Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Current Practices in Hyaluronic Acid Dermal Filler Treatment in Asia Pacific and Practical Approaches to Achieving Safe and Natural-Looking Results
Authors Corduff N, Juniarti L, Lim TS , Lin F, Mariwalla K, Pavicic T, Quiambao A , Siew TW, Suwanchinda A , Tseng FW , Vachiramon V , Youn CS, Ho WWS
Received 24 February 2022
Accepted for publication 17 June 2022
Published 1 July 2022 Volume 2022:15 Pages 1213—1223
DOI https://doi.org/10.2147/CCID.S363583
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jeffrey Weinberg
Niamh Corduff,1 Lanny Juniarti,2 Ting Song Lim,3 Frank Lin,4 Kavita Mariwalla,5 Tatjana Pavicic,6 Arnelle Quiambao,7 Tuck Wah Siew,8 Atchima Suwanchinda,9,10 Fang Wen Tseng,11 Vasanop Vachiramon,10 Choon Shik Youn,12 Wilson WS Ho13
1Cosmetic Refinement Clinic Geelong, Victoria, Australia; 2Miracle Aesthetic Clinic, Surabaya, Indonesia; 3Clique Clinic, Kuala Lumpur, Malaysia; 4Eastern Plastic Surgery, Victoria, Australia; 5Mariwalla Dermatology, New York, NY, USA; 6Private Practice for Dermatology & Aesthetics of Dr. Tatjana Pavicic, Munich, Germany; 7YouPlus Intelligent Aesthetics Clinic, BGC Taguig City, Philippines; 8Radium Medical Aesthetics, Singapore; 9Department of Medicine, Chulabhorn Hospital, Chulabhorn Royal Academy, Bangkok, Thailand; 10Division of Dermatology, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; 11Milano Aesthetic Clinic, Taoyuan City, Taiwan; 12Yemiwon Dermatologic Clinic, Seoul, Korea; 13The Specialists: Lasers, Aesthetic & Plastic Surgery, Central, Hong Kong
Correspondence: Niamh Corduff, Cosmetic Refinement Clinic Geelong, T9 400 Pakington St, Newtown VIC, Victoria, 3220, Australia, Tel +61 407122578, Email [email protected]
Abstract: Complications such as delayed inflammatory reactions (DIRs) and unnatural outcomes can sometimes arise from hyaluronic acid (HA) dermal filler treatments and can be challenging to address. Given the popularity of HA dermal fillers for aesthetic procedures, there is a need for preventive strategies to minimize these complications. Two hundred practitioners from 10 regions in Asia Pacific who administer HA fillers completed a survey on prevention of DIRs and unnatural outcomes. Thirteen global experts convened to evaluate the current practices and propose practical approaches for safe and appropriate use of HA dermal fillers. From the survey, the top three measures used to reduce the risk of DIRs included choosing an appropriate HA filler, aseptic technique, and patient selection. Key strategies employed to achieve natural-looking outcomes were treatment customization, understanding the rheological properties and behavior of different HA fillers, and being conservative in treatment approach. The panel developed a concise reference guide aimed at minimizing the risk of DIRs while achieving natural aesthetic outcomes. Five practical considerations were recommended: patient assessment and individualization of treatment plan, choice of an appropriate HA filler, adequate knowledge of facial anatomy, strict adherence to aseptic methods, and proper injection technique. The panel highlighted the need for education efforts to increase awareness of differential immunogenicity between HA fillers and to improve understanding on the importance of preserving aesthetic individuality for optimal results. These practical insights from the global experts support practitioners in optimizing safety and quality of aesthetic treatment with HA fillers.
Keywords: hyaluronic acid dermal filler, consensus, practical approaches, safe, natural-looking outcomes, current practices
Introduction
Hyaluronic acid (HA) dermal fillers are one of the most common nonsurgical modalities in aesthetic medicine due to their ease of administration, quick onset of action and minimal recovery time for patients.1 Although HA fillers are useful for facial rejuvenation and soft-tissue augmentation,2–4 complications such as delayed inflammatory reactions (DIRs) and aesthetically unpleasant outcomes can arise, in addition to common side effects like bruising, swelling and redness.5,6
While there are discrepancies in the definition of DIRs in the literature, in this article, DIRs are defined as large, tender, erythematous nodules with surrounding edema presenting ≥14 days after filler placement.7 Although DIRs are uncommon and may resolve spontaneously, more often than not, additional interventions are required to promote resolution; they can be challenging to treat and can lead to permanent sequelae if not managed properly.5,7
Aesthetically unpleasant outcomes manifest as overfilled appearance, surface irregularities, bumps/nodules, disproportionate face, distorted appearance, etc.5,6 They may be caused by overfilling of filler, inappropriate filler choice, and inappropriate placement of filler.5,6 While some of these unpleasant outcomes may resolve spontaneously, others require remedial interventions that may not necessarily restore the original anatomy and patient appearance.5,6
Therefore, it is best to take precautions to avoid these undesirable complications to improve patient safety and the quality of aesthetic outcomes. This article reports the current practices in HA dermal filler treatment in Asia Pacific pertaining to the knowledge of practitioners in preventing DIRs and achieving pleasant, natural-looking outcomes. It also includes relevant recommendations and a concise reference guide to support good practices among aesthetic practitioners who administer HA fillers.
Survey on HA Dermal Filler Treatment and Panel Discussion
An online survey was conducted to understand the current practices in HA dermal filler treatment in Asia Pacific. As the survey only collected information on treatment practices, ethics approval was not required. Practitioners from 10 regions in Asia Pacific completed the online survey between May and June 2021. A panel comprising 13 clinical experts with extensive experience in using HA fillers, known as the Senior Aesthetics Filler Experts (SAFE) council, convened in a virtual meeting in July 2021 to review and discuss the survey findings, and establish a consensus for safe and appropriate use of HA dermal fillers. Specifically, they proposed recommendations and practical approaches to prevent and manage DIRs and achieve natural-looking results. The panel consisted of aesthetic physicians, dermatologists, and plastic surgeons from Australia, Germany, Hong Kong, Indonesia, Malaysia, the Philippines, Singapore, South Korea, Taiwan, Thailand, and the United States. The voting results were graded as follows: strong consensus (>95% agreement); consensus (>75% to 95% agreement); majority consent (>50% to 75% agreement); and no majority consent (≤50% agreement). The definition of DIR described by Artzi and colleagues7 was adopted for this work.
Current Practices in HA Dermal Filler Treatment in Asia Pacific
Two hundred practitioners in Asia Pacific took part in the survey. They had an average of 9.4 (SD 4.8) years of experience in using HA fillers. Practitioners from South Korea (33%), Thailand (20%), and Taiwan (15%) made up 68% of the survey participants, whereas those from the Philippines (7%), Australia (6%), Indonesia (6%), Hong Kong (5%), Singapore (4%), Malaysia (2%), and New Zealand (2%) constituted the remaining 32%. The majority were aesthetic general physicians (64%) and the rest included dermatologists (27%), plastic surgeons (8%), and non-physician practitioners (1%).
Practitioners’ Views and Practices Pertaining to DIRs
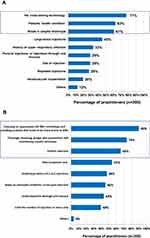
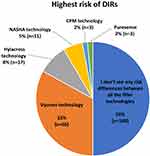
Close to three-quarters (74%) of the practitioners reported having encountered patients who had DIRs.7 When asked to rate the incidence of DIRs in their practice, 12% rated DIRs as common (1:100 patients), whereas 88% rated the incidence as low (1:1000 patients) or extremely low (<1:1000 patients). HA cross-linking technology, patients’ health condition, and break in aseptic technique were identified as the top three risk factors for DIRs (Figure 1A). Choosing an appropriate HA filler technology and avoiding products that seem to be more prone to DIRs, aseptic technique, and patient selection were the top three measures to reduce the risk of DIRs (Figure 1B). About half (52%) viewed HA fillers with a higher composition of low molecular weight-hyaluronic acid (LMW-HA) as associated with a higher risk of DIRs (Figure 2). Half of the practitioners indicated they do not perceive any difference in the risk of DIRs between different HA filler technologies. Of those who did, two-thirds perceived Vycross® technology to have the highest risk of DIRs (Figure 3).
In terms of the strategies used to manage these delayed reactions, broad spectrum antibiotics, oral corticosteroids, and intralesional hyaluronidase were most frequently used as early treatment options, whereas surgical excision was used as a last resort treatment (Figure 4).
 |
Figure 4 Strategies employed by practitioners to manage DIRs. Abbreviations: DIRs, delayed inflammatory reactions; 5-FU, fluorouracil; NSAIDs, nonsteroidal anti-inflammatory drugs. |
Practitioners’ Views and Practices Pertaining to Natural Outcomes
Nearly all practitioners indicated it is very important (92%) or rather important (5%) to create a natural outcome for their patients. Practitioners viewed overfilling (90%), surface irregularities (88%), bumps/nodules (86%), disproportionate face (81%), and distorted appearance (79%) as unnatural outcomes. They cited poor technique (such as large bolus injections and wrong layer of injection), and the use of HA fillers that do not integrate well into the underlying tissue as the main causes of unnatural outcomes in treatment with HA filler (Figure 5A). When were asked about the treatment strategies used to achieve natural outcomes with HA fillers, treatment customization, understanding the rheological properties and behavior of different HA fillers, and being conservative in treatment approach were the key strategies reported (Figure 5B).
Panel Perspectives
Overall, the panel shared that the views and practices of practitioners in Asia Pacific are largely in line with their experience. The panel added that although choosing an appropriate HA dermal filler and ensuring strict adherence to aseptic technique are important measures for minimizing the risk of DIRs, it is also essential for practitioners to have an adequate knowledge of facial anatomy, and to use proper injection techniques. Collectively, these measures are fundamental for achieving safe and natural outcomes for patients.5,8–11
The survey revealed limited awareness of differential immunogenicity between different HA fillers and the relevant influencing factors among practitioners in Asia Pacific despite published evidence.12–18 Although practitioners recognized the importance of choosing HA fillers that are less prone to DIRs for treatment, close to half did not perceive high composition of LMW-HA as relating to higher risk of DIRs and half did not seem to be aware of any difference in the risk of DIRs between different HA filler technologies (Figures 1B, 2 and 3). The panel acknowledged the need for educational efforts to improve knowledge on the different HA filler technologies and their associated risk of DIRs among practitioners in Asia Pacific to support them in making informed decisions when selecting an appropriate HA filler for their patients.
Consensus and Practical Approaches to Achieving Safe and Natural-Looking Results with HA Dermal Fillers
The panel shared their experiences and expertise with HA dermal filler treatment, and established a consensus for the safe and proper use of HA fillers. The consensus statements (Table 1)12–19 and the panel’s recommendations relating to HA filler treatment (described in the sections below) are summarized into a concise reference guide (Figure 6). The guide is not intended to provide detailed instructions on how to prevent DIRs or attain natural-looking results, which are already available in published literature,5,6,8–11,20,21 but rather to highlight practical considerations to support practitioners in customizing their treatment plans based on the unique conditions and needs of the individual patient.
 |
Table 1 Consensus Statements on HA Dermal Filler Treatment Pertaining to DIRs and Natural Outcomes |
 |
Figure 6 Practical considerations for creating an individualized treatment plan to achieve safe and natural-looking results. Abbreviation: HA, hyaluronic acid. |
Consensus on HA Dermal Filler Treatment Pertaining to DIRs
The panel expressed high level of agreement with statements 1–6 (Table 1). They recognized that most practitioners will encounter DIRs in their practice and pointed out that it is important for practitioners to adopt a proactive approach to avoid these complications, and be able to recognize and manage them when they do occur. The panel noted three important risk factors for DIRs—fillers with higher immunogenic potential, break in aseptic technique, and poor injection technique. In addition, the panel acknowledged that any condition or procedure that can cause a systemic or localized immune response such as known ongoing infections, dental procedures, vaccinations, etc, can also trigger the onset of DIRs. It was recommended that practitioners consider patients’ medical history and other medical procedures when creating a treatment plan for their patients8–10 (Figure 6). In the case of patients with an ongoing infection, filler treatment should be deferred until the condition is resolved. For patients who have other medical procedures such as dental procedures, vaccinations (including COVID-19 vaccinations), etc, it was recommended that such procedures be performed at least 2–4 weeks before or after filler treatment to minimize the risk of complications. The development of a DIR following COVID-19 vaccinations has been postulated to occur via the inactivation of angiotensin-converting enzyme receptor 2 (ACE2). Published case reports suggest the use of ACE inhibitors as a possible alternative to oral steroids for managing the reaction should it arise.22,23 The panel concurred with the recommendations from the ASDS guidance that was issued in response to reports of COVID-19 mRNA vaccine-related adverse events in patients with dermal filler.19 The majority of the panel members agreed that the risk of developing DIRs following filler treatment is rare. Hence, there was a strong consensus that such potential complications should not hinder patients from taking vaccines (including COVID-19 vaccines).
The panel pointed out that although HA fillers are generally well tolerated and have a favorable safety profile, there is differential immunogenicity between different HA fillers, which is influenced by several factors including the molecular weight of HA, crosslinking and manufacturing technologies, etc.24 The incidence of DIRs to HA filler treatments has been reported to range from around 0%–4.3% across different HA filler products.16,17 Hence, the panel recommended that practitioners be aware of the immunogenicity of different HA fillers and the influencing factors when selecting an appropriate filler for their patients. Reports have shown that HA dermal fillers with a higher composition of LMW-HA (<1000 kDa) are associated with a higher risk of DIRs.12,13 Published evidence suggests increased incidence of DIRs with Vycross® technology HA dermal fillers.12,16–18 One hypothesis for the greater inflammatory response relates to the high composition of LMW-HA in Vycross® fillers, which is proinflammatory and could trigger DIRs.12,14–18 Hence, it was recommended that practitioners consider a filler product containing a lower percentage of LMW-HA for treatment (Table 1).
The panel recommended three measures that are fundamental for avoiding DIRs—choosing an appropriate HA dermal filler with low immunogenic potential, ensuring strict adherence to aseptic technique, and using proper injection technique which requires an adequate knowledge of facial anatomy, in particular of deep spaces, different fat compartments, and muscle–bone relationship8–10 (Figure 6) (Table 1). The panel stressed that strict adherence to aseptic technique is important for minimizing the risk of DIRs. Injections can increase the chance of bacteria passing through the skin and causing a biofilm infection, which can induce the development of chronic nodules and granulomatous inflammation.25 Hence, it was recommended that patients’ skin be thoroughly cleaned and disinfected before injection. Aseptic technique should be adhered to throughout the procedure and practitioners should exercise continuous vigilance against possible contamination. Patients should be advised to delay putting on makeup and avoid touching the treated area for at least 12 hours after injection. The panel also addressed the importance of proper injection technique to avoid DIRs. Due to high numbers of bacteria in the perioral areas, practitioners are advised to take additional precaution when injecting these areas, and avoid injecting through the oral mucosa. Studies show that large bolus filler injections can trigger the occurrence of DIRs, and multiple injections can increase the risk of bacterial contamination,25,26 hence the panel recommended limiting filler volume and the number of injection sites, according to patient’s aesthetic needs, treatment area, and product selected. In line with published guidance,5,8 the panel pointed out that it is pertinent for practitioners to have a thorough knowledge of facial anatomy and a clear understanding of the appropriate depth and plane of injection. It is also important to understand how each filler intercalates with the skin at different depths.27–32 For example, what can be placed superficially and what will easily intersperse with fat in areas like the mid face.
The panel recognized that DIRs can present as a spectrum of clinical manifestations with varying etiology that require distinct treatments (Table 1). It was recommended that practitioners first assess patients to differentiate DIRs from non-inflammatory nodules. The development of DIR-related nodules can be attributed to biofilm and granulomatous reactions.25 The panel agreed that the strategies for treating DIRs can include broad-spectrum oral antibiotics, oral steroids, and/or intralesional hyaluronidase, with surgical excision used as a last resort.5,7,9,33,34 Oral antibiotics should be prescribed first, before oral steroids or intralesional hyaluronidase are considered. For severe DIRs, the majority of the panel recommended using a combination of broad-spectrum oral antibiotics, intralesional hyaluronidase, and oral steroids to manage the symptoms. However, a few panel members noted that they prefer to separate the use of oral antibiotics and oral steroids to distinguish pure hypersensitivity reaction from bacterial biofilm. For recalcitrant DIRs, it was agreed that intralesional hyaluronidase alone or in combination with 5-FU can be used for treatment. Triple combination including intralesional hyaluronidase, 5-FU, and triamcinolone can also be used, with surgical extraction reserved as a last resort. The panel recommended using ultrasonography to determine the location of the injected filler and to guide the precise delivery of hyaluronidase.35 The panel recommended treatment with broad-spectrum antibiotics for a week. Treatment duration with oral steroids is recommended to be between 1 and 2 weeks, depending on the severity. If intralesional steroid is administered, it is recommended to be given at every 3–4 weekly interval. Most of the panel members recommended weekly intralesional hyaluronidase injection.
Consensus on HA Dermal Filler Treatment Pertaining to Natural Outcomes
The panel agreed to define unnatural outcomes as overfilled appearance, surface irregularities, bumps/nodules, disproportionate face, and distorted appearance that manifest after filler treatment (Table 1). Considering patients can be distressed with such unpleasant outcomes and the potential risk of permanent sequelae,5,6 the panel stressed the importance for practitioners to adopt proactive strategies to achieve natural outcomes for their patients when using HA fillers in aesthetic procedures. They noted two key causes of unnatural outcomes—poor technique and inappropriate selection of HA fillers—which practitioners should be aware of.5,6
The panel recommended three important measures that practitioners should adopt for achieving aesthetically pleasant outcomes for their patients: choosing an appropriate HA dermal filler, having adequate knowledge of facial anatomy, and using proper injection technique5,8–11 (Figure 6). The panel stressed that filler treatment plans should be individualized, respecting the patient’s unique anatomical structure and avoiding a one-size-fits-all treatment approach, which can produce unnatural outcomes (Table 1). When choosing a HA dermal filler for treatment, the panel advised prioritizing product safety and effectiveness in achieving natural outcomes over durability of the filler product. Use of incorrect products for an indication may result in noninflammatory nodules.8 Hence, it is recommended that practitioners have a good understanding of the rheological properties and behavior of different HA fillers to aid them in selecting the appropriate product to address the unique aesthetic needs of the individual patient. The panel recommended selecting products that have better tissue integration for more natural results. The panel added that it is essential for practitioners to have detailed knowledge of facial anatomy, and a thorough understanding of the appropriate depth and plane of injection to ensure correct placement of the product into the appropriate tissue layer.8 The panel also addressed the importance of proper injection technique for achieving natural results. It is recommended to limit filler volume and inject slowly to introduce the product gently and evenly.8 The panel advised physicians to take a conservative approach to undercorrect to avoid an overfilled appearance.11,20 It is noted that while drastic results may be desired by patients, it is important to increase awareness on the possibility of irreversible distortion of the original tissue structure and facial contours with overcorrection.6 The panel acknowledged the need for educational efforts to improve understanding on the importance of preserving aesthetic individuality and proper injection procedures for optimal results.
Conclusions
This is the first report describing the current practices of HA dermal filler treatment in Asia Pacific on preventing DIRs and achieving natural-looking outcomes. The report also includes a concise reference guide for creating an individualized treatment plan aimed at minimizing the risk of DIRs while achieving natural aesthetic outcomes with HA fillers. More clinical evidence may still be needed to support the recommendations for the prevention of DIRs and unnatural results. Nonetheless, the current findings from the survey and the practical insights from the experts can raise awareness of these complications in clinical practice and support practitioners in optimizing the safety and quality of aesthetic treatment with HA fillers.
Acknowledgments
Medical writing and editorial support were provided by Tech Observer Asia Pacific Pte Ltd and was funded by Merz Aesthetics.
Disclosure
Dr Corduff serves as a lecturer and clinical advisor for Merz Aesthetics. Dr Lim, Dr Suwanchinda, Dr Tseng, and Dr Ho serve as speakers and advisory board members for Merz Aesthetics. Dr Lin serves as a speaker and advisory board member for Merz Aesthetics, BTL, and AbbVie. Dr Pavicic serves as a speaker and a member of advisory boards for Merz Aesthetic, AAT, J&J, and BTL, as well as conducts clinical studies for Merz Aesthetics, LG, AAT, Galderma, and AbbVie. Dr Quiambao has received honoraria from Merz Aesthetics and Teoxane Laboratories and serves as a speaker and regional advisory board member for Merz Aesthetics. Dr Siew serves as a trainer and lecturer for Merz Aesthetic. Dr Vachiramon serves as a speaker for Merz Aesthetic, LG Chem, Leo Pharma, and Biersdorf, L’Oreal, as well as a member in advisory boards for Merz Aesthetic, AbbVie, and L’Oreal. All authors have received honoraria from Merz Aesthetics for their contributions at the advisory board meeting and subsequent manuscript preparation.
The authors report no other conflicts of interest in this work.
References
1. McKee D, Remington K, Swift A, Lambros V, Comstock J, Lalonde D. Effective rejuvenation with hyaluronic acid fillers: current advanced concepts. Plast Reconstr Surg. 2019;143:1277e–1289e.
2. Gold MH. Use of hyaluronic acid fillers for the treatment of the aging face. Clin Interv Aging. 2007;2:369–376.
3. Jung YR, Hwang C, Ha JM, et al. Hyaluronic acid decreases lipid synthesis in sebaceous glands. J Invest Dermatol. 2017;137:1215–1222.
4. Prasetyo AD, Prager W, Rubin MG, Moretti EA, Nikolis A. Hyaluronic acid fillers with cohesive polydensified matrix for soft-tissue augmentation and rejuvenation: a literature review. Clin Cosmet Investig Dermatol. 2016;9:257–280.
5. Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295–316.
6. Lim TS Facial overfilled syndrome complications of inappropriate filler delivery; 2018. Available from: https://www.prime-journal.com/facial-overfilled-syndrome-complications-of-inappropriate-filler-delivery/.
7. Artzi O, Cohen JL, Dover JS, et al. Delayed inflammatory reactions to hyaluronic acid fillers: a literature review and proposed treatment algorithm. Clin Cosmet Investig Dermatol. 2020;13:371–378.
8. De Boulle K, Heydenrych I. Patient factors influencing dermal filler complications: prevention, assessment, and treatment. Clin Cosmet Investig Dermatol. 2015;8:205–214.
9. Heydenrych I, Kapoor KM, De Boulle K, et al. A 10-point plan for avoiding hyaluronic acid dermal filler-related complications during facial aesthetic procedures and algorithms for management. Clin Cosmet Investig Dermatol. 2018;11:603–611.
10. Philipp-Dormston WG, Goodman GJ, De Boulle K, et al. Global approaches to the prevention and management of delayed-onset adverse reactions with hyaluronic acid-based fillers. Plast Reconstr Surg Glob Open. 2020;8:e2730.
11. Rho NK, Chang YY, Chao YY, et al. Consensus recommendations for optimal augmentation of the Asian face with hyaluronic acid and calcium hydroxylapatite fillers. Plast Reconstr Surg. 2015;136:940–956.
12. Beleznay K, Carruthers JD, Carruthers A, Mummert ME, Humphrey S. Delayed-onset nodules secondary to a smooth cohesive 20 mg/mL hyaluronic acid filler: cause and management. Dermatol Surg. 2015;41:929–939.
13. Pérez-Pérez L, García-Gavín J, Wortsman X, Santos-Briz Á. Delayed adverse subcutaneous reaction to a new family of hyaluronic acid dermal fillers with clinical, ultrasound, and histologic correlation. Dermatol Surg. 2017;43:605–608.
14. Farwick M, Gauglitz G, Pavicic T, et al. Fifty-kDa hyaluronic acid upregulates some epidermal genes without changing TNF-alpha expression in reconstituted epidermis. Skin Pharmacol Physiol. 2011;24:210–217.
15. Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–461.
16. Artzi O, Loizides C, Verner I, Landau M. Resistant and recurrent late reaction to hyaluronic acid-based gel. Dermatol Surg. 2016;42:31–37.
17. Sadeghpour M, Quatrano NA, Bonati LM, Arndt KA, Dover JS, Kaminer MS. Delayed-onset nodules to differentially crosslinked hyaluronic acids: comparative incidence and risk assessment. Dermatol Surg. 2019;45:1085–1094.
18. Povolotskiy R, Oleck NC, Hatzis CM, Paskhover B. Adverse events associated with aesthetic dermal fillers: a 10-year retrospective study of FDA data. Am J Cosmetic Surg. 2018;35:143–151.
19. American Society for Dermatologic Surgery. Guidance regarding SARS-CoV-2 mRNA vaccine side effects in dermal filler patients; 2020. Available from: https://www.asds.net/Portals/0/PDF/secure/ASDS-SARS-CoV-2-Vaccine-Guidance.pdf.
20. van Loghem J, Sattler S, Casabona G, et al. Consensus on the use of hyaluronic acid fillers from the cohesive polydensified matrix range: best practice in specific facial indications. Clin Cosmet Investig Dermatol. 2021;14:1175–1199.
21. Lim T, Frank K, Hadjab B. Target-specific sandwich technique: facial rejuvenation leveraging CPM technology. J Cosmet Dermatol. 2021;00:1–13.
22. Sloan B. Lisinopril for delayed inflammatory responses to hyaluronic acid fillers after COVID-19 vaccinations. J Am Acad Dermatol. 2021;85:34.
23. Munavalli GG, Knutsen-Larson S, Lupo MP, Geronemus RG. Oral angiotensin-converting enzyme inhibitors for treatment of delayed inflammatory reaction to dermal hyaluronic acid fillers following COVID-19 vaccination-a model for inhibition of angiotensin II-induced cutaneous inflammation. JAAD Case Reports. 2021;10:63–68.
24. Owczarczyk-Saczonek A, Zdanowska N, Wygonowska E, Placek W. The immunogenicity of hyaluronic fillers and its consequences. Clin Cosmet Investig Dermatol. 2021;14:921–934.
25. Saththianathan M, Johani K, Taylor A, et al. The role of bacterial biofilm in adverse soft-tissue filler reactions: a combined laboratory and clinical study. Plast Reconstr Surg. 2017;139:613–621.
26. Glogau RG, Kane MA. Effect of injection techniques on the rate of local adverse events in patients implanted with nonanimal hyaluronic acid gel dermal fillers. Dermatol Surg. 2008;34(Suppl 1):S105–9.
27. Flynn TC, Sarazin D, Bezzola A, Terrani C, Micheels P. Comparative histology of intradermal implantation of mono and biphasic hyaluronic acid fillers. Dermatol Surg. 2011;37:637–643.
28. Goh AS, Kohn JC, Rootman DB, Lin JL, Goldberg RA. Hyaluronic acid gel distribution pattern in periocular area with high-resolution ultrasound imaging. Aesthet Surg J. 2014;34:510–515.
29. Micheels P, Besse S, Flynn TC, Sarazin D, Elbaz Y. Superficial dermal injection of hyaluronic acid soft tissue fillers: comparative ultrasound study. Dermatol Surg. 2012;38:1162–1169.
30. Micheels P, Besse S, Sarazin D. Two Crosslinking technologies for superficial reticular dermis injection: a comparative ultrasound and histologic study. J Clin Aesthet Dermatol. 2017;10:29–36.
31. Micheels P, Besse S, Sarazin D, et al. Ultrasound and histologic examination after subcutaneous injection of two volumizing hyaluronic acid fillers: a preliminary study. Plast Reconstr Surg Glob Open. 2017;5:e1222.
32. Tran C, Carraux P, Micheels P, Kaya G, Salomon D. In vivo bio-integration of three hyaluronic acid fillers in human skin: a histological study. Dermatology. 2014;228:47–54.
33. Shalmon D, Cohen JL, Landau M, Verner I, Sprecher E, Artzi O. Management patterns of delayed inflammatory reactions to hyaluronic acid dermal fillers: an online survey in Israel. Clin Cosmet Investig Dermatol. 2020;13:345–349.
34. Urdiales-Gálvez F, Delgado NE, Figueiredo V, et al. Treatment of soft tissue filler complications: expert consensus recommendations. Aesthetic Plast Surg. 2018;42:498–510.
35. Schelke LW, Decates TS, Velthuis PJ. Ultrasound to improve the safety of hyaluronic acid filler treatments. J Cosmet Dermatol. 2018;17:1019–1024.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.