-Exos can regulate cell migration, proliferation, and differentiation. Furthermore, MSC-Exos can remodel matrix synthesis and deliver signals and molecules to other cells.55,60–62 In studies on myocardial repair after acute myocardial infarction and autoimmune diseases, MSC-derived exosomes showed similar effects as MSCs, including anti-apoptosis, angiogenesis promotion, and immunomodulation.50,59,63 In some studies, MSC-derived exosomes (MSC-Exos) have shown better results than MSCs.59,64,65 MSC-Exos can prevent the challenges of microvascular obstruction, allogeneic rejection, and abnormal chromosomal differentiation of MSCs.59 MSC-Exos have been shown to enhance wound healing and tissue regeneration.49,66,67 MSC-Exos can exert beneficial effects in neurological, bone, renal, and heart diseases, as well as cancer.49,68–71 Additionally, MSC-Exos possess immunosuppressive properties and can effectively alleviate autoimmune diseases, such as multiple sclerosis, systemic lupus erythematosus, type-1 diabetes, uveitis, rheumatoid arthritis, and inflammatory bowel disease.59 Furthermore, MSC-Exos can be used as an alternative MSC-based therapy and play a role in the treatment of liver diseases.
As delivery vectors, exosomes can be isolated and prepared in various ways (Table 1). Exosomes can be isolated by differential ultracentrifugation and density gradient centrifugation, immunoaffinity chromatography, size exclusion chromatography, polymer precipitation, and microfluidic technologies. Differential ultracentrifugation and density gradient centrifugation are considered to be the “gold standard” for the isolation of exosomes.72 Different separation methods have their advantages and disadvantages, and the best separation method can be selected according to the actual needs and conditions. These methods can be used in combination to partially alleviate their limitations and improve extraction yield and purity to meet the needs of research and disease treatment.57 Table 1 provides a summary of the exosome isolation methods and a comparison of their advantages and disadvantages. Moreover, at present, biological cargoes can be loaded into MSC-Exos in various ways, including electroporation, transfection, and overexpression. Transfection is the most commonly used method to load RNA. Electroporation generally introduces hydrophilic cargoes into MSC-Exos, and overexpression is usually used to introduce proteins into MSC-Exos.73 The methods of drug encapsulation by exosomes and exosome culture vary.74 Exosomes treated in different ways have different effects on the liver disease when used as drug delivery vehicles. Electroporation is widely used for exosome drug loading, which has the best drug encapsulation efficiency.51 When norcantharidin (NCTD) is loaded into purified BMSC-Exos by electroporation, BMSC-Exos-NCTD provides a continuous and slow release of the drug.75 Li et al76 found that the 3D culture of hUCMSCs (3D-hUCMSCs) promoted cell yield and stemness maintenance. 3D culture of exosomes (3D-Exos) has a better anti-liver fibrosis effect than 2D-Exos.77 Compared to 2D-tumour-cell-derived microparticles (2D MPs), 3D-tumour-cell-derived microparticles (3D MPs) can achieve effective internalization into target cells, ultimately improving their ability to deliver drugs.78 The stability of proteins and microRNAs in MSC-Exos was significantly increased. Therefore, the retention of MSC-Exos in vivo was increased.79 The above reports have guiding significance for the future application of MSC-Exos in liver diseases, and we should pay attention to the effect of the way of MSC-Exos culture on the therapeutic effect. The above methods and technologies have laid a foundation for the large-scale preparation of exosomes and delivery of biological cargoes and drugs, making MSC-Exos-based delivery vectors feasible for the treatment of liver diseases.
 |
Table 1 Separation and Purification of MSC-Exos |
Application of Mesenchymal Stem Cell-Derived Exosome-Based Delivery Vectors in Liver Diseases
To apply MSC-Exos-based delivery vectors in the treatment of liver diseases, the following section will evaluate the role of MSC-Exos as delivery vectors in the treatment of liver diseases, including liver injury, liver failure, liver fibrosis, HCC, and ischemia and reperfusion (I/R) injury (Table 2) (Figure 1).
 |
Table 2 The Role of MSC-Exo-Based Delivery Vectors in the Treatment of Liver Diseases, as Discussed in the Text |
Liver Injury and Liver Failure
MSC-Exos can play an effective protective role in various organ damages, including spinal cord injury, traumatic brain injury, acute lung injury, and cardiac injury.109–113 Similarly, in recent years, researchers have found that MSC-Exos exhibit great promise for liver injury therapy. MSC-Exos have been shown to induce hepatoprotective effects against drug-induced liver injury. MSC-Exos inhibited the acetaminophen (APAP)- and hydrogen peroxide (H2O2)-induced hepatocyte apoptosis mainly through activation of antiapoptotic, proliferative, and regenerative responses by upregulation of Bcl-xL protein expression.114 MSC-Exos significantly attenuated CCl4-induced lipid peroxidation and reduced other iron ptosis markers, including decreased expression of SLC7A11 and increased expression of Ptgs2 and LOXs, thus alleviating CCl4-induced liver injury.115 Phosphoinositide 3-kinase (PI3K) plays a key role in the activation of the IL-6-related signaling pathway. hUCMSC-Exos rich in miR-455-3p can regulate the PIK3r1 gene, which encodes the PI3K subunit P85α and inhibits IL-6-related signaling pathways, thus inhibiting macrophage activation and alleviating acute liver injury.89 Furthermore, in an experimental model of autoimmune hepatitis (AIH), BMSC-Exos are effective in liver injury, which could be related to miR-223 regulation of NLRP3 and caspase-1.90
Acute liver failure (ALF) is a rare but life-threatening critical illness that usually results from viral infections and drug-induced liver injury.116 NLRP3 inflammasome has been identified as a potential mediator of hepatocyte damage, immune cell activation, and hepatitis amplification.117 AMSC-Exos can reduce the activation of NLRP3 inflammasome in macrophages through miR-17-mediated thioredoxin-interacting protein (TXNIP) inhibition. Therefore, AMSC-Exos play a protective role in lipopolysaccharide and d-galactosamine (LPS/D-GalN)-induced ALF.91 hUCMSC-Exos have been shown to significantly improve LPS/D-GalN-induced hepatitis by the downregulation of NLRP3.118 BMSC-Exos can promote autophagy and effectively reduce hepatocyte apoptosis in LPS/D-GaIN-induced ALF.119 In a study, MSC-Exos migrated to sites of injury and AML12 cells (a mouse hepatocyte cell line) after fulminant hepatic failure (FHF). Therefore, LPS/D-GalN-induced apoptosis of AML12 cells was inhibited. MSC-Exos significantly inhibited apoptosis in hepatocytes, improved liver function, and increased survival rates by reducing the number of mononuclear cells and the expression of caspase-3.120 Glutathione peroxidase1 (GPX1) is a critical antioxidant in the human body.121
In conclusion, MSC-Exos can be used as a delivery vector to overexpress target miRNAs and deliver these miRNAs to target tissues, thus playing a role in effectively reducing liver injury and liver failure. This may become a new approach to the treatment of liver injury and liver failure.
Liver Fibrosis
Liver fibrosis is caused by chronic liver damage, inflammation, and excess accumulation of extracellular matrix (ECM) components.122–124 Several studies have found that hepatic stellate cell (HSC) activation is a key driver of hepatic fibrosis.125–127 In a CCl4-induced liver fibrosis model, AMSCs translocated miR-181-5p to damaged hepatocytes by selectively transferring exosomes to mouse HSCs. In vitro analysis confirmed that miR-181-5p-rich AMSCs were secreted extracellularly and subsequently taken up by stem stellate cells, thus allowing miR-181-5p to be transferred. MSC-Exos could be used to deliver miRNAs to HSCs. miR-181-5p-modified AMSC-Exos effectively inhibited liver fibrosis by increasing autophagy of HSCs by inhibiting the STAT3/Bcl-2/Beclin 1 pathway and decreasing TNFα, IL-6, and IL-17 levels.92 CircDIDO1 (a circRNA derived from 2 to 6 exons of DIDO1 gene) mediated by MSC-Exos was confirmed to inhibit HSC activation by sponging miR-143-3p, which was an activator of the activation of the PTEN/AKT pathway.93 MSC-Exos induced the transformation of proinflammatory macrophages to the anti-inflammatory phenotype and subsequently reduced liver fibrosis. MiR-148a, as the therapeutic effector of MSC-Exos, regulated the STAT3 signaling pathway by directly targeting KLF6.94 Wang et al77 showed that miR-6766-3p in the exosomes derived from human embryonic stem cells (hESC-Exos) inactivates recombinant mothers against decapentaplegic (SAMD) signaling by restraining TGFβ type II receptor (TGFβRII) expression, consequently attenuating LX2 cell and HSC activation and suppressing liver fibrosis. By delivering miR-122 content into HSCs, exosomes led to altered expression of miR-122-target gene in HSCs, thereby enhancing the therapeutic effect of AMSCs on liver fibrosis.64 The signal transducer and activator of transcription 3 (STAT3) has been proven to be an important transcription factor related to the pathogenesis of liver fibrosis.96 Compared to scrambled siRNA control, siRNA-STAT3, or ASO-STAT3, MSC-Exos carrying siRNA or antisense oligonucleotide (ASO) treatments enhanced STAT3 targeting efficiency and suppressed STAT3 levels and extracellular matrix (ECM) deposition in established liver fibrosis in mice. Liver function was significantly restored.96 In addition to playing an effective role as a delivery vector for the treatment of liver fibrosis, MSC-Exos could inhibit liver fibrosis. Rong et al found that the expression of several proteins (PPARγ, Wnt3a, Wnt10b, and β-catenin) in the Wnt signaling pathway can be downregulated by BMSC-Exos, which in turn inhibited downstream gene (WISP1, Cyclin D1) expression. BMSC-Exos could inhibit the activation of HSCs through inhibition of the Wnt/β-catenin signaling pathway, inhibit the expression of α-SMA, alleviate liver inflammation, improve liver function, promote hepatocyte regeneration, reduce liver fibrosis, and improve liver function.128 Ferroptosis has been reported to play an important role in liver fibrosis.129 MSC-Exos can enhance HSCs ferroptosis through the exosome/BECN1/xCT/GPX4 pathway, thereby ameliorating liver injury and alleviating liver fibrosis.95 Altogether, the delivery vector based on MSC-Exos has a good effect in the treatment of liver fibrosis and is expected to become a new method for the treatment of liver fibrosis in the future.
Hepatocellular Carcinoma
Liver cancer is the most common fatal malignancy and the second leading cause of cancer-related death worldwide.130,131 In recent years, the incidence of hepatocellular carcinoma (HCC) is increasing.3,132 Tumor occurrence is closely related to the physiological state of the tumor microenvironment (TME), which is involved in tumor biology, tumorigenesis, development, and treatment response.133,134 Surprisingly, TME can be regulated by exosomes.135–137 Recently, researchers have found that MSC-Exos can exert an inhibitory effect on HCC through various pathways. Exosomes may be able to enhance or expand their therapeutic ability in cancer through chemical or biological modification.138 AMSC-Exos can mediate the delivery of miR-199a-3p between AMSCs and HCC cells, thus the sensitivity of HCC cells to chemotherapeutic drugs can be effectively improved by miR-199a-3p-modified AMSC-Exos by targeting the mTOR (a serine/threonine kinase) pathway.97 AMSC-Exos mediated miR-122 communication between AMSCs and HCC cells and further altered miR-122-target gene expression in HCC cells. By enhancing cell apoptosis and cell cycle arrest, the sensitivity of HCC cells could be enhanced by miR-122-modified AMSC-Exos.98 By downregulating E26 transformation specific-1 (EST1), the miR-338-3p-modified BMSC-Exos could delay the development of HCC, which inhibited the proliferation, invasion, and migration of HCC cells, and induced cell apoptosis.101 MiR-451a-modified hUCMSC-Exos inhibited the epithelial-mesenchymal transition of HCC cells by repressing ADAM10 (a target gene of miR-451a). By this means, hUCMSC-Exos inhibited paclitaxel resistance, cell cycle transition, proliferation, invasion, and migration of HCC cells, thereby promoting apoptosis of HCC cells.100 HCC can be inhibited by miR-125a and miR-125b, which repressed proliferation, stem cell properties, and migration of HCC cells through the CD90 pathway.139 Delivery of miR-125a/b by MSC-Exos may be a new therapeutic approach for HCC. In conclusion, MSC-Exos rich in different miRNAs can inhibit HCC cells through various effects, resulting in a therapeutic effect on HCC. MSC-Exo-based HCC treatment has a certain potential to become an alternative therapy for HCC. Compared with the anticancer drug, norcantharidin (NCTD) treatment alone, BMSC-Exos-NCTD delivery system showed a more significant antitumor effect, which was reflected in promoting cellular uptake, inducing cell cycle arrest, reducing tumor cell proliferation, and increasing apoptosis. Moreover, BMSC-Exo-NCTD increased cellular proliferation and inhibited hepatocyte oxidation without showing body toxicity.75 Additionally, adipose stem cell exosomes (ASC-Exos) have been shown to inhibit the hepatoma cell line growth and promote the normal liver cell line growth.51 Therefore, MSC-Exos have been hypothesized to have the ability to inhibit the growth of liver cancer cells and promote the growth of normal liver cells, thereby exerting a therapeutic effect on HCC.
Liver cancer stem cells (CSCs) are a unique subset of HCC cells with stem cell characteristics, which have the ability of self-renewal and differentiation.140,141 However, this role can be inhibited by MSC-Exos. Exosomes released by CSCs induced Nanog expression and regorafenib resistance in differentiated cells142 and induced tumor development and progression in vivo.137 Furthermore, exosomes can affect CSCs. Gu et al99 evidenced that the malignant behaviour of liver CSCs was blocked by exosomes through the C5orf66AS1/miR-127-3p/DUSP1/ERK axis. However, several researchers have previously found that MSCs promote HCC.143,144 MSCs interact with tumor cells in a myriad of ways that can support or suppress tumor growth. Klopp et al144 indicated that the effect of MSCs on tumors can be affected by several factors, including the heterogeneity of MSCs, the effects of propagating cells in vitro, the time of MSCs that enter the TME in vivo, and the variability of MSCs from patient to patient. This suggests that researchers should pay attention to the influence of the above factors on the results when studying the effect of MSC-Exos on HCC, because they may produce results that MSC-Exos have a promoting effect on HCC.
In summary, MSC-Exos inhibit HCC by delivering different types of miRNA, drug delivery, and blocking the stemness of liver CSCs, thereby achieving the therapeutic effect of HCC. However, attention should be paid to the potential role of MSC-Exos in promoting HCC to better apply them in clinical treatment.
Ischemia and Reperfusion Injury
Exosomes have been shown to play a protective role in organ ischemia and reperfusion (I/R) injury of organs, such as the brain and heart.145–147 Exosomes protect cardiomyocytes from acute myocardial I/R injury by transmitting survival signals to the ischemic myocardium and inhibiting cardiomyocyte apoptosis in vivo.148 Studies have shown that MSC-Exos are used as delivery carriers in the ischemia and reperfusion (I/R) injury of various organs, such as the brain, spinal cord, heart, kidney, and liver.149–152 hUCMSC-Exos regulated the glycogen synthase kinase 3β (GSK3β)-mediated Wnt/β-catenin pathway by delivering miR-1246 and finally alleviated hepatic I/R injury.102 Furthermore, hUCMSC-Exos could alleviate hepatic I/R injury by delivering miR-1246, targeting the IL-6/gp130/STAT3 axis to regulate the balance between Tregs and Th17 cells.103 hUCMSC-Exos-enriched miR-20a could alleviate hepatic I/R injury by alleviating the abnormal expression of genes related to apoptosis and autophagy.104 Ferroptosis was associated with the I/R injury of liver transplantation (LT) with a severe steatotic donor liver. Wu et al105 showed that heme oxygenase oxygen-1 (HO-1)-modified BMSC-Exos (HM-Exos) could inhibit hepatocyte ferroptosis and reduce graft hepatic I/R injury by delivering miR-124-3p to downregulate the Steap3 level. Additionally, HM-Exos could inhibit hepatocyte ferroptosis by delivering miR-29a-3p targeting Ireb2, ultimately reducing hepatic I/R injury.106 These studies provide a new way to solve the problem of future donor liver shortage. Furthermore, many studies have found that exosomes can reduce the inflammatory response, inhibit cell apoptosis, promote cell proliferation, and liver regeneration, thereby alleviating hepatic I/R injury.153–156 In summary, MSC-Exos-based delivery vectors can alleviate hepatic I/R injury by delivering multiple miRNAs, which may effectively improve the success rate of LT and the prognosis of patients.
Non-Alcoholic Fatty Liver Disease
Non-alcoholic fatty liver disease (NAFLD) is the main cause of chronic liver disease, and the liver-related mortality of patients with non-alcoholic steatohepatitis has increased in recent years.108 NAFLD affects more than one-third of the population.157 Exosomes from AMSCs attenuated white adipose tissue inflammation, systemic insulin resistance, dyslipidemia, and hepatic steatosis in a study on obese mice.158 Exosomes enriched in miR-223 inhibited NAFLD-associated liver fibrosis by transfer into hepatocytes to suppress the hepatic expression of fibrotic genes.159 hUCMSC-Exos rich in miR-627-5p improved glucose and lipid metabolism and alleviated liver injury by inhibiting FTO (fat mass and obesity-associated gene) expression, thereby meliorating the progression of NAFLD.107 Moreover, hepatocyte-derived exosomal miR-192-5p was shown to inhibit proinflammatory macrophage activation and disease progression in NAFLD.160 In conclusion, MSC-Exos can deliver multiple miRNAs, thereby slowing or halting the progression of NAFLD and improving the quality of life of patients.
Liver Regeneration
Exosomes have great potential for liver regeneration, tissue repair, and blood vessel formation. Exosomes, as nanocarriers, deliver active factors or small molecules to promote tissue repair. Preclinical studies of exosomes in tissue engineering and regenerative medicine have been performed in the fields of bone/cartilage repair, skin repair, and nerve, liver, kidney, and vascular tissue regeneration.161 Hepatic gene expression of cytokines and growth factors related to cell proliferation, angiogenesis, and anti-inflammatory response was upregulated by an MSC-conditioned culture medium (MSC-CM).162 In the early phase after surgical resection, MSC-derived factors promoted hepatocyte proliferation and regenerative responses. After patients have undergone extensive liver resection or liver transplantation, MSC-derived factors therapy could represent a feasible new strategy to promote liver regeneration.162 After partial hepatectomy in rats, miR-124 derived from hUCMSC-Exos can downregulate Foxg1 and promote liver regeneration.163 Xue et al found that ADMSC-Exos could promote vascular endothelial growth factor (VEGF) expression and angiogenesis by activating the protein kinase A (PKA) signaling pathway.164 The above studies provide new ideas for the application of MSC-Exos-based delivery vector in liver regeneration, which may benefit patients with extensive liver resection.
Advantages and Disadvantages of MSC-Exos
As a relatively novel treatment, exosomes have certain advantages over nano-drug. As mentioned above, exosomes have been shown to promote the growth of normal cell lines and inhibit the growth of hepatocellular carcinoma cell lines which were inhibited through several anti-inflammatory molecules. Compared with nano-drugs, exosomes are naturally secreted by cells, which have the advantages of low immunogenicity and immune rejection prevention.51 Unlike other lipid nanoparticles, exosomes have surfaces that are rich in membrane proteins, which can mediate adhesion and target functions between exosomes and plasma membrane of recipient cells, thereby regulating exosome uptake.38,161 These advantages may allow MSC-Exo-based delivery vectors to play a good therapeutic effect in the treatment of liver diseases. The use of MSCs as cell therapy carries some risks, such as potential tumorigenicity and immunological rejection.165 As a cell-free therapy, MSC-Exos can effectively reduce this risk. Although derived from MSCs, MSC-Exos sometimes have better results than MSCs.128 In addition, many in vivo studies have shown that MSC-derived exosomes can enter the liver.38 This suggests that MSC-Exo-based delivery vector is a promising alternative to MSC therapy, especially in liver disease.
In the treatment of liver diseases, MSC-Exo-based delivery vectors have a wider application space. Concurrently, we must admit that MSC-Exos should be used cautiously in the treatment of HCC because the role of MSC-Exos in tumor development has not been fully elucidated.137 The choice of exosome drug loading method and surface targeting peptide needs to be fully considered.138
MSC-Exos in Clinical Trials
Currently, 139 clinical trials of exosomes are available at www.ClinicalTrials.gov and nine of them are on MSC-Exos. However, currently, clinical trials on the treatment of liver diseases based on MSC-Exos are lacking. This situation is in part due to the fact that translating MSC-Exos therapy from preclinical studies to the clinic requires key parameters.65
Conclusions and Prospects
The above findings add substantially to our understanding of the therapeutic effect of MSC-Exo-based delivery vectors in liver disease. In the past years, MSC-Exo-based therapy has raised considerable concern. With in-depth research on cell-free therapy, exosomes have a broad application value in liver diseases, including drug delivery, liver cancer, and liver transplantation. MSC-Exos have shown therapeutic potential in various liver diseases and are expected to become a new treatment method for liver diseases. Besides, MSC-Exos can be used as a carrier for drug delivery to assist the more accurate delivery of clinically used drugs to target tissues, which not only improves the efficacy of drugs but also reduces systemic toxic side effects. As a kind of biological carrier, MSC-Exos provide a new idea for the current drug delivery scheme and expand the drug delivery system in the treatment of liver diseases. Therefore, MSC-Exos have the potential to become biological agents for the treatment of liver diseases. By delivering biological cargoes or drugs, MSC-Exos have the potential to be an alternative treatment option for various liver diseases, whether benign or malignant liver disease and early or advanced liver disease. MSC-Exo-based delivery vectors have been widely shown to reduce normal cell apoptosis, promote liver regeneration, increase autophagy of hepatic stellate cells, and inhibit the growth of hepatocellular carcinoma cells. However, MSC-Exos do not exhibit systemic toxicity. Through these mechanisms, MSC-Exos have shown good therapeutic effects in drug-induced liver injury, liver I/R injury, liver resection, HCC, and other liver diseases by delivering biological cargoes or drugs. At present, MSC-Exo-based liver disease therapies are still in the stage of in vitro research and animal models. The current challenges of large-scale production, quality control, long-term storage, cost, and safety of MSC-Exos have not been solved. Therefore, we need to conduct more in-depth research and analysis.50 The clinical application of MSC-Exos-based delivery vectors, as an emerging treatment for liver diseases, should be fully and effectively evaluated. More preclinical studies should be conducted to accumulate more data and prepare for relevant clinical studies. In the treatment of liver disease, investigators need to fully consider the safety and potential side effects of MSC-Exo-based therapy. Further studies on how to efficiently extract or prepare MSC-Exos on a large scale, reduce the cost, improve the loading efficiency of biological cargoes or drugs, and accurately exert therapeutic effects on effector cells or tissues are needed. In summary, MSC-Exos own high clinical translation and application value. In the future, the treatment of MSC-Exos as a biological carrier may not only assist the existing treatment options for liver diseases but also become a new treatment plan for liver diseases. MSC-Exo-based therapy has the potential to relieve the symptoms of patients with liver disease and improve their quality of life and prognosis.
Abbreviations
2D MPs, 2D-tumour-cell-derived microparticles; 2D-Exos, 2D culture of exosomes; 3D-Exos, 3D culture of exosomes; 3D-hUCMSCs, 3D culture of hUCMSCs; ADAM10, a target gene of miR-451a; AFM, atomic force microscopy; AIH, autoimmune hepatitis; ALF, Acute liver failure; AMSC-Exos, adipose tissue-derived mesenchymal stem cell-derived exosomes; AMSCs, adipose tissue-derived mesenchymal stem cells; APAP, acetaminophen; ASO, antisense oligonucleotide; Bcl-xL, One of the antiapoptotic genes; BECN1, a crucial regulator of ferroptosis; BMSC-Exos, bone marrow mesenchymal stem cell-derived exosomes; C5orf66AS1, one of long noncoding RNAs (lncRNAs); CircDIDO1, a circRNA derived from 2 to 6 exons of DIDO1 gene; CSCs, cancer stem cells; DUSP1, dual-specificity phosphatase 1; ECM, extracellular matrix; EST1, E26 transformation specific-1; EVs, Extracellular vesicles; FHF, fulminant hepatic failure; FTO, a fat mass and obesity-associated gene; gp130, The interaction between miR-1246 and interleukin 6 (IL-6) signal transducer; GPX1, Glutathione peroxidase1; GPX4, Glutathione peroxidase 4; GSK3β, glycogen synthase kinase 3β; HBV, Hepatitis B virus; HCC, hepatocellular carcinoma; hESC-Exos, exosomes derived from human embryonic stem cell; hiPSC-MSC-Exos, exosomes produced by human-induced pluripotent stem cell-derived mesenchymal stromal cells; HO-1, heme oxygenase oxygen-1; HSC, hepatic stellate cell; hUCMSC-Exos, human umbilical cord mesenchymal stem cell-derived exosomes; I/R injury, ischemia and reperfusion injury; IL-17, interleukin-17; IL-6, interleukin-6; Ireb2, Iron response element-binding protein 2; KLF6, Kruppel-like factor 6; LT, liver transplantation; LX2 cell, Human hepatic stellate cells; MSC-Exos, Exosomes derived from mesenchymal stem cells; MSCs, Mesenchymal stem cells; mTOR, a serine/threonine kinase; NAFLD, non-alcoholic fatty cell liver disease; NCTD, norcantharidin; NLRP3, nucleotide-binding and oligomerization domain-like receptor 3; NTA, Nanoparticle Tracking Analysis; PI3K, Phosphoinositide 3-kinase; PI3K, Phosphoinositide 3-kinase; PKA, protein kinase A; PTX, paclitaxel; ROS, oxygen species; SAMD, Recombinant Mothers Against Decapentaplegic; STAT3, Signal transducer and activator of transcription 3; TEM, transmission electron microscope; TGFβRII, TGFβ type II receptor; TME, tumor microenvironment; TNFα, Tumor necrosis factor α; TXNIP, thioredoxin-interacting protein; VEGF, vascular endothelial growth factor; α-SMA, Alpha-smooth muscle actin.
Funding
This work was supported by National Key Research and Development Program of China (No. 2021YFA1100500); Key Research & Development Plan of Zhejiang Province (No. 2019C03050); The Construction Fund of Key Medical Disciplines of Hangzhou (OO20200093).
Disclosure
The authors declare that they have no competing interests.
References
1. Y Z, Qm A, M M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15. doi:10.1038/nrgastro.2017.109
2. Yang X, Lu D, Wang R, et al. Single‐cell profiling reveals distinct immune phenotypes that contribute to ischaemia‐reperfusion injury after steatotic liver transplantation. Cell Prolif. 2021;54:e13116. doi:10.1111/cpr.13116
3. Xiao J, Wang F, Wong N-K, et al. Global liver disease burdens and research trends: analysis from a Chinese perspective. J Hepatol. 2019;71:212–221. doi:10.1016/j.jhep.2019.03.004
4. Mokdad AA, Lopez AD, Shahraz S, et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med. 2014;12:145. doi:10.1186/s12916-014-0145-y
5. Moon AM, Singal AG, Tapper EB. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin Gastroenterol Hepatol. 2020;18:2650–2666. doi:10.1016/j.cgh.2019.07.060
6. Wang F-S, Fan J-G, Zhang Z, et al. The global burden of liver disease: the major impact of China. Hepatology. 2014;60:2099–2108. doi:10.1002/hep.27406
7. Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975–2014, featuring survival. J Natl Cancer Inst. 2017;109. doi:10.1093/jnci/djx030
8. Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380:1450–1462. doi:10.1056/NEJMra1713263
9. Asrani SK, Devarbhavi H, Eaton J, et al. Burden of liver diseases in the world. J Hepatol. 2019;70:151–171. doi:10.1016/j.jhep.2018.09.014
10. Zhou J, Chen J, Wei Q, et al. The role of ischemia/reperfusion injury in early hepatic allograft dysfunction. Liver Transpl. 2020;26:1034–1048. doi:10.1002/lt.25779
11. Samuel D, Coilly A. Management of patients with liver diseases on the waiting list for transplantation: a major impact to the success of liver transplantation. BMC Med. 2018;16:113. doi:10.1186/s12916-018-1110-y
12. Yu J, Liu Z, Li C, et al. Regulatory T cell therapy following liver transplantation. Liver Transpl. 2021;27:264–280. doi:10.1002/lt.25948
13. Ding D-C, Shyu W-C, Lin S-Z. Mesenchymal stem cells. Cell Transplant. 2011;20:5–14. doi:10.3727/096368910X
14. Li A, Guo F, Pan Q, et al. Mesenchymal stem cell therapy: hope for patients with systemic lupus erythematosus. Front Immunol. 2021;12:728190. doi:10.3389/fimmu.2021.728190
15. Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–274.
16. Li N, Hua J. Interactions between mesenchymal stem cells and the immune system. Cell Mol Life Sci. 2017;74:2345–2360. doi:10.1007/s00018-017-2473-5
17. Lan T, Luo M, Wei X. Mesenchymal stem/stromal cells in cancer therapy. J Hematol Oncol. 2021;14:195. doi:10.1186/s13045-021-01208-w
18. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi:10.1126/science.284.5411.143
19. Han F, Wang C-Y, Yang L, et al. Contribution of murine bone marrow mesenchymal stem cells to pancreas regeneration after partial pancreatectomy in mice. Cell Biol Int. 2012;36:823–831. doi:10.1042/CBI20110680
20. Rose RA, Jiang H, Wang X, et al. Bone marrow-derived mesenchymal stromal cells express cardiac-specific markers, retain the stromal phenotype, and do not become functional cardiomyocytes in vitro. Stem Cells. 2008;26:2884–2892. doi:10.1634/stemcells.2008-0329
21. Qian H, Yang H, Xu W, et al. Bone marrow mesenchymal stem cells ameliorate rat acute renal failure by differentiation into renal tubular epithelial-like cells. Int J Mol Med. 2008;22:325–332.
22. Cho K-A, S-Y J, Cho SJ, et al. Mesenchymal stem cells showed the highest potential for the regeneration of injured liver tissue compared with other subpopulations of the bone marrow. Cell Biol Int. 2009;33:772–777. doi:10.1016/j.cellbi.2009.04.023
23. Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54. doi:10.1038/emm.2013.94
24. Volarevic V, Nurkovic J, Arsenijevic N, et al. Concise review: therapeutic potential of mesenchymal stem cells for the treatment of acute liver failure and cirrhosis. Stem Cells. 2014;32:2818–2823. doi:10.1002/stem.1818
25. Liu P, Qian Y, Liu X, et al. Immunomodulatory role of mesenchymal stem cell therapy in liver fibrosis. Front Immunol. 2023;13:1096402. doi:10.3389/fimmu.2022.1096402
26. Wang Y-H, D-B W, Chen B, et al. Progress in mesenchymal stem cell–based therapy for acute liver failure. Stem Cell Res Ther. 2018;9:227. doi:10.1186/s13287-018-0972-4
27. Hu C, Wu Z, Li L. Mesenchymal stromal cells promote liver regeneration through regulation of immune cells. Int J Biol Sci. 2020;16:893–903. doi:10.7150/ijbs.39725
28. Yi S, Cong Q, Zhu Y, et al. Mechanisms of action of mesenchymal stem cells in metabolic-associated fatty liver disease. Stem Cells Int. 2023;2023:3919002. doi:10.1155/2023/3919002
29. Yang X, Meng Y, Han Z, et al. Mesenchymal stem cell therapy for liver disease: full of chances and challenges. Cell Biosci. 2020;10:123. doi:10.1186/s13578-020-00480-6
30. Thirabanjasak D, Tantiwongse K, Thorner PS. Angiomyeloproliferative lesions following autologous stem cell therapy. J Am Soc Nephrol. 2010;21:1218–1222. doi:10.1681/ASN.2009111156
31. Elsharkasy OM, Nordin JZ, Hagey DW, et al. Extracellular vesicles as drug delivery systems: why and how? Adv Drug Deliv Rev. 2020;159:332–343. doi:10.1016/j.addr.2020.04.004
32. Urabe F, Kosaka N, Ito K, et al. Extracellular vesicles as biomarkers and therapeutic targets for cancer. Am J Physiol Cell Physiol. 2020;318:C29–C39. doi:10.1152/ajpcell.00280.2019
33. Harrell CR, Jovicic N, Djonov V, et al. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells. 2019;8:1605. doi:10.3390/cells8121605
34. Wang Y, Pang J, Wang Q, et al. Delivering antisense oligonucleotides across the blood‐brain barrier by tumor cell‐derived small apoptotic bodies. Adv Sci. 2021;8:2004929. doi:10.1002/advs.202004929
35. Zhou M, Y-J L, Tang Y-C, et al. Apoptotic bodies for advanced drug delivery and therapy. J Control Rel. 2022;351:394–406. doi:10.1016/j.jconrel.2022.09.045
36. Davidson SM, Boulanger CM, Aikawa E, et al. Methods for the identification and characterization of extracellular vesicles in cardiovascular studies: from exosomes to microvesicles. Cardiovasc Res. 2023;119:45–63. doi:10.1093/cvr/cvac031
37. Psaraki A, Ntari L, Karakostas C, et al. Extracellular vesicles derived from mesenchymal stem/stromal cells: the regenerative impact in liver diseases. Hepatology. 2022;75:1590. doi:10.1002/hep.32129
38. van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–228. doi:10.1038/nrm.2017.125
39. Marar C, Starich B, Wirtz D. Extracellular vesicles in immunomodulation and tumor progression. Nat Immunol. 2021;22:560–570. doi:10.1038/s41590-021-00899-0
40. Harding C, Stahl P. Transferrin recycling in reticulocytes: pH and iron are important determinants of ligand binding and processing. Biochem Biophys Res Commun. 1983;113:650–658. doi:10.1016/0006-291x(83)91776-x
41. Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967–978. doi:10.1016/0092-8674(83)90040-5
42. Zhang L, Yu D. Exosomes in cancer development, metastasis, and immunity. Biochim Biophys Acta Rev Cancer. 2019;1871:455–468. doi:10.1016/j.bbcan.2019.04.004
43. Munson P, Shukla A. Exosomes: potential in cancer diagnosis and therapy. Medicines. 2015;2:310–327. doi:10.3390/medicines2040310
44. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. doi:10.1126/science.aau6977
45. Lötvall J, Hill AF, Hochberg F, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:
46. Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487–514. doi:10.1146/annurev-biochem-013118-111902
47. Mathivanan S, Fahner CJ, Reid GE, et al. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40:D1241–D1244. doi:10.1093/nar/gkr828
48. Wang C, Zhou H, Wu R, et al. Mesenchymal stem cell-derived exosomes and non-coding RNAs: regulatory and therapeutic role in liver diseases. Biomedicine & Pharmacotherapy. 2023;157:114040. doi:10.1016/j.biopha.2022.114040
49. Hade MD, Suire CN, Suo Z. Mesenchymal stem cell-derived exosomes: applications in regenerative medicine. Cells. 2021;10:1959. doi:10.3390/cells10081959
50. Tang Y, Zhou Y, Li H-J. Advances in mesenchymal stem cell exosomes: a review. Stem Cell Res Ther. 2021;12:71. doi:10.1186/s13287-021-02138-7
51. Zhang X, Han C, Du B, et al. Isolation and identification of adipose stem cell exosomes and the study of its potential as drug delivery carrier in vitro. Appl Biochem Biotechnol. 2022;194:2594–2603. doi:10.1007/s12010-022-03835-6
52. Wiklander OPB, Nordin JZ, O’Loughlin A, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:
53. Kim MS, Haney MJ, Zhao Y, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. 2016;12:655–664. doi:10.1016/j.nano.2015.10.012
54. Tian T, Zhang H-X, He C-P, et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137–149. doi:10.1016/j.biomaterials.2017.10.012
55. Mianehsaz E, Mirzaei HR, Mahjoubin-Tehran M, et al. Mesenchymal stem cell-derived exosomes: a new therapeutic approach to osteoarthritis? Stem Cell Res Ther. 2019;10:340. doi:10.1186/s13287-019-1445-0
56. Lai RC, Yeo RWY, Lim SK. Mesenchymal stem cell exosomes. Semin Cell Dev Biol. 2015;40:82–88. doi:10.1016/j.semcdb.2015.03.001
57. Joo HS, Suh JH, Lee HJ, et al. Current knowledge and future perspectives on mesenchymal stem cell-derived exosomes as a new therapeutic agent. Int J Mol Sci. 2020;21:727. doi:10.3390/ijms21030727
58. Pan W, Chen H, Wang A, et al. Challenges and strategies: scalable and efficient production of mesenchymal stem cells-derived exosomes for cell-free therapy. Life Sci. 2023;319:121524. doi:10.1016/j.lfs.2023.121524
59. Shen Z, Huang W, Liu J, et al. Effects of mesenchymal stem cell-derived exosomes on autoimmune diseases. Front Immunol. 2021;12:749192. doi:10.3389/fimmu.2021.749192
60. Meirelles L, Fontes AM, Covas DT, et al. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–427. doi:10.1016/j.cytogfr.2009.10.002
61. Toh WS, Foldager CB, Pei M, et al. Advances in mesenchymal stem cell-based strategies for cartilage repair and regeneration. Stem Cell Rev Rep. 2014;10:686–696. doi:10.1007/s12015-014-9526-z
62. Toh WS, Lai RC, Hui JHP, et al. MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin Cell Dev Biol. 2017;67:56–64. doi:10.1016/j.semcdb.2016.11.008
63. Xiong -Y-Y, Gong Z-T, Tang R-J, et al. The pivotal roles of exosomes derived from endogenous immune cells and exogenous stem cells in myocardial repair after acute myocardial infarction. Theranostics. 2021;11:1046–1058. doi:10.7150/thno.53326
64. Lou G, Yang Y, Liu F, et al. MiR‐122 modification enhances the therapeutic efficacy of adipose tissue‐derived mesenchymal stem cells against liver fibrosis. J Cell Mol Med. 2017;21:2963–2973. doi:10.1111/jcmm.13208
65. Mendt M, Rezvani K, Shpall E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant. 2019;54:789–792. doi:10.1038/s41409-019-0616-z
66. Zhang J, Guan J, Niu X, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49. doi:10.1186/s12967-015-0417-0
67. Shabbir A, Cox A, Rodriguez-Menocal L, et al. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev. 2015;24:1635–1647. doi:10.1089/scd.2014.0316
68. Yoon YM, Lee JH, Song K-H, et al. Melatonin-stimulated exosomes enhance the regenerative potential of chronic kidney disease-derived mesenchymal stem/stromal cells via cellular prion proteins. J Pineal Res. 2020;68:e12632. doi:10.1111/jpi.12632
69. Grange C, Bellucci L, Bussolati B, et al. Potential applications of extracellular vesicles in solid organ transplantation. Cells. 2020;9:E369. doi:10.3390/cells9020369
70. Brossa A, Fonsato V, Grange C, et al. Extracellular vesicles from human liver stem cells inhibit renal cancer stem cell-derived tumor growth in vitro and in vivo. Int J Cancer. 2020;147:1694–1706. doi:10.1002/ijc.32925
71. Zhang Y, Chopp M, Meng Y, et al. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122:856–867. doi:10.3171/2014.11.JNS14770
72. Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219:396–405. doi:10.1016/j.jconrel.2015.07.030
73. Sun Y, Liu G, Zhang K, et al. Mesenchymal stem cells-derived exosomes for drug delivery. Stem Cell Res Ther. 2021;12:561. doi:10.1186/s13287-021-02629-7
74. Liang Y, Duan L, Lu J, et al. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183–3195. doi:10.7150/thno.52570
75. Liang L, Zhao L, Wang Y, et al. Treatment for Hepatocellular Carcinoma Is Enhanced When Norcantharidin Is Encapsulated in Exosomes Derived from Bone Marrow Mesenchymal Stem Cells. Mol Pharmaceutics. 2021;18:1003–1013. doi:10.1021/acs.molpharmaceut.0c00976
76. Li Y, Guo G, Li L, et al. Three-dimensional spheroid culture of human umbilical cord mesenchymal stem cells promotes cell yield and stemness maintenance. Cell Tissue Res. 2015;360:297–307. doi:10.1007/s00441-014-2055-x
77. Wang N, Li X, Zhong Z, et al. 3D hESC exosomes enriched with miR-6766-3p ameliorates liver fibrosis by attenuating activated stellate cells through targeting the TGFβRII-SMADS pathway. J Nanobiotechnology. 2021;19:437. doi:10.1186/s12951-021-01138-2
78. Liang Q, Bie N, Yong T, et al. The softness of tumour-cell-derived microparticles regulates their drug-delivery efficiency. Nat Biomed Eng. 2019;3:729–740. doi:10.1038/s41551-019-0405-4
79. Zhang K, Zhao X, Chen X, et al. Enhanced therapeutic effects of mesenchymal stem cell-derived exosomes with an injectable hydrogel for hindlimb ischemia treatment. ACS Appl Mater Interfaces. 2018;10:30081–30091. doi:10.1021/acsami.8b08449
80. Cvjetkovic A, Lötvall J, Lässer C. The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles. J Extracell Vesicles. 2014;3:23111. doi:10.3402/jev.v3.23111
81. Wang J, Chen D, Ho EA. Challenges in the development and establishment of exosome-based drug delivery systems. J Control Rel. 2021;329:894–906. doi:10.1016/j.jconrel.2020.10.020
82. Langevin SM, Kuhnell D, Orr-Asman MA, et al. Balancing yield, purity and practicality: a modified differential ultracentrifugation protocol for efficient isolation of small extracellular vesicles from human serum. RNA Biol. 2019;16:5–12. doi:10.1080/15476286.2018.1564465
83. Onódi Z, Pelyhe C, Terézia Nagy C, et al. Isolation of high-purity extracellular vesicles by the combination of iodixanol density gradient ultracentrifugation and bind-elute chromatography from blood plasma. Front Physiol. 2018;9:1479. doi:10.3389/fphys.2018.01479
84. Haraszti RA, Miller R, Stoppato M, et al. Exosomes produced from 3D cultures of MSCs by Tangential flow filtration show higher yield and improved activity. Mol Ther. 2018;26:2838–2847. doi:10.1016/j.ymthe.2018.09.015
85. Böing AN, van der Pol E, Grootemaat AE, et al. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J Extracell Vesicles. 2014;3:23430. doi:10.3402/jev.v3.23430
86. Rider MA, Hurwitz SN, Meckes DG. ExtraPEG: a polyethylene glycol-based method for enrichment of extracellular vesicles. Sci Rep. 2016;6:23978. doi:10.1038/srep23978
87. Wu M, Ouyang Y, Wang Z, et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc Natl Acad Sci U S A. 2017;114:10584–10589. doi:10.1073/pnas.1709210114
88. Lin Z, Wu Y, Xu Y, et al. Mesenchymal stem cell-derived exosomes in cancer therapy resistance: recent advances and therapeutic potential. Mol Cancer. 2022;21:179. doi:10.1186/s12943-022-01650-5
89. Shao M, Xu Q, Wu Z, et al. Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate IL-6-induced acute liver injury through miR-455-3p. Stem Cell Res Ther. 2020;11:37. doi:10.1186/s13287-020-1550-0
90. Chen L, Lu F, Chen D, et al. BMSCs-derived miR-223-containing exosomes contribute to liver protection in experimental autoimmune hepatitis. Mol Immunol. 2018;93:38–46. doi:10.1016/j.molimm.2017.11.008
91. Liu Y, Lou G, Li A, et al. AMSC-derived exosomes alleviate lipopolysaccharide/d-galactosamine-induced acute liver failure by miR-17-mediated reduction of TXNIP/NLRP3 inflammasome activation in macrophages. EBioMedicine. 2018;36:140–150. doi:10.1016/j.ebiom.2018.08.054
92. Qu Y, Zhang Q, Cai X, et al. Exosomes derived from miR‐181‐5p‐modified adipose‐derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J Cell Mol Med. 2017;21:2491–2502. doi:10.1111/jcmm.13170
93. Ma L, Wei J, Zeng Y, et al. Mesenchymal stem cell-originated exosomal circDIDO1 suppresses hepatic stellate cell activation by miR-141-3p/PTEN/AKT pathway in human liver fibrosis. Drug Deliv. 2022;29:440–453. doi:10.1080/10717544.2022.2030428
94. Tian S, Zhou X, Zhang M, et al. Mesenchymal stem cell-derived exosomes protect against liver fibrosis via delivering miR-148a to target KLF6/STAT3 pathway in macrophages. Stem Cell Res Ther. 2022;13:330. doi:10.1186/s13287-022-03010-y
95. Tan Y, Huang Y, Mei R, et al. HucMSC-derived exosomes delivered BECN1 induces ferroptosis of hepatic stellate cells via regulating the xCT/GPX4 axis. Cell Death Dis. 2022;13:319. doi:10.1038/s41419-022-04764-2
96. Tang M, Chen Y, Li B, et al. Therapeutic targeting of STAT3 with small interference RNAs and antisense oligonucleotides embedded exosomes in liver fibrosis. FASEB J. 2021;35:e21557. doi:10.1096/fj.202002777RR
97. Lou G, Chen L, Xia C, et al. MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. J Exp Clin Cancer Res. 2020;39:4. doi:10.1186/s13046-019-1512-5
98. Lou G, Song X, Yang F, et al. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol. 2015;8:122. doi:10.1186/s13045-015-0220-7
99. Gu H, Yan C, Wan H, et al. Mesenchymal stem cell-derived exosomes block malignant behaviors of hepatocellular carcinoma stem cells through a lncRNA C5orf66-AS1/microRNA-127-3p/DUSP1/ERK axis. Hum Cell. 2021;34:1812–1829. doi:10.1007/s13577-021-00599-9
100. Xu Y, Lai Y, Cao L, et al. Human umbilical cord mesenchymal stem cells-derived exosomal microRNA-451a represses epithelial–mesenchymal transition of hepatocellular carcinoma cells by inhibiting ADAM10. RNA Biol. 2021;18:1408–1423. doi:10.1080/15476286.2020.1851540
101. Li YH, Lv MF, Lu MS, et al. Bone marrow mesenchymal stem cell-derived exosomal MiR-338-3p represses progression of hepatocellular carcinoma by targeting ETS1. J Biol Regul Homeost Agents. 2021;35:617–627. doi:10.23812/20-638-A
102. Xie K, Liu L, Chen J, et al. Exosomes derived from human umbilical cord blood mesenchymal stem cells improve hepatic ischemia reperfusion injury via delivering miR-1246. Cell Cycle. 2019;18:3491–3501. doi:10.1080/15384101.2019.1689480
103. Xie K, Liu L, Chen J, et al. Exosomal miR-1246 derived from human umbilical cord blood mesenchymal stem cells attenuates hepatic ischemia reperfusion injury by modulating T helper 17/regulatory T balance. IUBMB Life. 2019;71:2020–2030. doi:10.1002/iub.2147
104. Zhang L, Song Y, Chen L, et al. MiR-20a-containing exosomes from umbilical cord mesenchymal stem cells alleviates liver ischemia/reperfusion injury. J Cell Physiol. 2020;235:3698–3710. doi:10.1002/jcp.29264
105. Wu L, Tian X, Zuo H, et al. miR-124-3p delivered by exosomes from heme oxygenase-1 modified bone marrow mesenchymal stem cells inhibits ferroptosis to attenuate ischemia–reperfusion injury in steatotic grafts. J Nanobiotechnology. 2022;20:196. doi:10.1186/s12951-022-01407-8
106. Li X, Wu L, Tian X, et al. miR-29a-3p in exosomes from heme oxygenase-1 modified bone marrow mesenchymal stem cells alleviates steatotic liver ischemia-reperfusion injury in rats by suppressing ferroptosis via iron responsive element binding protein 2. Oxid Med Cell Longev. 2022;2022:6520789. doi:10.1155/2022/6520789
107. Cheng L, Yu P, Li F, et al. Human umbilical cord-derived mesenchymal stem cell-exosomal miR-627-5p ameliorates non-alcoholic fatty liver disease by repressing FTO expression. Hum Cell. 2021;34:1697–1708. doi:10.1007/s13577-021-00593-1
108. Wang XJ, Malhi H. Nonalcoholic fatty liver disease. Ann Intern Med. 2018;169:ITC65–ITC80. doi:10.7326/AITC201811060
109. Wen Z, Mai Z, Zhu X, et al. Mesenchymal stem cell-derived exosomes ameliorate cardiomyocyte apoptosis in hypoxic conditions through microRNA144 by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 2020;11:36. doi:10.1186/s13287-020-1563-8
110. Xiao K, He W, Guan W, et al. Mesenchymal stem cells reverse EMT process through blocking the activation of NF-κB and Hedgehog pathways in LPS-induced acute lung injury. Cell Death Dis. 2020;11:863. doi:10.1038/s41419-020-03034-3
111. Li L, Zhang Y, Mu J, et al. Transplantation of human mesenchymal stem-cell-derived exosomes immobilized in an adhesive hydrogel for effective treatment of spinal cord injury. Nano Lett. 2020;20:4298–4305. doi:10.1021/acs.nanolett.0c00929
112. Das M, Mayilsamy K, Mohapatra SS, et al. Mesenchymal stem cell therapy for the treatment of traumatic brain injury: progress and prospects. Rev Neurosci. 2019;30:839–855. doi:10.1515/revneuro-2019-0002
113. Liu W-Z, Z-J M, J-R L, et al. Mesenchymal stem cell-derived exosomes: therapeutic opportunities and challenges for spinal cord injury. Stem Cell Res Ther. 2021;12:102. doi:10.1186/s13287-021-02153-8
114. Tan CY, Lai RC, Wong W, et al. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther. 2014;5:76. doi:10.1186/scrt465
115. Lin F, Chen W, Zhou J, et al. Mesenchymal stem cells protect against ferroptosis via exosome-mediated stabilization of SLC7A11 in acute liver injury. Cell Death Dis. 2022;13:271. doi:10.1038/s41419-022-04708-w
116. Bernal W, Wendon J. Acute liver failure. N Engl J Med. 2013;369:2525–2534. doi:10.1056/NEJMra1208937
117. Zhan C, Lin G, Huang Y, et al. A dopamine-precursor-based nanoprodrug for in-situ drug release and treatment of acute liver failure by inhibiting NLRP3 inflammasome and facilitating liver regeneration. Biomaterials. 2021;268:120573. doi:10.1016/j.biomaterials.2020.120573
118. Jiang L, Zhang S, Hu H, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate acute liver failure by reducing the activity of the NLRP3 inflammasome in macrophages. Biochem Biophys Res Commun. 2019;508:735–741. doi:10.1016/j.bbrc.2018.11.189
119. Zhao S, Liu Y, Pu Z. Bone marrow mesenchymal stem cell-derived exosomes attenuate D-GaIN/LPS-induced hepatocyte apoptosis by activating autophagy in vitro. Drug Des Devel Ther. 2019;13:2887–2897. doi:10.2147/DDDT.S220190
120. Chen L, Xiang B, Wang X, et al. Exosomes derived from human menstrual blood-derived stem cells alleviate fulminant hepatic failure. Stem Cell Res Ther. 2017;8:9. doi:10.1186/s13287-016-0453-6
121. Brigelius-Flohé R, Maiorino M. Glutathione peroxidases. Biochim Biophys Acta. 2013;1830:3289–3303. doi:10.1016/j.bbagen.2012.11.020
122. Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38(Suppl 1):S38–S53. doi:10.1016/s0168-8278(02)00429-4
123. Parola M, Pinzani M. Liver fibrosis: pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med. 2019;65:37–55. doi:10.1016/j.mam.2018.09.002
124. Hu X, Ge Q, Zhang Y, et al. A review of the effect of exosomes from different cells on liver fibrosis. Biomedicine & Pharmacotherapy. 2023;161:114415. doi:10.1016/j.biopha.2023.114415
125. Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411. doi:10.1038/nrgastro.2017.38
126. Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev. 2017;121:27–42. doi:10.1016/j.addr.2017.05.007
127. Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18:151–166. doi:10.1038/s41575-020-00372-7
128. Rong X, Liu J, Yao X, et al. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway. Stem Cell Res Ther. 2019;10:98. doi:10.1186/s13287-019-1204-2
129. Sui M, Jiang X, Chen J, et al. Magnesium isoglycyrrhizinate ameliorates liver fibrosis and hepatic stellate cell activation by regulating ferroptosis signaling pathway. Biomed Pharmacother. 2018;106:125–133. doi:10.1016/j.biopha.2018.06.060
130. Anwanwan D, Singh SK, Singh S, et al. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 2020;1873:188314. doi:10.1016/j.bbcan.2019.188314
131. Sia D, Villanueva A, Friedman SL, et al. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology. 2017;152:745–761. doi:10.1053/j.gastro.2016.11.048
132. Yang JD, Hainaut P, Gores GJ, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589–604. doi:10.1038/s41575-019-0186-y
133. Roma-Rodrigues C, Mendes R, Baptista PV, et al. Targeting tumor microenvironment for cancer therapy. Int J Mol Sci. 2019;20:840. doi:10.3390/ijms20040840
134. Dai J, Su Y, Zhong S, et al. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther. 2020;5:145. doi:10.1038/s41392-020-00261-0
135. Steinbichler TB, Dudás J, Riechelmann H, et al. The role of exosomes in cancer metastasis. Semin Cancer Biol. 2017;44:170–181. doi:10.1016/j.semcancer.2017.02.006
136. Sauter ER. Exosomes in lymph and cancer. Transl Cancer Res. 2017;6. doi:10.21037/16277
137. Alzahrani FA, El-Magd MA, Abdelfattah-Hassan A, et al. Potential effect of exosomes derived from cancer stem cells and MSCs on progression of DEN-induced HCC in rats. Stem Cells Int. 2018;2018:8058979. doi:10.1155/2018/8058979
138. Zhu L, Sun H-T, Wang S, et al. Isolation and characterization of exosomes for cancer research. J Hematol Oncol. 2020;13:152. doi:10.1186/s13045-020-00987-y
139. Wang Y, Wang B, Xiao S, et al. miR-125a/b inhibits tumor-associated macrophages mediated in cancer stem cells of hepatocellular carcinoma by targeting CD90. J Cell Biochem. 2019;120:3046–3055. doi:10.1002/jcb.27436
140. Lee TK-W, Guan X-Y, Ma S. Cancer stem cells in hepatocellular carcinoma - from origin to clinical implications. Nat Rev Gastroenterol Hepatol. 2022;19:26–44. doi:10.1038/s41575-021-00508-3
141. X-L M, Hu B, Tang W-G, et al. CD73 sustained cancer-stem-cell traits by promoting SOX9 expression and stability in hepatocellular carcinoma. J Hematol Oncol. 2020;13:11. doi:10.1186/s13045-020-0845-z
142. Huang H, Hou J, Liu K, et al. RAB27A-dependent release of exosomes by liver cancer stem cells induces Nanog expression in their differentiated progenies and confers regorafenib resistance. J Gastroenterol Hepatol. 2021;36:3429–3437. doi:10.1111/jgh.15619
143. Zhu W, Xu W, Jiang R, et al. Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Exp Mol Pathol. 2006;80:267–274. doi:10.1016/j.yexmp.2005.07.004
144. Klopp AH, Gupta A, Spaeth E, et al. Concise review: dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells. 2011;29:11–19. doi:10.1002/stem.559
145. Vicencio JM, Yellon DM, Sivaraman V, et al. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J Am Coll Cardiol. 2015;65:1525–1536. doi:10.1016/j.jacc.2015.02.026
146. Song Y, Li Z, He T, et al. M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics. 2019;9:2910–2923. doi:10.7150/thno.30879
147. Wu X, Iroegbu CD, Peng J, et al. Cell death and exosomes regulation after myocardial infarction and ischemia-reperfusion. Front Cell Dev Biol. 2021;9:673677. doi:10.3389/fcell.2021.673677
148. Wang Y, Zhang L, Li Y, et al. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int J Cardiol. 2015;192:61–69. doi:10.1016/j.ijcard.2015.05.020
149. Zhao J, Li X, Hu J, et al. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc Res. 2019;115:1205–1216. doi:10.1093/cvr/cvz040
150. Cheng C, Chen X, Wang Y, et al. MSCs‑derived exosomes attenuate ischemia-reperfusion brain injury and inhibit microglia apoptosis might via exosomal miR-26a-5p mediated suppression of CDK6. Mol Med. 2021;27:67. doi:10.1186/s10020-021-00324-0
151. Li R, Zhao K, Ruan Q, et al. Bone marrow mesenchymal stem cell-derived exosomal microRNA-124-3p attenuates neurological damage in spinal cord ischemia-reperfusion injury by downregulating Ern1 and promoting M2 macrophage polarization. Arthritis Res Ther. 2020;22:75. doi:10.1186/s13075-020-2146-x
152. Cao J-Y, Wang B, Tang -T-T, et al. Exosomal miR-125b-5p deriving from mesenchymal stem cells promotes tubular repair by suppression of p53 in ischemic acute kidney injury. Theranostics. 2021;11:5248–5266. doi:10.7150/thno.54550
153. Du Y, Li D, Han C, et al. Exosomes from human-induced pluripotent stem cell–derived mesenchymal stromal cells (hiPSC-MSCs) protect liver against hepatic ischemia/ reperfusion injury via activating sphingosine kinase and sphingosine-1-phosphate signaling pathway. CPB. 2017;43:611–625. doi:10.1159/000480533
154. Nojima H, Freeman CM, Schuster RM, et al. Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. J Hepatol. 2016;64:60–68. doi:10.1016/j.jhep.2015.07.030
155. Nong K, Wang W, Niu X, et al. Hepatoprotective effect of exosomes from human-induced pluripotent stem cell-derived mesenchymal stromal cells against hepatic ischemia-reperfusion injury in rats. Cytotherapy. 2016;18:1548–1559. doi:10.1016/j.jcyt.2016.08.002
156. Yang B, Duan W, Wei L, et al. Bone marrow mesenchymal stem cell-derived hepatocyte-like cell exosomes reduce hepatic ischemia/reperfusion injury by enhancing autophagy. Stem Cells Dev. 2020;29:372–379. doi:10.1089/scd.2019.0194
157. Mahmoudi A, Butler AE, Jamialahmadi T, et al. The role of exosomal miRNA in nonalcoholic fatty liver disease. J Cell Physiol. 2022;237:2078–2094. doi:10.1002/jcp.30699
158. Zhao H, Shang Q, Pan Z, et al. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes. 2017;67:235–247. doi:10.2337/db17-0356
159. Hou X, Yin S, Ren R, et al. Myeloid cell-specific IL-6 signaling promotes miR-223-enriched exosome production to attenuate NAFLD-associated fibrosis. Hepatology. 2021;74:116–132. doi:10.1002/hep.31658
160. Liu X-L, Pan Q, Cao H-X, et al. Lipotoxic hepatocyte-derived exosomal MicroRNA 192-5p activates macrophages through rictor/Akt/forkhead box transcription factor O1 signaling in nonalcoholic fatty liver disease. Hepatology. 2020;72:454–469. doi:10.1002/hep.31050
161. Huang J, Xiong J, Yang L, et al. Cell-free exosome-laden scaffolds for tissue repair. Nanoscale. 2021;13:8740–8750. doi:10.1039/d1nr01314a
162. Fouraschen SMG, Pan Q, de Ruiter PE, et al. Secreted factors of human liver-derived mesenchymal stem cells promote liver regeneration early after partial hepatectomy. Stem Cells Dev. 2012;21:2410–2419. doi:10.1089/scd.2011.0560
163. Song X-J, Zhang L, Li Q, et al. hUCB-MSC derived exosomal miR-124 promotes rat liver regeneration after partial hepatectomy via downregulating Foxg1. Life Sci. 2021;265:118821. doi:10.1016/j.lfs.2020.118821
164. Xue C, Shen Y, Li X, et al. Exosomes derived from hypoxia-treated human adipose mesenchymal stem cells enhance angiogenesis through the PKA signaling pathway. Stem Cells Dev. 2018;27:456–465. doi:10.1089/scd.2017.0296
165. Shiota G, Itaba N. Progress in stem cell-based therapy for liver disease. Hepatol Res. 2017;47:127–141. doi:10.1111/hepr.12747
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.