Back to Journals » Open Access Rheumatology: Research and Reviews » Volume 11
Current Perspectives On Emerging Biomarkers For Rheumatoid Arthritis-Associated Interstitial Lung Disease
Authors Amigues I , Ramadurai D, Swigris JJ
Received 27 February 2019
Accepted for publication 24 September 2019
Published 15 October 2019 Volume 2019:11 Pages 229—235
DOI https://doi.org/10.2147/OARRR.S166070
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Chuan-Ju Liu
Isabelle Amigues,1 Deepa Ramadurai,2 Jeffrey J Swigris3
1Division of Rheumatology, National Jewish Health, Denver, CO, USA; 2Department of Medicine, University of Colorado Anschutz Medical Campus, Aurora, CO, USA; 3Division of Pulmonary, Critical Care and Sleep Medicine, Interstitial Lung Disease Program, National Jewish Health, Denver, CO, USA
Correspondence: Jeffrey J Swigris
National Jewish Health, 1400 Jackson Street, Denver, CO 80206, USA
Email [email protected]
Abstract: Rheumatoid arthritis (RA) is a common systemic autoimmune disease whose fibro-inflammatory manifestations may affect a number of tissues and organs, including the lungs. In fact, interstitial lung disease (ILD) is a leading cause of mortality among patients with RA. RA-related interstitial lung disease (RA-ILD) most often presents in an injury pattern called usual interstitial pneumonia (UIP), which portends a relatively worse prognosis than other less commonly occurring patterns of RA-ILD, like non-specific interstitial pneumonia (NSIP). Biomarkers from serum or bronchoalveolar lavage fluid could aid in the identification of patients at risk for RA-ILD, the detection of patients most likely to develop the UIP pattern of RA-ILD, and the prediction of disease behaviour over time. Notably, the use of highly sensitive serologic biomarkers, including rheumatoid factor (RF) and antibodies targeting cyclic citrullinated peptides, while somewhat specific for RA joint disease, have only limited utility as biomarkers for RA-ILD. Candidate biomarkers for RA-ILD include these and other autoantibodies as well as certain genes and molecules that hold promise as biomarkers in other forms of ILD. In this manuscript, we summarize the state of knowledge on biomarkers for the development and progression of RA-ILD.
Keywords: rheumatoid arthritis, interstitial lung disease, pulmonary fibrosis, usual interstitial pneumonia, biomarker, antibody
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease that afflicts approximately 1% of the US population.1 Although RA principally affects the joints, causing symmetric pain, stiffness, swelling, and limited motion and function of multiple joints, its fibro-inflammatory manifestations may develop in other organs. RA is complicated by lung manifestations, such as interstitial lung disease (ILD), in up to 60% of patients with RA;2,3 however, clinically significant RA-related ILD (RA-ILD) is believed to occur in about 10%.4 The factors that drive the development or progression of clinically significant ILD in patients with RA are poorly understood. While overall survival in RA has improved dramatically in the last 20 years, in part due to earlier diagnosis and in part from more effective therapies for inflammatory arthritis, the respiratory manifestations, and particularly ILD, have become the leading cause of mortality in patients with RA.5
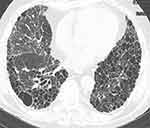
Major subtypes of RA-ILD are defined by their histopathological and/or high-resolution computed tomography (HRCT) patterns. Usual interstitial pneumonia (UIP) (characterized on high-resolution computed tomography (HRCT) by predominantly basal, subpleural, and patchy honeycombing, reticular opacities, and traction bronchiectasis without nodularity, consolidation or extensive ground glass opacities) is the most common subtype,6 and it carries a poor prognosis (Figure 1).7 Nonspecific interstitial pneumonia (NSIP), cryptogenic organizing pneumonia (COP), and acute interstitial pneumonia (AIP) are other subtypes of RA-ILD that are seen with far less frequency than UIP.
Although there is much to learn about the pathophysiology of RA-ILD, it is commonly thought that the systemic autoimmune process activates the lung’s molecular pathways, including certain cytokines, chemokines, and growth factors that drive aberrant wound healing mechanisms, including differentiation and proliferation of fibroblasts, increased synthesis and deposition of extracellular matrix (ECM) and increased activity of matrix metalloproteinases (MMP), that ultimately result in ILD.8 Fibroblasts appear to play a somewhat similar role in the pathogenesis of synovitis. Whether and how autoantibodies that target citrullinated proteins are directly involved in the pathogenesis of RA or RA-ILD is not entirely clear, but interestingly, in one study, investigators showed that citrullinated vimentin peptides were present in tissue samples from the lungs of certain patients with RA and synovial biopsies from other patients with RA.9
Early diagnosis of RA-ILD is important so that treatment and necessary surveillance can be initiated. No controlled trials of therapy for RA-ILD have been completed; however, analyses suggest certain drugs may be safe and effective for patients with RA-ILD. For example, in a retrospective study of 700 patients with RA, rituximab was well-tolerated and associated with stabilization or improvement of pulmonary function tests in the 56-subject subgroup with RA-ILD.10 Anti-fibrotic drugs that have been approved for the treatment of idiopathic pulmonary fibrosis (IPF) – a condition with many features that overlap with RA-ILD; namely, the UIP pattern of fibrosis – may hold promise for patients with RA-ILD,11 and at the time of writing, there is a multi-national, placebo-controlled trial of pirfenidone enrolling subjects with RA-ILD (NCT02808871).
The World Health Organization (WHO), in coordination with the United Nations and the International Labor Organization, has defined a biomarker as “any substance, structure, or process that can be measured in the body or its products and influence or predict the incidence of outcome or disease”. Thus, the first step in biomarker identification and analysis is to confirm that a candidate biomarker is associated with the clinical condition of interest. The second, and possibly most important step, is to confirm the robustness of the association and determine what clinically meaningful information it provides that is otherwise not readily available. Ultimately, a biomarker should influence the health outcome for the patient in a meaningful way.
Finding valid biomarkers for RA-ILD could help identify patients at risk, promote earlier diagnosis, predict the subtype of RA-ILD most likely to develop, forecast disease behaviour over time and guide appropriate surveillance and therapeutic strategies. A number of candidate biomarkers have already been identified (Table 1), and research is ongoing to assess their performance and to identify additional candidates. In this manuscript, we summarize the state of knowledge on biomarkers for RA-ILD.
 |
Table 1 Summary Of Biomarkers In RA-ILD, Known And Experimental |
Biomarkers For RA-ILD
Commercially Available Serological Biomarkers
Although the European League Against Rheumatism (EULAR)/American College of Rheumatology (ACR) 2010 criteria for RA were specifically developed to “facilitate the study of persons at earlier stages of the disease”, they are typically used to establish a clinical diagnosis of RA.12 These criteria include the presence of at least one swollen joint (synovitis), the absence of an alternative diagnosis to explain the symptoms, and a total score of ≥6 from four different domains; joints involved, serologic abnormality, elevated acute phase reactants, and symptom duration.12 The EULAR/ACR 2010 criteria for RA include two serologic biomarkers: rheumatoid factor (RF) and anti-citrullinated peptide antibodies (ACPA).
In patients with RA, a diagnosis of RA-ILD can usually be made by taking a thorough history (to rule out other causes for ILD) and integrating clinical data, most importantly, findings on HRCT and pulmonary function tests (PFTs), ideally within a multidisciplinary discussion among pulmonology, radiology, and rheumatology.13–15 It is well-known that patients with RA have a preclinical period of autoimmunity during which RA-related autoantibodies, including RF and/or ACPA, are present in the blood before clinically evident synovitis.12 Confusion arises in the not infrequent situation when patients present with ILD, are found to have serologic markers for RA – but no articular disease. Some such patients will eventually develop characteristic RA joint disease, occasionally several years after ILD is diagnosed; in retrospect, they had preclinical RA, although an argument could be made that they had RA all along, and ILD was initially its sole manifestation. Other patients with ILD and RF or ACPA positivity never develop joint disease, and thus, are never diagnosed with RA.16,17 Multiple studies have demonstrated higher tiers of RF and ACPA in patients who had RA-ILD versus those without ILD, suggesting these antibodies could have some utility as biomarkers and may contribute to the pathophysiology of the associated ILD.18–20
Rheumatoid Factor
The rheumatoid factor (RF) is an antibody directed at the Fc domain of IgG molecules and was one of the first serologic biomarkers discovered for RA. It has low specificity for RA: it occurs in other autoimmune diseases, chronic infections such as hepatitis C, and in 15% of the general population, particularly in ever-smokers.21 Nevertheless, seropositivity for RF is a risk factor for the development of RA-ILD,22 and elevated levels of RF in the serum, sputum, and bronchoalveolar lavage fluid (BALF) appear to be modestly associated with RA-ILD.23–25
ACPA (Anti-Citrullinated Protein Antibodies)
Precisely how RA develops is unknown. Protein citrullination, a form of post-translational modification, appears to be a cornerstone in the pathogenic process leading to RA, and it is tightly linked with autoimmunity in RA. Several autoantibodies that target cyclic citrullinated peptides (CCP) have been described in RA, including autoantibodies to citrullinated vimentin, fibrinogen, type II collagen, histone and enolases. Although there is much to learn about the pathogenic roles of ACPA, their presence in BALF and sputum23 suggests that the lungs may be an antigenic source for ACPA production. Compared with RF, ACPA are more commonly present in the preclinical or early stages of RA.
There is growing evidence that ACPA contribute directly to the pathogenesis of joint disease in RA through direct targeting of synovial antigens.24,25 ACPA may be pathogenic in the lungs as well. Among 252 patients with RA, high ACPA levels were associated with a 1.5-fold increase in the odds of lung disease as defined by HRCT.26 Seropositivity for ACPA, particularly among ever-smokers, is associated with subclinical evidence of ILD in community-dwelling adults.27 Whether such patients should be regarded as having RA (with no joint involvement – and ILD as the sole manifestation) or simply considered at risk for RA, or whether, in these patients, ACPA are generated as a non-specific response to lung injury, is entirely unclear. Related autoantibodies, namely anti-citrullinated alpha enolase peptide-1 (anti-CEP-1) antibodies are present in 40% of patients with RA and may be a marker of erosive joint disease and RA-ILD.28
Commercially available ACPA assays have undergone several modifications over the past decade. These have included changes to the antigens and the ability to detect IgA ACPA (historically, assays only identified IgG antibodies). Compared to IgA-ACPA, IgG-ACPA positivity is more strongly associated with RA joint disease.29 But, commercially available tests for ACPA do not distinguish whether IgA, IgG or both are detected. In recently completed but as yet unpublished work, our group found that serum ACPA positivity, particularly for IgA-ACPA, is present in up to 30% of patients with idiopathic pulmonary fibrosis (IPF). IPF is one of the most common forms of ILD; by definition, patients with IPF do not have RA (any other systemic autoimmune disease, or any known cause for pulmonary fibrosis), but it is interesting that like the majority of patients with RA-ILD, UIP is the injury pattern in IPF.
There is much to unravel about ACPA, like whether the IgA isoform of ACPA is specific for lung disease, or whether the development of joint inflammation characteristic of RA (according to EULAR/ACR criteria) in a patient with pre-existing ILD requires transition from IgA- to IgG-ACPA. Among all-comers from the general population without synovitis on physical exam, serum ACPA positivity is predictive of developing classifiable RA in the future.30,31 Our unpublished data suggest 10% of patients with IPF and ACPA positivity develop RA joint disease within three years of the identification of positive serology. In summary, it appears RF and ACPA are sensitive for RA and RA-ILD. However, neither is specific, so although many data are intriguing, neither possesses the performance characteristics of an excellent biomarker for RA-ILD.
New Serological Biomarkers
Other serologic biomarkers have been studied in patients with RA-ILD, but at present, none have large-scale, robust data to prove their utility in clinical practice. Given the similarities between IPF and RA-ILD, including them sharing the same injury pattern and risk factor (smoking), ivestigators have begun to examine whether molecultes that hold promise as biomarkers in IPF might also be useful in RA-ILD. What follows is a discussion about some of the most promising candidates.
Markers Of Diagnostic Utility
Harlow and colleagues reported the association between RA-ILD and serum autoantibodies that target citrullinated heat shock protein 90 alpha (Hsp90α) or beta (Hsp90β). These antibodies were 90% specific for RA-ILD, and were not found in patients with IPF or in healthy controls.32 HSP90 has also been found in BALF of patients with RA-ILD.
Chen and colleagues found that serum levels of matrix metalloproteinase (MMP)-7 and C-X-C motif chemokine 10 (CXCL10) were elevated in patients with RA-ILD and not in patients who had RA without ILD.33 Doyle and co-investigators evaluated a biomarker signature composed of MMP-7, pulmonary and activation-regulation chemokine, and surfactant protein D (SP-D) that improved detection of subclinical and clinically overt RA-ILD.34
Fu and colleages found that compared to healthy controls, lysyl oxidase-like 2 (LOXL2) levels were higher in subjects with RA (whether they had ILD or not).35 There was no difference in LOXL2 levels between subjects with RA-ILD and those with RA but no ILD. Among subjects with RA-ILD, LOXL2 levels were significantly higher in those with ILD for less than three months compared to those with ILD longer than three months.
The gain-of-function MUC5B promoter variant rs35705950 is not only the strongest genetic risk factor for IPF,36 it has been associated with RA-ILD and may play a role in its development.37 Specifically, the odds of having the MUC5B promoter variant were over three-fold greater among patients with RA-ILD than patients with RA but no ILD (95% CI, 1.8 to 5.4; P=7.4×10−5). The association was even stronger when RA-ILD was confined to subjects with either UIP or “possible” UIP on HRCT (odds ratio=6.1, 95% CI, 2.9 to 13.1; P=2.5×10−6).37 Notably, the MUC5B promoter variant was not a risk factor for RA itself. Whether the MUC5B promoter variant is associated with progression of RA-ILD is unknown, but studies are ongoing.
Biomarkers That Predict Disease Progression
Peptidylarginine deiminases (PAD) are enzymes that promote citrullination. Five PAD isotypes exist; each has its own tissue-specific expression profiles. PAD2 and PAD4 have been linked to RA pathogenesis: the enzymes have been detected in the synovial tissue of patients with RA, and they are believed to play a role in the generation of citrullinated autoantigens targeted by ACPAs. In certain circumstances, autoantibodies are formed against PAD.
While PAD enzyme expression and citrullinated proteins are elevated in the lungs of chronic smokers without RA, Cappelli and colleagues found that a smoking history was not associated with anti-PAD4 antibodies in patients with RA.38 Compared to patients with established RA (in whom anti-PAD4 antibodies may be associated with disease duration and ACPA positivity), anti-PAD4 antibodies are found less frequently in ACPA positive individuals without RA (ie, those putatively at risk for RA).38 This suggests anti-PAD4 antibodies may be amplifiers of disease severity rather than initiators of it. In one study, a subgroup of patients with severe (erosive) RA were found to have anti-PAD4 antibodies that cross-reacted with the related isoenzyme PAD3 (anti-PAD3/4XR).39 These anti-PAD3/4XR antibodies have been linked to ILD in RA patients, even after accounting for confounding factors such as smoking history.18
Krebs von den Lungen 6/MUC1 (KL-6) is a mucin-like glycoprotein, classified as MUC1, which has profibrotic and anti-apoptotic effects on lung fibroblasts. Serum KL-6 was found to be elevated in RA-ILD (8 of 9 patients in a study with “active pneumonitis” versus only 2 of 168 of patients without active pneumonitis)40 and may be particularly helpful for early detection of ILD progression. Serum KL-6 levels were shown to correlate with the severity of RA-ILD on computed tomography in 47 RA patients.41 In another study, Lee and colleagues utilized KL-6 to monitor patients with connective tissue disease-related ILD, including 41 patients with RA-ILD; KL-6 levels were higher in those with ILD than those without ILD.42 In a study looking at the use of tumor necrosis factor (TNF) inhibitors in RA-ILD, investigators observed that increasing KL-6 was a marker for worsening ILD.43
Some biomarkers used in patients with cancer (e.g., CA19-9, CA15-3, CA 125) have been found to be elevated in patients with RA-ILD compared to those with RA without ILD. For example, in one study of subjects with RA, elevated CA-125 was associated with a 6-fold increase in the odds of ILD.44 Serum anti-malondialdehyde-acetaldehyde (anti-MAA) antibodies (IgA and IgM) have also been found to be higher in patients with RA-ILD than patients with RA alone or COPD.45 Investigations suggest genetic mutations and/or polymorphisms may hold promise as useful biomarkers for RA-ILD.
In summary, like RF and ACPA, each of these candidate molecules has some data to suggest an association with RA-ILD. However, additional research is needed to clarify those associations and support validity if any is to be considered a clinically useful biomarker for RA-ILD.
Conclusion
There are multiple promising candidate serological, clinical and genetic biomarkers for RA-ILD, and yet as it stands, none have demonstrated acceptable feasibility or performance characteristics to reliably identify RA-ILD or predict its longitudinal behavior. More validation data are required, including those comparing RA-ILD patients to patients with other categories of fibrotic ILD. To advance a biomarker to clinical use, it must possess specificity for RA-ILD and should be easily obtainable and cost effective. The hope is that such biomarkers will identify patients at highest risk for RA-ILD and those most likely to progress. The suspicion is that the identification of biomarkers with excellent performance characteristics would advance understanding of the pathophysiology of RA-ILD and ultimately inform the development of targeted therapies for this devastating manifestation of RA.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gabriel SE, Crowson CS, Kremers HM, et al. Survival in rheumatoid arthritis: a population-based analysis of trends over 40 years. Arthritis Rheum. 2003;48(1):54–58. doi:10.1002/art.10705
2. Gabbay E, Tarala R, Will R, et al. Interstitial lung disease in recent onset rheumatoid arthritis. Am J Respir Crit Care Med. 1997;156(2 Pt 1):528–535. doi:10.1164/ajrccm.156.2.9609016
3. Yuksekkaya R, Celikyay F, Yilmaz A, et al. Pulmonary involvement in rheumatoid arthritis: multidetector computed tomography findings. Acta Radiol. 2013;54(10):1138–1149. doi:10.1177/0284185113491566
4. Olson AL, Swigris JJ, Sprunger DB, et al. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Respir Crit Care Med. 2011;183(3):372–378.
5. Sparks JA, Chang SC, Liao KP, et al. Rheumatoid arthritis and mortality among women during 36 years of prospective follow-up: results from the nurses’ health study. Arthritis Care Res (Hoboken). 2016;68(6):753–762. doi:10.1002/acr.22752
6. Tansey D, Wells AU, Colby TV, et al. Variations in histological patterns of interstitial pneumonia between connective tissue disorders and their relationship to prognosis. Histopathology. 2004;44(6):585–596. doi:10.1111/j.1365-2559.2004.01896.x
7. Solomon JJ, Ryu JH, Tazelaar HD, et al. Fibrosing interstitial pneumonia predicts survival in patients with rheumatoid arthritis-associated interstitial lung disease (RA-ILD). Respir Med. 2013;107(8):1247–1252. doi:10.1016/j.rmed.2013.05.002
8. Shaw M, Collins BF, Ho LA, Raghu G. Rheumatoid arthritis-associated lung disease. Eur Respir Rev. 2015;24(135):1–16. doi:10.1183/09059180.00008014
9. Ytterberg AJ, Joshua V, Reynisdottir G, et al. Shared immunological targets in the lungs and joints of patients with rheumatoid arthritis: identification and validation. Ann Rheum Dis. 2015;74(9):1772–1777. doi:10.1136/annrheumdis-2013-204912
10. Md Yusof MY, Kabia A, Darby M, et al. Effect of rituximab on the progression of rheumatoid arthritis-related interstitial lung disease: 10 years’ experience at a single centre. Rheumatology. 2017;56(8):1348–1357. doi:10.1093/rheumatology/kex072
11. Rosenbloom J, Mendoza FA, Jimenez SA. Strategies for anti-fibrotic therapies. Biochim Biophys Acta. 2013;1832(7):1088–1103. doi:10.1016/j.bbadis.2012.12.007
12. Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi:10.1002/art.27584
13. Assayag D, Elicker BM, Urbania TH, et al. Rheumatoid arthritis-associated interstitial lung disease: radiologic Identification of usual interstitial pneumonia pattern. Radiology. 2014;270(2):583–588. doi:10.1148/radiol.13130187
14. Hunninghake GW, Zimmerman MB, Schwartz DA, et al. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2001;164(2):193–196. doi:10.1164/ajrccm.164.2.2101090
15. Raghu G, Mageto YN, Lockhart D, Schmidt RA, Wood DE, Godwin JD. The accuracy of the clinical diagnosis of new-onset idiopathic pulmonary fibrosis and other interstitial lung disease: a prospective study. Chest. 1999;116(5):1168–1174. doi:10.1378/chest.116.5.1168
16. Fischer A, du Bois R. Interstitial lung disease in connective tissue disorders. Lancet. 2012;380(9842):689–698. doi:10.1016/S0140-6736(12)61079-4
17. Homma Y, Ohtsuka Y, Tanimura K, et al. Can interstitial pneumonia as the sole presentation of collagen vascular diseases be differentiated from idiopathic interstitial pneumonia? Respiration. 1995;62(5):248–251. doi:10.1159/000196457
18. Giles JT, Danoff SK, Sokolove J, et al. Association of fine specificity and repertoire expansion of anticitrullinated peptide antibodies with rheumatoid arthritis associated interstitial lung disease. Ann Rheum Dis. 2014;73(8):1487–1494. doi:10.1136/annrheumdis-2012-203160
19. Mori S, Koga Y, Sugimoto M. Different risk factors between interstitial lung disease and airway disease in rheumatoid arthritis. Respir Med. 2012;106(11):1591–1599. doi:10.1016/j.rmed.2012.07.006
20. Zhu J, Zhou Y, Chen X, Li J. A metaanalysis of the increased risk of rheumatoid arthritis-related pulmonary disease as a result of serum anticitrullinated protein antibody positivity. J Rheumatol. 2014;41(7):1282–1289. doi:10.3899/jrheum.131341
21. Wolfe F, Cathey MA, Roberts FK. The latex test revisited. Rheumatoid factor testing in 8,287 rheumatic disease patients. Arthritis Rheum. 1991;34(8):951–960. doi:10.1002/art.1780340804
22. Kelly CA, Saravanan V, Nisar M, et al. Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics–a large multicentre UK study. Rheumatology. 2014;53(9):1676–1682. doi:10.1093/rheumatology/keu165
23. Willis VC, Demoruelle MK, Derber LA, et al. Sputum autoantibodies in patients with established rheumatoid arthritis and subjects at risk of future clinically apparent disease. Arthritis Rheum. 2013;65(10):2545–2554. doi:10.1002/art.38066
24. Deane KD, Norris JM, Holers VM. Preclinical rheumatoid arthritis: identification, evaluation, and future directions for investigation. Rheum Dis Clin North Am. 2010;36(2):213–241. doi:10.1016/j.rdc.2010.02.001
25. Demoruelle MK, Deane K. Antibodies to citrullinated protein antigens (ACPAs): clinical and pathophysiologic significance. Curr Rheumatol Rep. 2011;13(5):421–430. doi:10.1007/s11926-011-0193-7
26. Aubart F, Crestani B, Nicaise-Roland P, et al. High levels of anti-cyclic citrullinated peptide autoantibodies are associated with co-occurrence of pulmonary diseases with rheumatoid arthritis. J Rheumatol. 2011;38(6):979–982. doi:10.3899/jrheum.101261
27. Bernstein EJ, Barr RG, Austin JHM, et al. Rheumatoid arthritis-associated autoantibodies and subclinical interstitial lung disease: the multi-ethnic study of atherosclerosis. Thorax. 2016;71(12):1082–1090. doi:10.1136/thoraxjnl-2016-208932
28. Alunno A, Bistoni O, Pratesi F, et al. Anti-citrullinated alpha enolase antibodies, interstitial lung disease and bone erosion in rheumatoid arthritis. Rheumatology. 2018;57(5):850–855. doi:10.1093/rheumatology/kex520
29. Kokkonen H, Mullazehi M, Berglin E, et al. Antibodies of IgG, IgA and IgM isotypes against cyclic citrullinated peptide precede the development of rheumatoid arthritis. Arthritis Res Ther. 2011;13(1):R13. doi:10.1186/ar3237
30. Fischer A, Solomon JJ. du Bois RM, et al. Lung disease with anti-CCP antibodies but not rheumatoid arthritis or connective tissue disease. Respir Med. 2012;106(7):1040–1047. doi:10.1016/j.rmed.2012.03.006
31. Majka DS, Deane KD, Parrish LA, et al. Duration of preclinical rheumatoid arthritis-related autoantibody positivity increases in subjects with older age at time of disease diagnosis. Ann Rheum Dis. 2008;67(6):801–807. doi:10.1136/ard.2007.076679
32. Harlow L, Gochuico BR, Rosas IO, et al. Anti-citrullinated heat shock protein 90 antibodies identified in bronchoalveolar lavage fluid are a marker of lung-specific immune responses. Clin Immunol. 2014;155(1):60–70. doi:10.1016/j.clim.2014.08.004
33. Chen J, Doyle TJ, Liu Y, et al. Biomarkers of rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheumatol. 2015;67(1):28–38. doi:10.1002/art.38904
34. Doyle TJ, Patel AS, Hatabu H, et al. Detection of rheumatoid arthritis-interstitial lung disease is enhanced by serum biomarkers. Am J Respir Crit Care Med. 2015;191(12):1403–1412. doi:10.1164/rccm.201411-1950OC
35. Fu Q, Bai Y, Liu Y, Zhou J, Zheng Y. The serum level and significance of lysyl oxidase-like 2 in patients with rheumatoid arthritis-associated interstitial lung disease. Clin Rheumatol. 2018;37(1):193–198. doi:10.1007/s10067-017-3878-0
36. Seibold MA, Wise AL, Speer MC, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364(16):1503–1512. doi:10.1056/NEJMoa1013660
37. Juge PA, Lee JS, Ebstein E, et al. MUC5B promoter variant and rheumatoid arthritis with interstitial lung disease. N Engl J Med. 2018;379(23):2209–2219. doi:10.1056/NEJMoa1801562
38. Cappelli LC, Konig MF, Gelber AC, Bingham CO
39. Darrah E, Giles JT, Ols ML, Bull HG, Andrade F, Rosen A. Erosive rheumatoid arthritis is associated with antibodies that activate PAD4 by increasing calcium sensitivity. Sci Transl Med. 2013;5(186):186ra165. doi:10.1126/scitranslmed.3005370
40. Oyama T, Kohno N, Yokoyama A, et al. Detection of interstitial pneumonitis in patients with rheumatoid arthritis by measuring circulating levels of KL-6, a human MUC1 mucin. Lung. 1997;175(6):379–385.
41. Kinoshita F, Hamano H, Harada H, et al. Role of KL-6 in evaluating the disease severity of rheumatoid lung disease: comparison with HRCT. Respir Med. 2004;98(11):1131–1137. doi:10.1016/j.rmed.2004.04.003
42. Lee JS, Lee EY, Ha YJ, Kang EH, Lee YJ, Song YW. Serum KL-6 levels reflect the severity of interstitial lung disease associated with connective tissue disease. Arthritis Res Ther. 2019;21(1):58. doi:10.1186/s13075-019-1835-9
43. Nakashita T, Ando K, Kaneko N, Takahashi K, Motojima S. Potential risk of TNF inhibitors on the progression of interstitial lung disease in patients with rheumatoid arthritis. BMJ Open. 2014;4(8):e005615. doi:10.1136/bmjopen-2014-005615
44. Wang T, Zheng XJ, Ji YL, Liang ZA, Liang BM. Tumour markers in rheumatoid arthritis-associated interstitial lung disease. Clin Exp Rheumatol. 2016;34(4):587–591.
45. England BR, Duryee MJ, Roul P, et al. Malondialdehyde-acetaldehyde adducts and antibody responses in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheumatol. 2019;71(9):1483–1493. doi:10.1002/art.40900
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.