Back to Journals » International Journal of Women's Health » Volume 12
Current Perspectives of Prenatal Sonographic Diagnosis and Clinical Management Challenges of Nuchal Cord(s)
Authors Sherer DM , Ward K , Bennett M , Dalloul M
Received 15 April 2020
Accepted for publication 17 July 2020
Published 10 August 2020 Volume 2020:12 Pages 613—631
DOI https://doi.org/10.2147/IJWH.S211124
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
David M Sherer, Kayana Ward, Michelle Bennett, Mudar Dalloul
Division of Maternal–Fetal Medicine, Department of Obstetrics and Gynecology, State University of New York (SUNY), Downstate Health Sciences University, Brooklyn, NY, USA
Correspondence: David M Sherer
Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, State University of New York (SUNY), Downstate Health Sciences University, 450 Clarkson Avenue, Box 24, Brooklyn, NY, USA
Tel +1 718 270-2081
Fax +1 718 270-4122
Email [email protected]
Abstract: Umbilical cord accidents preceding labor are uncommon. In contrast, nuchal cords are a very common finding at delivery, with reported incidences of a single nuchal cord of approximately between 20% and 35% of all singleton deliveries at term. Multiple loops occur less frequently, with reported incidence rates inverse to the number of nuchal cords involved. Rare cases of up to 10 loops of nuchal cord have been reported. While true knots of the umbilical cord have been associated with a 4– 10-fold increased risk of stillbirth, nuchal cord(s) are most often noted at delivery of non-hypoxic non-acidotic newborns, without any evidence of subsequent adverse neonatal outcome. Prior to ultrasound, nuchal cords were suspected clinically following subtle (spontaneous or evoked) electronic fetal heart rate changes. Prenatal sonographic diagnosis, initially limited to real-time gray-scale ultrasound, currently entails additional sonographic modalities, including color Doppler, power Doppler, and three-dimensional sonography, which have enabled increasingly more accurate prenatal sonographic diagnoses of nuchal cord(s). In contrast to true knots of the umbilical cord (which are often missed at sonography, reflecting the inability to visualize the entire umbilical cord, and hence are often incidental findings at delivery), nuchal cord(s), reflecting their well-defined and sonographically accessible anatomical location (the fetal neck), lend themselves with relative ease to prenatal sonographic diagnosis, with increasingly high sensitivity and specificity rates. While current literature supports that single (and possibly double) nuchal cords are not associated with increased adverse perinatal outcome, emerging literature suggests that cases of ≥ 3 loops of nuchal cords or in the presence of a coexisting true knot of the umbilicus may be associated with an increased risk of stillbirth or compromised neonatal status at delivery. This commentary will address current perspectives of prenatal sonographic diagnosis and clinical management challenges associated with nuchal cord(s) in singleton pregnancies.
Keywords: prenatal ultrasound, nuchal cord
Introduction
Umbilical cord accidents preceding labor are uncommon. Nevertheless, approximately 20% of stillbirths at autopsy have been attributed to fatal compromise of the umbilical circulation.1–3 A study of 121 stillbirths noted that umbilical cord pathology (including stricture, true knots, strangulation of the fetus, and prolapse) was the third most common cause of stillbirth in the second trimester (16.42%), following unexplained (29.85%) and congenital anomalies and malformations (19.4%).1 Umbilical cord pathology is, however, the leading cause of stillbirth in the third trimester (33.33%) and of overall total stillbirth (23.97%).1 Lehtonen et al, reporting causes of stillbirth in Finland (where the rate of recording causes of stillbirth is exceptionally high), noted that “severe constricting loops or knots” accounted for 16/98 (16.3%) of stillbirth cases.2 Most recently, the NICHD Stillbirth Collaborative Research Network Group in 2020 reported that of 496 stillbirths with complete cause of death analysis, using the Initial Causes of Fetal Death (INCODE) classification system, 94 (19%, 95% CI 16–23%) were associated with umbilical cord abnormality.3 Forty-five (48%) had compromised fetal microcirculation, 27 (29%) had cord entanglement (nuchal, body, or shoulder cord), 26 (27%) had knots, torsions, or stricture, and five (5%) had cord prolapse.3
Detailed histopathological criteria for umbilical cord blood flow restriction in cases of unexplained stillbirth were presented by Parast et al in 2008.4 Correlating clinical and autopsy data with gross and histological placental findings from a series of presumptive cord accidents, these authors proposed minimal histological findings suggestive of cord compromise, which included vascular ectasia and thrombosis within the umbilical cord, chorionic plate, and/or stem villi. Of 27/62 cases reviewed whose cause of death was determined to be other than cord accident, 3/62 (11%) met all histological criteria for cord accident (specificity of 89%). In contrast, of the remaining 25 cases of stillbirth with an undetermined cause of death, 13/25 (52%) met the criteria for cord accident (P=0.0038).4
The sensitivity and specificity of these criteria were later investigated in 2012 in a separate study with a review of an independent set of stillbirth cases.5 Histopathology from 26 cases (in which cord accident was deemed the cause of death) and 62 controls (in which the cause of death was anything other than cord accident) was assessed. Of the 62 stillbirth controls, only four (6%) met the complete criteria for cord accident (specificity 4%). In contrast, of the 26 cases with a cause of death related to cord accident, 16 met the minimal criteria (sensitivity 62%) and 12 met the complete criteria (sensitivity 46%).5 Thus, these histological criteria identify cases of cord accident as a cause of stillbirth with a relatively high specificity, confirming the potential utility of these criteria for a diagnosis of cord accident, and further emphasize the importance of detailed placental examination in the evaluation of all stillbirths.5
Potential adverse outcomes associated with nuchal cord(s) are better understood with detailed knowledge of the embryology, anatomy, and array of (uncommon) anomalies of the umbilical cord. For these, the reader is referred to our Commentary published in this journal in 2020, regarding current perspectives of prenatal sonographic diagnosis and clinical management challenges of true knot of the umbilical cord.6
The umbilical cord may be correctly viewed as an extension of the fetal cardiovascular system. It is important to recognize that the umbilical vessels (both arteries and vein) are protected by unique inherent anatomical features of the umbilical cord, including the length of the umbilical cord, Wharton’s jelly, the presence of two arteries, coiling, and suspension in amniotic fluid, which all contribute to protective buffering of the cord from twisting, shearing, and compressive forces throughout gestation, and especially during labor and delivery.6 An additional protective mechanism from the potential effects of compression upon the umbilical arteries is the presence of a 1.5–2 cm shunt between the umbilical arteries within 3 cm of the placental cord insertion, the Hyrtl anastomosis, which is present in approximately 96% of umbilical cords.7 This arterial anastomosis equalizes pressures between the respective umbilical arteries before entering the placenta and functions as a safety valve in the event of placental compression or blockage of an umbilical artery, and has been confirmed with prenatal ultrasound.7–9 The important (and possibly critical) safety effect of this anastomosis will be detailed later in conjunction with the (not uncommon) association of a single umbilical artery (the most common true congenital anomaly in humans) and nuchal cord(s).
Predisposing factors of nuchal cord(s) and a true knot(s) of the umbilical cord are similar and include an excessively long umbilical cord (prone to entanglement) and the presence of each of these entities (separately, and at times coexisting in the same pregnancy).6,10-16 Accordingly, predisposing risk factors include excessively long umbilical cords, polyhydramnios, excessive fetal movements, gestational diabetes, multiparity, post-term pregnancy, marginal cord insertion, and male fetuses.6,10-16 Ogueh et al, in a 2006 study of 57,853 singleton deliveries, noted 13,717 deliveries (23.71%) with a least one nuchal cord and additional risk factors, including induction of labor, slow progress of labor, and shoulder dystocia.14 In general, an umbilical cord length of >70–80 cm is considered a predisposing factor for the formation of nuchal cord(s).14 A case of five loops of nuchal cord (resulting in stillbirth) with an excessively long umbilical cord measuring 190 cm was reported by Dursun et al.16, Notwithstanding, the pathogenesis of the formation of nuchal cords is not entirely clear. Although it appears that fetal movements may result in cord entanglement (most commonly nuchal cord) and that excessive fetal movements and long umbilical cords predispose to entanglement, it remains unclear why some fetuses develop nuchal cord(s) while others do not.6,10 Whereas true knots of the umbilical cord are infrequent (<1% of singleton deliveries at term) and have been associated with a 4–10-fold increase in the likelihood of stillbirth (and are often not depicted at prenatal sonography owing to the inability to depict the umbilical cord throughout its entire length with ultrasound), loops of umbilical cord around the fetal neck – nuchal cords – are relatively frequent occurrences.
In general, the incidence of nuchal cord(s) increases linearly with advancing gestational age, ranging between 15.8% and 30% of all singleton deliveries at term.17–20 Schäffer et al in 2005 reported an incidence of nuchal cords in term and post-term deliveries of 33.7% and 35%, respectively.20 The finding that the reported incidence of nuchal cord rises considerably toward term possibly reflects increasing fetal activity and gradually decreasing amniotic fluid volume, or both.21,22 The incidence of multiple nuchal cords is considerably lower (approximately 5% or lower for double nuchal cords, and in general is inverse to the increasing number of loops involved).20,23,24 The incidence of a quadruple nuchal cord of 0.1% of all deliveries was reported by Dipple.24 Rare cases of between five and 10 loops of nuchal cord have been reported.16,25-33 Of note, of these anecdotal cases of high-order multiple loops of nuchal cord, the cases with five and 10 loops of nuchal cord were associated with stillbirth, presumably due to the multiple nuchal cords.16,33 In the cases with six, seven, eight, and nine loops of nuchal cord, Cesarean deliveries of liveborn neonates were reported.28–32
Prior to prenatal sonographic diagnosis of nuchal cords, various indirect (either spontaneous or evoked) diagnostic modalities were utilized in the prenatal or intrapartum prediction of the presence of nuchal cords.34–40 In contrast, prenatal ultrasound enables direct imaging diagnosis throughout gestation, independent of fetal heart rate changes.
Two types of nuchal cord have been reported by Giacomello:41 type A, a freely sliding pattern, which may become reduced spontaneously; and, in contrast, type B, a nuchal cord that encircles the fetal neck in a locked fashion, which is unlikely to reduce spontaneously.16,41-43 Nuchal cords have also been designated “tight” versus “loose”, according to whether or not the nuchal cord(s) can be manually reduced (delivered over the fetal head).44
This commentary will address current perspectives of prenatal sonographic diagnosis and management challenges associated with nuchal cord(s) in singleton pregnancies.
Nuchal Cord(s) in the First Trimester
Effects of the presence of a nuchal cord in the first trimester include effects upon nuchal translucency measurement and flow within the ductus venosus.
Nuchal Translucency
Schaefer et al in 1998 reviewed 316 cases in which nuchal translucency was assessed and determined that inadvertent inclusion of the nuchal cord added a mean of 0.8 mm to the nuchal translucency measurement.45 These authors concluded that the presence of a nuchal cord may bias the results of nuchal translucency measurement and suggested that application of color Doppler imaging may decrease the false-positive rate in screening for fetal aneuploidy by nuchal translucency measurement.45 Maymon et al described two cases in which transient increased thickness of the nuchal translucency was attributed to the presence of a nuchal cord, and concurred that the possible presence of a nuchal cord should be considered in the work-up of cases of increased nuchal translucency.46 Scheier et al in 2007 prospectively assessed the nuchal translucency measurement of 53 fetuses with nuchal cord between 11 and 3/7 and 13 and 6/7 weeks’ gestation.47 These authors noted a wide scattering of measurements in fetuses with nuchal cord in comparison to the same fetuses (after a median interval of 132 minutes) when the nuchal cord has reduced spontaneously, and concurred that the presence of a nuchal cord prevents the accurate calculation of true nuchal translucency.47
Ductus Venous Assessment
Conflicting reports regarding this potential effect of a nuchal cord have been published. Plasencia et al studied 1174 normal non-selected singleton fetuses between 11 and 13 and 6/7 weeks’ gestation, noting a nuchal cord in 6.73% of cases.48 These authors found a lower pulsatility index in fetuses without a nuchal cord in comparison to those with a nuchal cord (P<0.001).48 In contrast, Petousis et al, in a later study of 1974 fetuses between 11 and 13 and 6/7 weeks’ gestation, noted a significantly higher incidence of nuchal cord between 13 and 13 and 6/7 weeks’ gestation of 24.7% (n=119) compared to between 12 and 12 and 6/7 weeks’ gestation of 16.5% (n=1920) and between 11 and 11 and 6/7 weeks’ gestation of 7.9% (n=26) (P<0.01).49 In this study, no correlation was noted between the presence of a nuchal cord and the ductus venosus pulsatility index.49
Nuchal Cord(s) in the Mid-Trimester
Tepper et al determined the incidence of cord entanglement around any part of the fetal body in 237 singleton fetuses between 13 and 16 weeks’ gestation.50 Cord entanglement was defined when one or more of the following were noted: one or more loops of cord around the neck, hand, leg, thorax, abdomen, and/or shoulder. Umbilical cord entanglement was noted in 149 patients (62.9%). Of those, 64 (42.9%) were nuchal cords, 23 (15.4%) around the legs, 19 (12.7%) around the hands, seven (4.8%) around the abdomen (Figure 1), and 36 (24.2%) around other body parts (thorax, shoulder, and pelvis).50 The authors noted that this incidence was higher than the incidence of cord entanglement later in pregnancy and should be therefore considered a part of early normal fetal development.
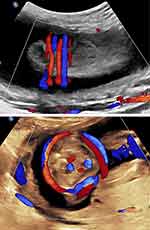 |
Figure 1 Mid-trimester axial image of the fetal body. Note two loops of cord around the lower fetal abdomen. Upper panel: Axial image. Lower panel: Sagittal image. |
Effects of the presence of a nuchal cord in the mid-trimester include an effect upon the nuchal fold thickness measurement (associated with an increased risk of fetal trisomy 21). Lee et al in 2003 first reported that among 548 fetuses assessed between 16 and 24 weeks’ gestation the nuchal fold was thicker among fetuses in the presence of a nuchal cord.51 This observation was further supported in 2012 by Ozhavukcu and Haliloglu, who studied 242 fetuses between 8 and 24 weeks’ gestation and reported mean nuchal fold thickness measurements of 4.66±0.64 mm and 4.36±0.79 mm for fetuses with and without nuchal cords, respectively (P=0.049).52 Thus, it appears prudent to obtain this measurement in the absence of a nuchal cord if possible.
Mid-trimester stillbirth attributed to nuchal cord entanglement has been reported rarely (in association with “multiple tight loops of nuchal cord and a markedly thinned and elongated fetal neck”).31,53,54 Other than attention to the above-mentioned potential indirect sonographic effects of nuchal cords during the mid-trimester, no benefit in clinical outcome has been reported with such early diagnosis of this condition, which commonly reduces spontaneously with advancing gestational age, as described by Tepper et al.50 Any potential impact upon perinatal outcome therefore remains limited to the third trimester, which will be detailed in the following section.
Nuchal Cord(s) in the Third Trimester
Prenatal Sonographic Diagnosis
Although reported initially with real-time, gray-scale ultrasound involving both sagittal and axial images of the fetal neck area (noting the vascular structures) of the nuchal cord,26,39,42,55,56 the gold-standard (indirect) gray-scale sonographic sign associated with nuchal cord, the “divot sign”, was reported by Ranzini et al in 1999.57 These authors described that in the presence of a nuchal cord, sagittal scanning of the posterior aspect fetal neck will depict scalloping of the fetal subcutaneous tissue – an indentation or “imprint” of the nuchal cord compressing the fetal subcutaneous tissue below the nuchal cord (Figure 2).57 This sonographic finding, reported as “a marked impression of the nuchal skin below the umbilical cord”, was described by Verdel and Exalto in association with a stillbirth and tight nuchal cord at delivery.58 The sonographic marked impression of the umbilical cord in the nuchal skin area was incorrectly attributed to “strangulation”. Rarely, in the presence of multiple loops ≥3 the number of “divots” may be lower than the number of nuchal loops in that some of the loops may overlap other loops and not necessarily the fetal skin. Given the sonolucent appearance of the umbilical vessels (despite utilization of high-resolution equipment), real-time ultrasound requires a high degree of suspicion in order not to inadvertently miss the presence of a nuchal cord. For accurate diagnosis as mentioned, both axial and sagittal imaging of the fetal neck are required. Although it may appear logical to infer that the presence of a divot sign (versus the absence of this sign in association with a nuchal cord) may indicate a tight nuchal cord at delivery, this is not the case. To our knowledge, there is no association between the presence of the divot sign and a tight nuchal cord at delivery.
Color and power Doppler imaging and three-dimensional ultrasound with direct sonographic depiction of nuchal cord(s) (and the precise number of loops) have enabled direct diagnosis without relying on the presence of indirect, associated sonographic changes – the subcutaneous indentation and “divot” sign. Although the latter remains a useful screening tool for the presence of nuchal cord(s), upon suspicion of such, further documentation with either color, power Doppler or three-dimensional ultrasound appears prudent (Figure 3). Color Doppler clearly has resulted not only in confirmation of nuchal cord(s) but also notably higher sensitivity and specificity rates for this condition than were previously possible with real-time, gray-scale ultrasound alone.
 |
Figure 3 Three-dimensional left posterior oblique view of the fetal head and neck. Note left fetal ear and mandible. Triple loop (of uncoiled) nuchal cord is clearly visible. |
Sensitivity
Utilizing color Doppler imaging at third-trimester sonographic imaging, Jauniaux et al in 1992 correctly identified 28 (72%) single and 17 (94%) multiple nuchal cords found at birth.59 The overall sensitivity of color imaging in the prenatal detection of nuchal cord(s) was greater than for gray-scale (79% compared with 33%). The sensitivity of color imaging was also higher after 36 weeks than before 36 weeks (93% compared with 67%).59
Morgan-Ortiz et al in 1997 assessed 114 pregnant women in labor for the presence of a nuchal cord, noting a sensitivity of 80% (95% CI 72.66–87.34%), specificity of 96% (95% CI 92.91–99.09%), and positive and negative predictive values of 87% and 94%, respectively.60 These authors concluded that the high specificity of 96% implies that sonography may be utilized as a screening test for the identification of pregnancies with nuchal cord.60
Qin et al in 2000 similarly assessed 180 patients with color Doppler imaging versus gray-scale ultrasound for the presence of a nuchal cord prior to labor. Sixty-two patients (34%) had a nuchal cord at delivery.61 The sensitivity of color Doppler imaging in detecting a nuchal cord was 96.8% and significantly higher (P<0.05) than gray-scale imaging alone.60
Romero-Gutiérrez et al, similarly utilizing color Doppler imaging, assessed 132 low-risk women for the presence of a nuchal cord, 50 (37%) of whom had a nuchal cord at delivery. Sensitivity, specificity, and positive predictive and negative predictive values were 92%, 87%, 81%, and 95%, respectively.62
Aksoy, in a limited study in 2003, demonstrated the sensitivity of color Doppler sonography in depicting a nuchal cord of 95% (18/19 fetuses), with a specificity of 92% (45/49), and positive and negative predictive values of 82% (18/22) and 98% (45/46), respectively.63
Hanoaka et al in 2002 compared real-time (two-dimensional), color Doppler and three-dimensional sonography in predicting the presence of nuchal cord at delivery of 120 women, 30 of whom had a single nuchal cord, four a double nuchal cord, and one a triple nuchal cord.64 Real-time, color Doppler, and three-dimensional sonography detected 24 (69%), 29 (83%), and 25 (71%), respectively, of the cases of nuchal cord. No significant differences in overall diagnostic indices of each diagnostic modality were noted, and the authors concluded that three-dimensional surface imaging does not provide enhanced diagnostic capabilities in the detection of nuchal cord(s).64
Peregrine et al in 2005 performed gray-scale and color Doppler imaging prospectively upon 289 women immediately prior to induction of labor to assess the presence of nuchal cord(s).65 A nuchal cord was present in 52 cases (18% of all deliveries). A single nuchal cord was noted in 14.5%, double loop in 2.8%, and triple loop in 0.7% of cases, although this difference did not meet clinical significance (relative risk=0.61; 95% CI 0.32–1.18). The sensitivity of ultrasound in diagnosing a nuchal cord was 37%, with specificity, and positive and negative predictive values of 80%, 29%, and 85%, respectively. The sensitivity of ultrasound in diagnosing a nuchal cord when there was more than one loop present was 60% compared to 37% when only one loop was present.65
Markov et al in 2007, in a study of 86 singleton pregnancies between 37 and 42 weeks’ gestation, noted sensitivity, specificity, and positive and negative predictive values of 68.1%, 77.2%, 53.5, and 86%, respectively.66 These authors, similarly to Peregrine et al,65 noted that the detection rate of multiple loops of nuchal cord was higher than that of single loops.66
Possible Confounding Factors
Single Umbilical Artery
Single umbilical artery is the most common true congenital anomaly of humans, with an incidence of approximately 0.63–1% of newborns (B).7,67 Friebe-Hoffmann et al detailed a retrospective analysis of 1169 women with singleton pregnancies diagnosed with single umbilical artery.68 Of the 169 fetuses, 989 (84.6%) were noted to have an isolated single umbilical artery while 180 (15.4%) presented with additional structural and/or chromosomal anomalies. Fetuses with single umbilical artery had lower birth weight (2825 grams versus 3220 grams, P<0.00) and higher rates of preterm delivery before 34 weeks’ gestation (13.7% versus 3.8%, P<0.001), and weighed <5th centile (21.6% versus 9.3%, P<0.001). In 60 children (5.1%), chromosomal or structural anomalies were detected postpartum.68 Isolated single umbilical artery was also noted in another study to be an independent predictor of adverse perinatal outcomes in term neonates. In this study, isolated single umbilical artery was noted in 786 (0.3%) of 233,123 deliveries.69 Adverse outcomes associated with isolated single umbilical artery included placental abruption (OR=3.4), umbilical cord true knot (OR=3.5), cord prolapse (OR=2.8), induction of labor and Cesarean delivery (OR=1.5 and OR=1.9), respectively, compared to the control group, and perinatal mortality rates were higher both antenatally (intrauterine fetal death OR=8.1) and postnatally (postpartum death OR=6.1).69
As mentioned in the Introduction, the presence of two umbilical arteries can be viewed as an inherent protection system of the fetus in the event of antepartum compression of the umbilical cord. In this scenario, the Hyrl anastomosis between the two umbilical arteries in close proximity to the placental insertion of the cord enables potential unpaired continuation of placental perfusion despite compression or, alternatively, cessation of flow in one of the umbilical arteries. This potential cord compression safety mechanism is clearly absent in the presence of a single umbilical artery. Upon follow-up of a fetus with a known single umbilical artery further complicated by the presence of a double nuchal cord, we were therefore not surprised at electronic fetal monitoring to note a prolonged fetal bradycardia necessitating immediate Cesarean delivery of an uncompromised neonate.70 We speculated that the umbilical cord compression with the double nuchal cord may have been considerably less tolerable by the fetus, given the absence of the second “protective” umbilical artery.70 Of note, we have observed similar occurrences of double nuchal cord in association with a single umbilical artery with prolonged fetal heart rate monitoring in the third trimester, necessitating immediate Cesarean delivery of non-compromised neonates on at least two other separate occasions. Although, to our knowledge, the potential impact of a single umbilical artery and possible associated diminished capacity of the fetus to tolerate compression of the nuchal cord(s) has not been reported by others, we maintain increased fetal surveillance (prolonged fetal heart rate monitoring) when noting the coexistence of these two otherwise unassociated events [nuchal cord(s) in the presence of a established single umbilical artery] occurring simultaneously, and are more likely to deliver upon noticing fetal heart rate changes.
Decreased Wharton’s Jelly
Studying the sonographic cross-sectional area of the umbilical cord and subtracting the vascular area from the overall umbilical cord area, Ghezzi et al in 2001 established a nomogram regarding the sonographic measurement of Wharton’s jelly in 659 fetuses between 15 and 42 weeks’ gestation.71 These authors noted that the Wharton’s jelly area increases as a function of gestational age, and is correlated with fetal size up to 32 weeks’ gestation.71
In three subsequent studies, this group of investigators noted that prenatal sonographic depiction of a lean umbilical cord may serve as a simple marker for fetuses destined to be small for gestational age at birth.72 The proportion of subsequent small for gestational age fetuses at birth exhibiting a lean umbilical cord was higher than those with a normal umbilical cord (11.5% versus 2.6%, P<0.05), and fetuses with a lean umbilical cord had a 4.4-fold higher risk of being small for gestational age at delivery than those with a normal umbilical cord. The incidence of meconium-stained amniotic fluid at delivery was higher in fetuses with a lean umbilical cord versus those with a normal umbilical cord (14.6% versus 3.1%, P<0.001). The proportion of infants who had a 5-minute Apgar score <7 was higher among those with a lean umbilical cord than among those with a normal umbilical cord (5.2% versus 1.3%, P<0.05). In addition, considering only women admitted in labor with intact membranes and who delivered an appropriate for gestational age infant, the proportion of fetuses with oligohydramnios at delivery was higher among those who had a lean umbilical cord than among those with a normal umbilical cord (17.6% versus 1.3%, P<0.01).72
In 2003, the same group of investigators compared 84 growth-restricted fetuses with 168 appropriate for gestational age fetuses, and noted that the prevalence of lean umbilical cords (cross-sectional area <10th centile for gestational age) was significantly higher among growth-restricted fetuses versus appropriate for gestational age fetuses (73.8% versus 11.3%, P<0.001).73
Raio et al in 1999 noted that a decrease in Wharton’s jelly is frequently observed in cases of single umbilical artery, itself a possible risk factor for fetuses with nuchal cord (see “Single Umbilical Artery”, above).74 These authors suggested that the association of increased perinatal morbidity and mortality observed in cases of single umbilical artery even in the absence of congenital or chromosomal abnormalities may reflect consequences of the reduced amount of Wharton’s jelly.74 Thus, the combined pathophysiology of single umbilical artery and a decreased amount of Wharton’s jelly possibly represents a combination of compromised inherent teleological protective mechanisms of the umbilical cord and thus results in the increase in adverse perinatal outcomes of fetuses, further complicated by the presence of nuchal cords, and especially multiple nuchal cords.
Decreased Umbilical Cord Coiling
Coiling of the umbilical cord, present from the mid-first trimester, is another teleological protective mechanism protecting the umbilical cord from lateral shearing and potential compressive forces. Realizing the absence of these protective mechanisms of the umbilical cord with decreased coiling, Strong et al noted clinical findings suggesting that the fetus with non-coiled umbilical blood vessels is at increased risk for perinatal morbidity and mortality.75 Strong et al, in an effort to quantitate umbilical vascular coiling, described the coiling index by dividing the total number of complete umbilical vascular coils by the umbilical cord length in centimeters.76 The mean umbilical coiling index was 0.21±0.07 (SD) coils per centimeter.76 These authors noted an increased risk of adverse perinatal outcomes associated with decreased coiling of the umbilical cord. Specifically, among those whose umbilical coiling index values were ≤10th centile, there was a significantly greater incidence of karyotype abnormalities (P=0.04), meconium-staining (P=0.03), and operative delivery for fetal distress (P=0.03). There was a significantly greater incidence of moderate or severe variable fetal heart rate decelerations for those whose umbilical coiling index value was either ≤10th centile (0.1 coils/cm) or ≥90th centile (0.3 coils/cm).76 These investigators later noted that among 200 consecutive liveborn neonates the mean umbilical coiling index among those with nuchal cords (0.18±0.09 coils/cm) was significantly lower than among the group without nuchal entanglement (0.21±0.07 coils/cm, P=0.01).77 Among those with umbilical coiling indices ≤0.1 coils/cm, 42% had nuchal cords, while only 4.8% of infants with umbilical cords with indices ≥0.3 coil/cm had nuchal cords (P=0.007). These authors concluded that an association exists between the density of umbilical vascular coiling and nuchal entanglement of the cord.77
Nuchal cord(s) noted either with prenatal ultrasound or, alternatively, at delivery may be coiled or uncoiled. Potential clinical implications of decreased coiling within the loops of nuchal cord are unclear. In our prospective study of a set of 115 patients with well-established dates and singleton, appropriate for gestational age, non-anomalous fetuses between 28 and 41 weeks’ gestation and nuchal cord(s) (103 fetuses with a single loop and 12 a double nuchal cord), we later performed an analysis of outcomes according to the presence or absence of coiling within the nuchal cord(s) at the time of prenatal sonographic diagnosis.78 No significant differences in perinatal outcomes were noted, although it should be mentioned that this subset analysis did not reach statistical power to substantiate this negative finding.
It appears the potential role of decreased umbilical cord coiling in the formation of nuchal cord(s) and/or the potential increased risk of adverse perinatal outcome associated with nuchal cords exhibiting decreased coiling versus fetuses with nuchal cord(s) with normal coiling currently remains elusive, although clearly the absent coiling may suggest or indicate that these umbilical cords are more prone to potential compression.
Decreased Amniotic Fluid Volume (Oligohydramnios)
Strong et al noted that among 70 women delivering infants with nuchal cords, there was a significantly higher incidence of meconium-stained amniotic fluid and severe variable fetal heart rate decelerations/fetal bradycardia among patients who were noted to have intrapartum oligohydramnios (defined by an amniotic fluid index ≤5 cm).79 It appears that the decreased amount of amniotic fluid is likely associated with decreased resistance of the umbilical cord vasculature to the transient effects of uterine contraction-associated compression.
Fetal Growth
The possible association between nuchal cords and fetal growth restriction remains unclear. Three relatively large studies did not find an association between nuchal cord and birthweight.80–82 In 1993, Lipitz et al studied outcomes of 12,697 births and noted that a double nuchal cord occurred in 8.3% of cases with no effect upon birth weight.80 These authors noted that the incidence of nuchal cord was significantly lower (P<0.0006) in infants with birth weights <2000 grams. Carey and Rayburn in 2003 similarly studied 13,256 deliveries, noting single nuchal and double nuchal cords in 3230 (24.4%) and 504 (3.8%) of deliveries, respectively.81 The mean birth weight was no different in the presence of single, multiple, or no nuchal cord(s) (3206 grams, versus 3135 grams, and 3252 grams, respectively (P=0.7)).81 Mastrobattista et al in 2005 studied 4426 neonates of whom 691 and 84 had one or double nuchal cords at delivery, versus 3651 neonates without nuchal cords. There were no significant differences in birth weight.82
In contrast, other studies note that diminished fetal growth has been associated with nuchal cord(s). Osak et al assessed 10,509 singleton term deliveries excluding pregnancies complicated by pregnancy-induced hypertension, fetal growth restriction, and diabetes.83 Of the infants studied, 2699 (25.7%) had a nuchal cord at delivery. Birth weights of infants with a nuchal cord were slightly lower (3481±467 grams versus 3548±142 grams, P<0.01). These authors reported a “dose effect”, with infants with double nuchal cords or higher having lower birth weights.83
Sornes in 1995 studied the outcomes of 11,201 births and noted that the presence of umbilical cord encirclements is associated with fetal growth restriction, with the severity of the growth restriction being positively related to the number of encirclements.84
Bord et al in 2007 reported a case of marked fetal growth restriction and multiple nuchal cords.85 Following depiction of severe early symmetrical fetal growth restriction with a quadruple nuchal cord at 30 weeks’ gestation, electronic fetal heart rate monitoring revealed repeated severe variable decelerations necessitating Cesarean delivery of a non-asphyxiated, markedly growth-restricted neonate weighing 840 grams with a tight quadruple nuchal cord.85
Hoh et al in 2012, in data regarding 150 cases with nuchal cords (300 cases without nuchal cords, 124 cases of single nuchal cord, and 26 with multiple nuchal cords), noted that the birth weight of infants with multiple nuchal cords was significantly lower than that of infants without nuchal cords (3317±24 grams versus 3054±55 grams, P=0.0008).86
Similarly, we followed a patient with a persistent quadruple nuchal cord throughout the third trimester (Figure 4).87 Repeat sonographic evaluations at 2-week intervals demonstrated gradual continued decelerating fetal growth. Detailed work-up for fetal growth restriction was negative. The patient declined elective delivery at 37 weeks, indicated owing to continued fall-off of growth. Following depiction of a triple nuchal cord at 40 weeks’ gestation (Figure 5), the patient underwent induction of labor at her request. Intrapartum non-reassuring fetal heart rate necessitated Cesarean delivery of a female infant weighing 2350 grams (<3rd centile for gestational age), with a tight triple nuchal cord.87
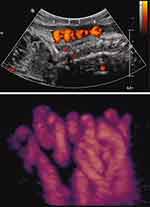 |
Figure 4 Sagittal image of breech-presenting fetus at 27 weeks’ gestation. Upper panel: Power Doppler image of quadruple nuchal cord. Lower panel: Power Doppler three-dimensional image of above. Note coiled nuchal cords. Reproduced with permission from Sherer DM, Dalloul M, Sabir S, London V, Haughton M, Abulafia O. Persistent quadruple nuchal cord throughout the third trimester associated with decelerating fetal growth. Ultrasound Obstet Gynecol. 2017;49(3):409–410. Copyright © 2016 ISUOG. Published by John Wiley & Sons Ltd.87 |
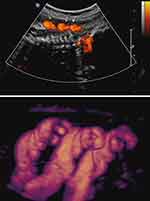 |
Figure 5 Sagittal image of the same fetus depicted in Figure 4 now vertex-presenting at 40 weeks’ gestation, with decelerated fetal growth (and now overt fetal growth restriction). Upper panel: Power Doppler image of triple nuchal cord. Lower panel: Power Doppler three-dimensional image of above. Note the presence of coiled nuchal cords. Reproduced with permission from Sherer DM, Dalloul M, Sabir S, London V, Haughton M, Abulafia O. Persistent quadruple nuchal cord throughout the third trimester associated with decelerating fetal growth. Ultrasound Obstet Gynecol. 2017;49(3):409–410. Copyright © 2016 ISUOG. Published by John Wiley & Sons Ltd.87 |
True Knot of the Umbilical Cord with Coexisting Nuchal Cord
Two recent reports have addressed prenatal sonographic diagnosis of coexisting true knot of the umbilical cord and nuchal cord.11,12 We reported a series of three such cases in the thirdtrimester, in which the true knot of the umbilical cord was located within the loop of the nuchal cord itself (Figure 6).11 Following admission for continuous fetal monitoring and administration of intramuscular antenatal steroids to decrease overall prematurity-associated neonatal morbidities, in each of these three cases (at 36–37 weeks’ gestation) fetal heart rate monitoring disclosed prolonged fetal bradycardia, leading to Cesarean delivery of uncompromised infants (in which all three cases had coexisting nuchal cords with a true knot of the umbilical cord located precisely as depicted by prenatal ultrasound within the nuchal cord loop) (Figure 6).11
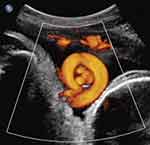 |
Figure 6 Power Doppler sagittal view of vertex-presenting fetus at 37 weeks’ gestation (fetal cranium on the right). Note depiction of true knot of umbilical cord (“smiley” sign), within the nuchal cord. Reproduced with permission from Sherer DM, Dalloul M, Ward K, et al. Coexisting true umbilical cord knot and nuchal cord: possible cumulative increased risk of adverse perinatal outcome. Ultrasound Obstet Gynecol. 2017;50(3):404–405. Copyright © 2016 ISUOG. Published by John Wiley & Sons Ltd.11 |
Tight Nuchal Cord
Nuchal cords have also been designated “tight” versus “loose”, the implication being that “tight” nuchal cord(s) are more likely to be prone to, or associated with, vascular compromise prior to or during labor, or at delivery.44, 88, Since the designation “tight” versus “loose” is made according to whether or not the nuchal cord(s) can be manually reduced (delivered over the fetal head), it is inferred that a “tight” nuchal cord cannot be diagnosed with prenatal ultrasound. Worded differently, prenatal sonography cannot predict whether or not nuchal cord(s) will be manually reducible at delivery.
Umbilical cord compression leads to obstruction of blood flow in the thin-walled umbilical vein, while blood continues to be delivered in a pulsatile fashion through the thicker-walled umbilical arteries. This may result in cases of “tight” nuchal cord in neonatal hypovolemia, acidemia, subsequent respiratory distress, and anemia.88,89 Physical examination upon delivery of such neonates may reveal overall pallor, dusky facies, petechiae, tachycardia, and hypotension. These clinical findings have been considered similar to those noted in association with non-lethal strangulation in the adult. Peesay described a possible syndrome, tight cord around the neck (tCAN) syndrome.43 Physical neonatal cardiorespiratory and neurological features, such as facial duskiness, skin abrasion of the neck due to tight nuchal cord, facial petechiae, subconjunctival hemorrhage, pallor of body below the tight nuchal cord, and respiratory distress and hypotonia, appear as a result of tight nuchal cord. Peesay grades tCAN syndrome (grades 1–4) based on the severity of clinical signs and symptoms.43
Martinez-Biarge et al in 2016 assessed intrapartum risk factors for neonatal arterial ischemic stroke.90 These authors noted that compared with controls, mothers of infants with neonatal arterial stroke were more likely to have experienced more intrapartum complications, including a tight nuchal cord at delivery (15% versus 6%), and concluded that intrapartum events may have a more significant role in the pathogenesis of neonatal arterial ischemic stroke than previously considered.90
Henry et al in 2013 performed a retrospective comparison utilizing electronic data of all deliveries during a 6-year period (2005–2010) in a multihospital system in the western United States.44 Each birth was recorded at delivery as having a tight nuchal cord, loose nuchal cord, or no nuchal cord. “Tight” was defined as the inability to manually reduce the loop over the fetal head and “loose” as the ability to manually reduce the loop over the head. Of 219,337 live births, 71.8% (n=157,492) had no nuchal cord, 21.6% (n=47,364) had a loose nuchal cord, and 6.6% (n=14,481) had a tight nuchal cord at delivery. No significant difference was noted in the neonatal outcome parameters assessed, including gestational age at delivery, birth weight, shoulder dystocia, admissions to the neonatal intensive care unit (NICU), dopamine in the first 24 hours of life, hemoglobin in the first 24 hours of life, RBC transfusion in the first 24 hours of life, and neonatal deaths in the hospital, between infants with no nuchal cord, and loose or tight nuchal cords at delivery. Among neonates born with very low birth weights (<1500 grams) (n=2005), 87.3% (n=1751) had no nuchal cord, 9.2% (n=184) had a loose nuchal cord, and 3.5% (n=70) had a tight nuchal cord at delivery. Similarly, in this subset group of neonates, no significant differences were found in the outcome parameters assessed, including gestational age at delivery, birth weight, Apgar scores at 1 or 5 minutes, intraventricular hemorrhage grade ≥3, retinopathy of prematurity grade ≥3, periventricular hemorrhage, or deaths in the hospital. Unfortunately, this retrospective study was unable to provide an objective assessment of the degree of nuchal cord tightness (including whether or not attempts at reducing the cord manually were performed). In addition, no information was provided regarding outcomes of deliveries with multiple nuchal cords, as this “was charted inconsistently”.44 Another drawback of this retrospective analysis is that no information whatsoever is given regarding mode of delivery. Clearly, the inclusion of only vaginal births may have skewed data in a favorable fashion. Conversely, if indeed Cesarean deliveries were included, separate analyses of neonatal outcomes of these deliveries, in our assessment, would have been critical, as potentially fetuses with tight nuchal cords (and possible intrapartum umbilical cord compression associated non-reassuring fetal status) resulting in Cesarean delivery may have revealed important clinical observations regarding tight nuchal cords. Nevertheless, despite clear lacking clinical data, these authors concluded that a tight nuchal cord at delivery is not associated with clinically significant adverse neonatal outcomes. While clearly the vast majority of neonates with single or multiple, loose or tight, nuchal cords are unaffected at delivery, this is by no means a given, and in our assessment these conclusions disregard emerging data and likely represent an oversimplification. Zhao et al in 2015 attempted to quantitate the prenatal sonographic assessment of “tightness of nuchal cord”.91 Among fetuses with nuchal cord (n=82), these authors chose the sonographic cross-section of the cord at the deepest impression of the nuchal cord (“divot” sign). They then created a connecting line between the two sides of the skin edges around the cord, and measured the depth from this line to the deepest edge of the impression (median depth=6.5 mm). Subsequently, they calculated the proportion of the depth divided by the area of the cord under the connecting line (between the skin edges), as representative of the “tightness” of the nuchal cord. Dividing the group of fetuses with nuchal cord into those with tight (n=41) versus loose nuchal cord (n=41), no significant correlations between the depth of the deepest impression or the proportion of the depth to area under the skin connecting line and fetal distress were noted.91 Nonetheless, clearly, labor, and especially descent of the fetal head in the birth canal, and in particular the residual length of umbilical cord, are parameters that may directly affect the tightness of the nuchal cord at delivery, which currently cannot be ascertained by prenatal sonography.
Thus, we are not aware of any prenatal objective qualification of the tightness of nuchal cord(s), other than possibly the prenatal sonographic depiction by Doppler flow velocimetry of umbilical cord vascular impairment, presumably due to compression (“tightness”), leading to delivery at 30 weeks’ gestation.92 Furthermore, Qin et al in 2000 assessed intrapartum color sonography, and although the sensitivity of color Doppler in detecting nuchal cords was 96.8%, tight or loose nuchal cords at delivery could not be distinguished by ultrasound.61
Miscellaneous
Induction of Labor
Ghi et al in 2007 assessed the possible impact of the presence of a nuchal cord upon induction of labor in 184 women, and noted that among women undergoing cervical ripening only multiparity and a favorable Bishop score were predictors of successful induction of labor.93 The presence of a nuchal cord, in 32% of women (159/184), was not associated with an increased risk of induction failure.
Shoulder Dystocia
Flamm in 1999 cautioned regarding the potential “catastrophic complication” of nuchal cord in association with shoulder dystocia.94 Ogueh et al noted that shoulder dystocia occurred more commonly in association with nuchal cord, especially when the nuchal cord was tight.14 These authors speculate that the nuchal cord may have prevented the fetus from descending through the birth canal, although they did caution regarding potential selective observer recording bias, in that events surrounding shoulder dystocia are more likely to be “documented more conscientiously”.
Polyhydramnios
Perlitz et al reported acute polyhydramnios at term associated with multiple nuchal cords and speculated that the triple nuchal cord may have limited fetal swallowing, similarly to the proposed association of swallowing impairments reported with fetal goiters, cervical teratomas, or skin abnormalities.95 Although this possible association appears plausible, it should be noted that despite the relatively high incidence of multiple nuchal cords, this observation has not been reported by others. Also of note, in their description, the authors fail to report the status of the fetal stomach, an important finding, as the presence of a fluid-distended fetal stomach would indicate unaffected fetal swallowing despite the presence of a triple nuchal cord.
External Cephalic Version
Al-Kouatly et al reported awaiting the spontaneous uncoiling of a double nuchal cord (documented at 36 weeks) prior to successfully performing external cephalic version of a patient with a breech-presenting fetus at 38 weeks’ gestation.96 However, nuchal cords are not considered an absolute contraindication for external cephalic version. Boujenah et al in 2017, in a retrospective analysis of cases with attempted external cephalic version between 1998 and 2015, noted that the success or failure of external cephalic version was not associated with an increased risk of cord accident (nuchal cord or prolapse).97
Perinatal Outcome
Clearly, the vast majority of infants with nuchal cord(s) at delivery are uncompromised. Review of the literature essentially confirms the complete absence of adverse perinatal outcomes, with comparable outcomes of infants delivered with or without nuchal cords.19,82,98
Few publications support significantly different outcomes, yet those that do report such results acknowledge reassuring overall unaffected neonatal outcomes despite these differences. Among such studies, Schäffer et al, noting rates of 34% and 35% of nuchal cords in patients delivering between 37 and 41 weeks’ gestation compared with those after 41 weeks’ gestation, respectively, detected that a significantly lower number of infants with nuchal cords were assigned 1-minute Apgar scores <7, with no difference in 5-minute Apgar scores or NICU admissions.20 Similar results regarding significantly lower 1-minute, but not 5-minute Apgar scores among infants born with nuchal cords were presented by Sheiner et al.99 Since fetuses with nuchal cord(s) exhibit a higher incidence of non-reassuring fetal heart rate patterns (due to transient umbilical cord compression), it appears that these lower 1-minute (but not 5-minute) Apgar scores may reflect such transient changes without affecting outcome. In the case–control study of Hankins et al, the umbilical artery pH levels at delivery were 7.25 versus 7.27 in infants with versus those without nuchal cords (P<0.05). Among infants born with a nuchal cord, the umbilical artery acidemia was usually mixed (68%) or respiratory in origin (23%), and pure metabolic acidemia was infrequent (9%).100
Miser in 1992 performed a retrospective case–control study of 706 infants born in a community hospital, in which the study group consisted of 167 (23.7%) deliveries complicated by a nuchal cord versus 523 control infants.98 Single, double, and triple nuchal cords occurred in 21.7%, 1.7%, and 0.3% of the deliveries, respectively. Fetal bradycardia and variable decelerations of the fetal heart rate occurred significantly more often in the nuchal cord group (18.6% versus 9.6%). Despite this finding, there were no significant differences in the incidence of operative deliveries or 1- or 5-minute Apgar scores; however, infants with nuchal cords weighed significantly less than controls.
Rhoades et al, in a population-based study of 3000 newborns with nuchal cords, after exclusion of selected obstetric complications, noted an increased risk of fetal distress, meconium-stained amniotic fluid, a 5-minute Apgar score <7, and assisted ventilation for <30 minutes.15 Although hospital charges were slightly greater than those without, hospital stays did not differ significantly. These authors concluded that although certain adverse perinatal outcomes are increased in infants with nuchal cords at delivery, the absence of longer hospital stays suggests that the effects of nuchal cord are likely transient.
Ghosh and Gudmundsson in 2008 utilized color Doppler imaging on 202 patients with post-term pregnancies and detected 69 patients with nuchal cord. Clinicians were blinded to the sonographic finding. Nuchal cords were noted in 69 (34.2%) patients.101 There was no significant increased risk for 1- and 5-minute Apgar scores <7, umbilical artery pH <7.1, umbilical vein pH <7.20, umbilical vein base excess <–11, perinatal death, Cesarean delivery, or NICU admissions. One (intrapartum) perinatal death occurred in the group of patients with nuchal cord (at 42 and 6/7 weeks’ gestation), and was attributed to asphyxia. Despite the relatively low number of patients in this study, the authors state that these data suggest that routine screening for nuchal cord will not improve the outcome of labor and delivery in post-term pregnancies.
Larson et al in 1995 reported that compared with a single or no nuchal cord, pregnancies with multiple entanglement were more likely to exhibit an abnormal intrapartum fetal heart rate pattern during advanced labor (P<0.001) and were more likely than controls to require a low or mid-forceps delivery (P<0.001), and to have meconium-stained amniotic fluid (P=0.013), a low 1-minute Apgar score (P<0.01), and umbilical artery pH <7.1 (OR= 2.2, P=0.013).102
Jauniaux et al in 1995 compared perinatal data of 550 fetuses with nuchal cord at delivery with controls matched for gestational age, maternal age, and parity, dividing the study group into fetuses with single versus multiple nuchal cords. The perinatal mortality rate, arterial pH levels <7.16, venous pH <7.2, and Apgar scores <7 at 5 and 10 minutes of life were similar in both groups.103 However, they noted a significantly higher incidence of 1-minute Apgar score <7, meconium-stained amniotic fluid, emergency Cesarean delivery, need for neonatal resuscitation. and admission to NICU in the nuchal cord group compared with controls. Multiple nuchal cords were noted as the main factor for the higher incidence of these complications and the only considered etiology for the three cases of stillbirth in this group, all of whom presented in the preceding week with decreased fetal movements.
Similarly, a number of publications have suggested less than optimal outcomes regarding multiple (at least three) nuchal cords. Schreiber et al, in a retrospective cohort study of 42,798 women with singleton, vertex vaginal deliveries between 24 and 43 weeks’ gestation, noted 3809 (8.9%) single nuchal cords, 1035 (2.42%) double nuchal cords, and 258 (0.6%) with three loops.104 Nuchal cord with three loops compared to no nuchal cord was associated with a higher incidence of intrauterine fetal death (1.9%), Apgar scores <7 at 1 and 5 minutes (7.4% and 2.3%, respectively), and a higher rate of operative vaginal deliveries (17.5%). Nuchal cord with two or three loops was associated with a higher incidence of fetal growth restriction (10.2% and 11.6%, respectively). Notably, in a multiple logistic regression analysis, nuchal cord with three loops was an independent risk factor for operative vaginal delivery and Apgar score <7 at 1 minute of life. A single nuchal cord was not associated with adverse perinatal outcomes.104
Mariya et al in 2018 studied a total of 2156 term deliveries, and assessed umbilical cord numbers not only for nuchal cord but also for trunk and limb cord entanglement, classifying cases into three groups: no loop (n=1458), single loop (n=594), and multiple loops (n=104). Umbilical artery pH levels and base excess levels were significantly “unfavorable” (P=0.002 and P<0.001, respectively) in entanglement cases, especially in the multiple loops group.105 A significantly higher percentage of neonates in the multiple loops group required oxygen at delivery (P<0.001).
Schreiber et al in 2019 reported 258 (0.6%) cases with three loops of nuchal cord, and noted a higher incidence of stillbirth (1.9%), Apgar scores <7 at 1 and 5 minutes (7.4% and 2.3%, respectively), and a higher rate of operative vaginal births (7.5%).104
Önderglu et al in 2008 found that incidences of oligohydramnios, fetal growth restriction, intrapartum anomalies, and Apgar scores <7 at 1 minute were not significantly different between cases with versus those without nuchal cords.106 However, umbilical cord pH (7.32 versus 7.30, P=0.048), partial pressure of oxygen (37.4±18.1 versus 31.7±14.4, P=0.01), and oxygen saturation (57±2.8 versus 48.3±20.4, P=0.005) were significantly lower in the nuchal cord group. Furthermore, the number of 1-minute Apgar scores <7 was significantly higher in the group with multiple nuchal cords (31.3% versus 15.6%, P=0.04). These authors suggested that while the presence of a single nuchal cord may negatively affect umbilical cord gases without significant perinatal complications, multiple nuchal cords may increase the development of intrapartum complications and lower Apgar scores.106
Hoh et al in 2012 reported that multiple nuchal cords (≥3) were associated with a higher incidence of intrapartum non-reassuring fetal heart rate, meconium-stained amniotic fluid, NICU admission, and emergency Cesarean delivery.86
Sepulveda in 2019 reported 10 singleton fetuses with triple nuchal cord after 24 weeks’ gestation (a prevalence of 1 in 506, or 0.2%).107 Four cases detected after 36 weeks’ gestation were delivered by Cesarean and the presence of the triple nuchal cord was confirmed at delivery. Cases diagnosed at <36 weeks’ gestation were followed expectantly, and in 83% of these cases the nuchal cord reduced by at least one loop. Overall, 8/10 cases were delivered by Cesarean and in only two of these deliveries was the sole indication the presence of the triple nuchal cord.
Neurodevelopmental Performance at 1 Year
Clapp et al studied the neurodevelopmental status of 190 infants of women with clinically normal antenatal courses, within 1 month of their first birthday. Infants were grouped according to the presence of a “symptomatic” nuchal cord during labor (abnormal fetal heart rate patterns or meconium).108 A symptomatic nuchal cord was noted in 24% of the 190 cases. At 1 year of age, scores on the Bayley Scales of Infant Development were slightly but significantly (P<0.1) lower in the infants delivered with asymptomatic nuchal cord. The mental index was 116±9 versus 120±7 and the psychomotor index was 101±11 versus 107±9. These cases were accentuated (P=0.9) when the symptomatic cases complicated by extreme tightness, multiple loops, or antenatal detection were compared to symptomatic cases without these additional complications (overall index 110±8 versus 105±10). There were no between-group differences in multiple potential confounding obstetric or demographic variables. These data were considered to suggest that symptomatic nuchal cord(s) at delivery may be associated with a subclinical deficit in neurodevelopmental performance at 1 year of age.108
Cerebral Palsy
Cerebral palsy is the most common neuromotor developmental disability in childhood, affecting approximately 2–3/1000 liveborn children. Cerebral palsy is a heterogeneous condition with various clinical types, co-morbidities, central nervous system imaging patterns, and underlying genetic variants.109 Few cases of cerebral palsy are solely a result of birth-related severe hypoxia or ischemia. The prevalence of cerebral palsy has remained constant despite widespread application of intrapartum fetal heart rate monitoring, increasing Cesarean delivery rates, and overall enhanced neonatal care.
Nelson and Grether in 1998 examined the association of cerebral palsy with conditions that can interrupt oxygen supply to the fetus as a potential primary event.110 In this research, a population-based case–control study was performed in four Californian counties (between 1983 and 1985) comparing birth records of 46 children with spastic cerebral palsy without recognized brain lesions and 378 randomly selected control children weighing ≥2500 grams at birth and surviving to 3 years of age. Eight of the 46 children had otherwise unexplained spastic cerebral palsy, and 15 of the 378 births had births complicated by tight nuchal cords (OR for quadriplegia=18, 95% CI 6.2–48). Other potentially asphyxiating conditions were uncommon, and were associated with spastic diplegia or hemiplegia. Level of care, oxytocin for augmentation, and surgical delivery did not alter the association of potentially asphyxiating conditions with spastic quadriplegia. Intrapartum abnormalities were common in both children with cerebral palsy and controls, and did not distinguish between them These authors concluded that potentially asphyxiating conditions, chiefly tight nuchal cord, were associated with a considerable proportion of unexplained spastic quadriplegia but not with diplegia or hemiplegia.110
Nielsen et al in 2008, in a case–control study assessing 271 singletons with spastic cerebral palsy and 217 singleton controls matched by gestational age group, investigated the association of asphyxia-related conditions (reduced blood flow or blood oxygen levels in the fetus) with spastic cerebral palsy, considering different age groups and the timing of risk.111 Placental and umbilical cord complications accounted for the majority of asphyxia conditions. In multivariate analysis, placental infarction was significantly associated with a four-fold increased risk of spastic quadriplegia and nuchal cord was significantly associated with a three-fold increased risk for spastic cerebral palsy overall. The combination with small for gestational age bestowed an especially increased high risk for spastic quadriplegia. Placental and umbilical cord complications were present in 21% of cases and 12% of controls.111
Greenwood and Impey in 2002 attempted to determine whether the association of cerebral palsy with nuchal cord is the result of recording bias.112 These authors studied 68 cases of cerebral palsy and 157 controls (consisting of singleton infants delivered at term, matched for gestational age and hospital of birth). Cerebral palsy was associated with tight nuchal cord (OR=2.8, 95% CI 1.1–6.8). Where nuchal cord was recorded at the discretion of the obstetrician (37 cases, 97 controls), there was an association between tight nuchal cord and cerebral palsy (OR=5.4, 95% CI 1.4–20.4), and in controls only, betweenApgar score <7 at 1 minute (OR=16.9, 95% CI 1.4–456.3). In the hospital where records included a check box for nuchal cord (31 cases, 60 controls), an association between cerebral palsy and nuchal cord could not be demonstrated (OR=1.4, 95% CI 0.4–4.9). Similarly, there was no apparent association between nuchal cord and Apgar score <7 at 1 minute (OR=2.6, 95% CI 0.4−15.9) in controls. The authors therefore concluded that the presence of nuchal cord is subject to recording bias, which in retrospective studies may lead to an association of cerebral palsy with nuchal cord that may not be evident with systematic documentation.112
Gutvirtz et al in 2019 similarly evaluated the incidence of cerebral palsy in children born with and without nuchal cord.113 These authors conducted a population-based cohort analysis including all singleton deliveries during >20 years at a single tertiary medical center. The incidence of cerebral palsy in children up to 18 years of age was evaluated. During the study period, 243,682 singleton deliveries met the inclusion criteria. Of these, 14.1% (n=34,332) were diagnosed with nuchal cord at birth. Rates of cerebral palsy were comparable between the groups (0.1% versus 0.1%, OR=1.03, 95% CI 0.69–1.52, P=0.89). Kaplan–Meier survival curves demonstrated no significant differences in cumulative incidences of cerebral palsy for children born with and those without nuchal cord (log rank P=0.92). A Cox proportional hazards model controlled for preterm delivery, maternal age, diabetes, and hypertensive disorders showed no association between nuchal cord and cerebral palsy (adjusted HR=1.06, 95% CI 0.71–1.57, P=0.77).113
With these conflicting data, the possible association between the presence of nuchal cord(s) at delivery and subsequent cerebral palsy remains unclear. It should be noted that all of the above studies were retrospective analyses and suffer the inherent weaknesses of such study design. In addition, none of these studies (including the last, larger study of 34,332 singleton infants with nuchal cords) refer to multiple nuchal cords, which clearly may incur increased risks of adverse neonatal outcome, including but not limited to cerebral palsy. Prospective studies, which include this possibly important (and clearly designated) clinical parameter, are needed to clarify this possible (causal) association.
Unusual Adverse Neonatal Outcomes Associated with Nuchal Cord(s)
Dural Sinus Dilatation/Ectasia
Rare, yet severe neonatal complications have been reported in association with nuchal cords. Katz et al reported “ectasia” of the dural sinuses in an asphyxiated neonate delivered with three tight nuchal cords. Dilated dural sinuses included the superior sagittal sinus, torcular Herophili, vein of Galen, and straight sinus, and were thought to have resulted from prolonged jugular venous distention created by the multiple tight nuchal cords.114 The infant subsequently developed multisystem failure and succumbed on the third day of life. Symmetric periventricular hyperechogenicity was considered a result of hypoxic or ischemic injury. These authors suggested that upon notcing nuchal cords, the status of the dural sinuses should be assessed. They also recommended that dural sinus ectasia in the presence of nuchal cords may suggest a chronic condition and delivery should be considered.114 Aziz and Strizke reported a similar case in which an asphyxiated infant (umbilical artery pH=6.93), delivered with a tight double nuchal cord, was shown by magnetic resonance imaging to have a large transient dural sinus dilatation.115 The neonate received positive pressure ventilation with 100% oxygen, inhaled nitric oxide, and hypothermia for neuroprotection. Follow-up imaging confirmed the absence of intracranial vascular malformations and normalization of the dilated sinus within 2 weeks of life. Similarly, these authors attributed the marked dural sinus dilatation to intracerebral vascular stasis due to the tight nuchal cord.115 Our prospective study of 105 fetuses with nuchal cords (with gestational age-matched controls) did not show increased intracranial vascular resistance of fetuses with nuchal cord in the third trimester of pregnancy.78 In contrast, the above two reports of dural sinus dilatation in newborns with multiple loops of nuchal cords followed deliveries complicated by the notation of “tight” nuchal cords at delivery. Butenandt et al describe eight children who manifested panhypopituitarism, severe growth hormone deficiency, or neurosecretory dysfunction for growth hormone, who all had a common background of umbilical cord encirclement.116 Notwithstanding that the etiology of the endocrinopathy could have been primary malformation of the pituitary gland, apoplexy of the pituitary, or hypothalamic dysfunction, these authors speculated that the last of these is predominant in cases of umbilical cord entanglement.116
In contrast to the above severe intracranial manifestations possibly associated with nuchal cords(s) at delivery, a prospective study of a set of 115 patients with well-established dates and singleton, appropriate for gestational age, non-anomalous fetuses between 28 and 41 weeks’ gestation, and nuchal cord(s) (103 fetuses with a single loop and 12 with a double nuchal cord) in comparison with gestational age-matched controls, showed no significant differences in gestational age at delivery, incidence of meconium-stained amniotic fluid, birth weight, mode of delivery, neonatal gender, 1- and 5-minute Apgar scores, umbilical artery pH, and base excess levels. This study, which included Doppler velocimetry of the fetal middle cerebral artery, demonstrated that prenatal cerebral vascular resistance is unaffected by the presence of nuchal cord(s).78
Summary
Prenatal sonographic diagnosis of nuchal cord(s) is currently readily available with real-time, color, and power Doppler, and three-dimensional technology. Nuchal cord(s) represent a dynamic condition in utero, and may form or alternatively undergo spontaneous resolution at times within days, if not hours. Therefore, 100% prenatal sensitivity and specificity values in prenatal sonographic diagnosis of nuchal cord(s) at delivery are neither expected nor attainable. Nevertheless, current (acceptable) sensitivity rates continue to increase.
Given the previously described abundant protective mechanisms of the extracorporeal component of the fetal cardiovascular system, the umbilical vessels are exceptionally protected from potential compressive and/or shearing forces. It is not surprising, therefore, that the vast majority of fetuses with nuchal cord(s) are not at increased risk of adverse perinatal outcome prior to or during labor. This notwithstanding, emerging data suggest that multiple loops of nuchal cord (≥3), especially in the presence of compounding factors, mainly consisting of compromised inherent protective mechanisms of the umbilical cord vasculature, or in the presence of a placental insufficiency-associated fetal growth restriction, or coexisting true knot of the umbilical cord, the risk for stillbirth or compromised neonatal status at delivery appears likely to be increased. Assuming that all labors are conducted with continuous electronic fetal monitoring, no clinical advantage has been proven regarding intrapartum depiction of nuchal cord(s), as any potential compromise of the umbilical cord will manifest primarily with fetal heart rate changes and, if warranted, actions will be taken secondary to these changes even in the absence of imaging depiction of the presence of nuchal cord(s). Thus, if any clinical advantages are to be attributed to prenatal diagnosis of nuchal cord(s), these would entail increased antepartum fetal surveillance to decrease potential stillbirth in selected cases. In our assessment, third-trimester prenatal sonographic diagnosis of nuchal cord(s) should be documented, reported, and discussed with the patient. Although there is currently a clear absence of prospective (randomized) data, we believe that prenatal sonographic diagnosis of multiple loops of nuchal cord (≥3) should lead to antepartum testing for fetal well-being. Potential confounding factors associated with an increased risk for adverse outcome should always be sought, and in their presence, increased fetal surveillance may be warranted even in the case of a single nuchal cord. Simply negating the potential clinical benefit of prenatal diagnosis of nuchal cord(s), given the usual uneventful outcomes associated with this condition at delivery, in our belief represents a likely unethical, scientifically incorrect oversimplification. Close prenatal fetal surveillance appears to be indicated in selected cases, and consideration should be given to delivery if and when indicated.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Liu LC, Huang HB, Yu MH, Su HY. Analysis of intrauterine fetal demise – A hospital based study in Taiwan over a decade. Taiwan J Obstet Gynecol. 2013;52:546–550.
2. Lehtonen T, Marrkula T, Soidinsalo P, Otonkonski S, Laine J. Causes of stillbirth in Turku, Finland, 2001–2011. Pediatr Dev Pathol. 2017;20(1):5–15. doi:10.1177/1093526616686236
3. Hammad IA, Blue NR, Allhouse AA, et al.; NICHD Stillbirth Collaborative Research Network Group. Umbilical cord abnormalities and stillbirth. Obstet Gynecol. 2020;135:644–652. doi:10.1097/AOG.0000000000003676
4. Parast MM, Crum CP, Boyd TK. Placental histologic criteria for umbilical blood flow restriction in unexplained stillbirth. Hum Pathol. 2008;39(6):948–953. doi:10.1016/j.humpath.2007.10.032
5. Ryan ED, Trivedi N, Bernirschke K, Lacoursiere DY, Parast MM. Placental histologic criteria for diagnosis of cord accident: sensitivity and specificity. Pediatr Dev Pathol. 2012;15(4):275–280. doi:10.2350/11-12-1127-OA.1
6. Sherer DM, Amoabeng O, Dryer A, Dalloul M. Current perspectives of prenatal sonographic diagnosis and clinical management challenges of true knot of the umbilical cord. Int J Womens Health. 2020;12:221–233. doi:10.2147/IJWH.S192260
7. Benirschke K, Kaufman P. Anatomy and pathology of the umbilical cord and major fetal vessels. In: Benirschke K, Kaufman P, editors. Pathology of the Human Placenta.
8. Raio L, Ghezzi F, Di Naro E, Franchi M, Bruhwiler H. Prenatal assessment of the Hyrtl anastomosis and evaluation of its function: case report. Hum Reprod. 1999;14(7):1890–1893. doi:10.1093/humrep/14.7.1890
9. Raio L, Ghezzi F, Di Naro E, Balestrei D, Duria P, Scheinder H. In-utero characterization of the blood flow in Hyrtl anastomosis. Placenta. 2001;22(6):597–601. doi:10.1053/plac.2001.0685
10. Sherer DM, Manning FA. Prenatal ultrasonographic diagnosis of conditions associated with potential umbilical cord compression. Am J Perinatol. 1999;16(9):445–458. doi:10.1055/s-1999-6807
11. Sherer DM, Dalloul M, Ward K, et al. Coexisting true umbilical cord knot and nuchal cord: possible cumulative increased risk of adverse perinatal outcome. Ultrasound Obstet Gynecol. 2017;50(3):404–405. doi:10.1002/uog.17389
12. Gurau D, Zaltz A, Yoo WK, Rahmani MR. All tied up and nowhere to go: report of a figure-eight umbilical cord complex true knot and triple nuchal cord detected on antenatal sonography. J Ultrasound Med. 2016;35:1361–1363. doi:10.7863/ultra.15.09044
13. Nkwabong E, Ndoumbe Mballo J, Dobbit JS. Risk factors for nuchal cord entanglement at delivery. Int J Gynaecol Obstet. 2018;4(1):108–112. doi:10.1002/ijgo.12421
14. Ogueh O, Al-Tarkait A, Vallerand D, et al. Obstetrical factors related to nuchal cord. Acta Obstet Gynecol Scand. 2006;85(7):810–814. doi:10.1080/00016340500345428
15. Rhoades DA, Latza U, Mueller BA. Risk factors and outcomes associated with nuchal cord. A population-based study. J Reprod Med. 1999;44:39–45.
16. Dursun P, Salman MC, Ozyuncu O, Aksu T. Nuchal cord type B associated with an excessively long umbilical cord as a cause of stillbirth. Clin Exp Obstet Gynecol. 2004;31(2):58–59.
17. Spellacy WN, Graven H, Fisch RO. The umbilical cord and complications of true knot, nuchal cords, and cords around the body. Am J Obstet Gynecol. 1966;94:1136–1142. doi:10.1016/0002-9378(66)90777-0
18. Larson JD, Rayburn WF, Harlan VL. Nuchal cord entanglements and gestational age. Am J Perinatol. 1997;14:555–557. doi:10.1055/s-2007-994333
19. Clapp JF, Stepanchak W, Hashimoto K, Ehrenberg H, Lopez B. The natural history of nuchal cords. Am J Obstet Gynecol. 2003;189:488–493. doi:10.1067/S0002-9378(03)00371-5
20. Schäffer L, Burkhardt T, Zimmermann R, Kurmanavicius J. Nuchal cords in term and postterm deliveries – do we need to know? Obstet Gynecol. 2005;106(1):23–28. doi:10.1097/01.AOG.0000165322.42051.0f
21. Crawford JS. Cord around the neck: incidence and sequelae. Acta Paediatr. 1962;51:594–603. doi:10.1111/j.1651-2227.1962.tb06586.x
22. Crawford JS. Cord around the neck: further analysis of incidence. Acta Paediatr. 1964;53:553–557. doi:10.1111/j.1651-2227.1964.tb07267.x
23. Shui KP, Eastman NJ. Coiling of the umbilical cord around the fetal neck. J Obstet Gynaecol Br Emp. 1957;64:227–228. doi:10.1111/j.1471-0528.1957.tb02625.x
24. Dipple AL. Maligned umbilical cord entanglements. Am J Obstet Gynecol. 1964;88:1012–1019. doi:10.1016/S0002-9378(16)35085-2
25. Cho FN, Liu CB, Li JY, Carey JR, Liou WS. Absent fetal movement and brain sparing effect associated with multiple tight nuchal cords. Taiwan J Obstet Gynecol. 2013;52:457. doi:10.1016/j.tjog.2013.06.009
26. Sherer DM, Abramowicz JS, Hearn B, Jones AP, Woods JR. Prenatal sonographic diagnosis, assessment, and management of a fetus with a quadruple nuchal cord at 39 weeks’ gestation. Am J Perinatol. 1991;8(6):383–384. doi:10.1055/s-2007-999421
27. Steinfeld JD, Ludmir J, Eife S, Robbins D, Samuels P. Prenatal detection and management of a quadruple nuchal cord. A case report. J Reprod Med. 1992;37(12):989–991.
28. Hinkson L, Pahlitzsch T, Henrich W. Sextuple rings of nuchal cord by breech presentation: a warning sign. Ultrasound Obstet Gynecol. 2019;54(6):843–844. doi:10.1002/uog.2036
29. Judy C, Bell LA. A case of seven nuchal loops and a review of the literature. Mo Med. 2005;102(6):569–570.
30. Wang Y, Le Ray C, Audibert F, Wagner MS. Management of nuchal cord with multiple loops. Obstet Gynecol. 2006;112(2 Pt 2):460–461. doi:10.1097/AOG.Ob03e3181fd75c
31. Dodds M, Windrim R, Kingdom J. Complex umbilical cord entanglement. J Matern Fetal Neonatal Med. 2012;25(9):1827–1829. doi:10.3109/14767058.2011.644361
32. Duffy S, Cochrane R. A difficult delivery associated with a nuchal cord found nine times around the fetal neck. J Obstet Gynaecol. 2007;27(8):859–860. doi:10.1080/01443610701748336
33. Milan DB, Konan J, Kouakou KC, Angoi V, Gbary E, Itoua C. Severe antenatal strangulation and sudden fetal death occurs at term. Clin Exp Obstet Gynecol. 2016;43(1):6.
34. Anyaegbunam A, Brustman L, Divon M, Langer O. The significance of antepartum variable decelerations. Am J Obstet Gynecol. 1986;155:707–710. doi:10.1016/S0002-9378(86)80003-5
35. Lyndrup J, Schouenborg L. Cord entanglement in monoamniotic twin pregnancies. Eur J Obstet Gynecol Reprod Biol. 1987;26:275–278. doi:10.1016/0028-2243(87)90080-3
36. Simmons JN, Rufleth P, Lewis PE. Identification of nuchal cords during nonstress testing. J Reprod Med. 1985;30:97–100.
37. Mendez-Bauer C, Troxell RM, Roberts JE, et al. A clinical test for diagnosing nuchal cords. J Reprod Med. 1987;32:924–927.
38. Sherer DM, Menashe M, Sadovsky E. Severe fetal bradycardia caused by external vibratory acoustic stimulation. Am J Obstet Gynecol. 1988;159:334–335. doi:10.1016/S0002-9378(88)80079-6
39. Sherer DM, Abramowicz JS, Hearn-Stebbins B, Woods JR. Sonographic verification of a nuchal cord following a vibratory acoustic stimulation-induced severe variable fetal heart rate deceleration with expedient abdominal delivery. Am J Perinatol. 1991;8:345–346. doi:10.1055/s-2007-999411
40. Anyaegbunam A, Dichik A, Stoessel R, Mikhail MS. Vibroacoustic stimulation of the fetus entering the second stage of labor. Obstet Gynecol. 1994;83:963–966. doi:10.1097/00006250-199406000-00013
41. Giacomello F. Ultrasound determination of nuchal cord in breech presentation. Am J Obstet Gynecol. 1988;156:531–532. doi:10.1016/S0002-9378(88)80123-6
42. Collins JH. Nuchal cord type A and type B. Am J Obstet Gynecol. 1997;177(1):94. doi:10.1016/S0002-9378(97)70444-7
43. Peesay M. Nuchal cord and its implications. Matern Health Neonatol Perinatol. 2017;3:28. doi:10.1186/s40748-017-0068-7
44. Henry E, Andres RL, Christensen RD. Neonatal outcomes following a tight nuchal cord. J Perinatol. 2013;33:231–234. doi:10.1038/jp.2012.79
45. Schaefer M, Laurichesse-Delmas H, Ville Y. The effect of nuchal cord on nuchal translucency measurements at 10–14 weeks. Ultrasound Obstet Gynecol. 1998;11:271–273. doi:10.1046/j.1469-0705.1998.11040271.x
46. Maymon R, Herman A, Dreazen E, Tovbin Y, Bukovsky I, Weintraub Z. Can nuchal cord cause transient increased nuchal translucency. Hum Reprod. 1999;14:56–59.
47. Scheier M, Egle D, Himmel I, et al. Impact of nuchal cord on measurement of fetal nuchal translucency thickness. Ultrasound Obstet Gynecol. 2007;30:197–200. doi:10.1002/uog.4064
48. Plasencia W, Lopez P, Esparaza M, Garcia R, Barber MA, Garcia JA. Influence of nuchal cord on ductus venosus assessment at 11 to 3 + 6 weeks’ gestation. Ultrasound Obstet Gynecol. 2010;35:263–266. doi:10.1002/uog.7564
49. Petousis S, Margioula-Siarkou C, Mamopoulos A, et al. Does first-trimester nuchal cord affect the blood flow in the ductus venosus? A prospective observational study. J Matern Fetal Neonatal Med. 2018;31:3115–3118. doi:10.1080/14767058.2017.13651
50. Tepper R, Kidron D, Aviram R, Markovitch O, Hershkovitz R. High incidence of cord entanglement during early pregnancy detected by three-dimensional sonography. Am J Perinatol. 2009;26(5):379–382. doi:10.10/s-0028-1110090
51. Lee PR, Won HS, Chung JY, Shin HJ, Kim A. The variables affecting nuchal skin-fold thickness in mid-trimester. Prenat Diagn. 2003;23(1):60–64. doi:10.1002/pd.522
52. Ozkavukcu E, Haliloglu N. The effect of nuchal cord on nuchal fold thickness measured in the second trimester. Eur J Radiol. 2012;81(2):e23–5. doi:10.1016/j.erad.2010.12.090
53. Ergin RH, Yayla M, Ergin AS. Fetal demise to cord entanglement in the early second trimester. Proc (Bayl Univ Med Cent). 2014;27(2):143–144. doi:10.1080/08998280.2014.11929092
54. Gambir PS, Gupte S, Kamat AD, Patankar A, Kulkarni VD, Phadke MA. Chronic umbilical cord entanglements causing intrauterine fetal demise in the second trimester. Pediatr Dev Pathol. 2011;14(3):252–254. doi:10.2350/10-06-0846-CR.1
55. Joupilla P, Kirkinen P. Ultrasonic diagnosis of nuchal encirclement by the umbilical cord: a case a methodological report. J Clin Ultrasound. 1982;10(2):59–62. doi:10.1002/jcu.1870100205
56. Collins JH, Collins CL, Weckwerth SR, De Angelis L. Nuchal cords: timing of prenatal diagnosis and duration. Am J Obstet Gynecol. 1995;173:768. doi:10.1016/0002-9378(95)90337-2
57. Ranzini AC, Walters CA, Vintzileos AM. Ultrasound diagnosis of nuchal cord; the gray-scale divot sign. Obstet Gynecol. 1999;93(Pt 2):854.
58. Verdel MJC, Exalto N. Tight nuchal coiling of the umbilical cord causing fetal death. J Clin Ultrasound. 1994;22:64–66. doi:10.1002/jcu.1870220115
59. Jauniaux E, Mawissa C, Peellaerts C, Rodesch F. Nuchal cord in normal third-trimester pregnancy: a color Doppler imaging study. Ultrasound Obstet Gynecol. 1992;2(6):417–419. doi:10.1046/j.1469-0705.1992.02060417.x
60. Morgan-Ortiz F, Rodriguez-Ontiveros C, Chang-Batiz H, Avila-Vergara MA. Evaluation of ultrasound as a diagnostic test in nuchal encirclement by the umbilical cord during labor. Ginecol Obstet Mex. 1997;65:529–532.
61. Qin Y, Wang CC, Lau TK, Rogers MS. Color sonography: a useful technique in the identification of nuchal cord during labor. Ultrasound Obstet Gynecol. 2000;15(5):413–417. doi:10.1046/j.1469-0705.2000.00113.x
62. Romero-Gutierrez G, Estrada Razo S, Chavez Curiel A, Ponce de Leon AL. Color flowmetry values in fetuses with nuchal cord encirclement. Ginecol Obstet Mex. 2000;68:401–407.
63. Askoy U. Prenatal color Doppler sonographic evaluation of nuchal encirclement by the umbilical cord. J Clin Ultrasound. 2003;3(9):473–477.
64. Hanoaka U, Yanagihara T, Tanaka H, Hata T. Comparison of three-dimensional, two dimensional and color Doppler ultrasound in predicting the presence of nuchal cord at birth. Ultrasound Obstet Gynecol. 2002;19(5):47. doi:10.1046/j.0960-7692.2001.00612.x
65. Peregrine E, O’Brien P, Jauniaux E. Ultrasound detection of nuchal cord prior to labor induction and the risk of Cesarean section. Ultrasound Obstet Gynecol. 2005;25(2):60–64. doi:10.1002/uog.1767
66. Markov D, Milanova K, Dimitrov A, Marinov B, Nikolov A, Ivanov S. Nuchal cord between 37 and 42 completed weeks of gestation – diagnosis and prognostic value. Akush Ginecol (Sofia). 2007;46(7):3–10.
67. Heifetz SA. Single umbilical artery: a statistical analysis of 237 autopsy cases and review of the literature. Perspect Pediatr Pathol. 1984;8:345–378.
68. Friebe-Hoffmann U, Hiltmann A, Friedl TWP, et al. Prenatally diagnosed single umbilical artery (SUA) – retrospective analysis of 1169 fetuses. Ultraschall Med. 2019;40(2):221–229. doi:10.1055/s-0043-123463
69. Gutvirtz G, Walfisch A, Beharier O, Sheiner E. Isolated single umbilical artery is an independent risk factor for perinatal mortality and adverse outcomes in neonates. Arch Gynecol Obstet. 2016;294(5):931–935. doi:10.1007/s00404-016-4088-8
70. Sherer DM, Khoury-Collado F, Dalloul M, et al. Recurrent antepartum compression of a single artery double nuchal cord necessitating cesarean delivery. Am J Perinatol. 2005;22(8):437–440. doi:10.1055/s-2005-916286
71. Ghezzi F, Raio L, Di Naro E, Franch M, Balestreri D, D’Addario V. Nomogram of Wharton’s jelly as depicted in the sonographic cross-section of the umbilical cord. Ultrasound Obstet Gynecol. 2001;18(2):121–125. doi:10.1046/j.1469-0705.2001.00468.x
72. Raio L, Ghezzi F, Di Naro E, et al. Prenatal diagnosis of a lean umbilical cord: a simple marker for the fetus at risk of being small for gestational age at birth. Ultrasound Obstet Gynecol. 1999;3(3):176–180. doi:10.1046/j.1469-0705.1999.13030176.x
73. Ghezzi RL, Di Naro E, Duwe DG, Cromi A, Scheider H. Umbilical cord morphologic characteristics and umbilical artery Doppler parameters in intrauterine growth-restricted fetuses. J Ultrasound Med. 2003;22(12):1341–1347. doi:10.7863/jum.2003.22.12.1341
74. Raio L, Ghezzi F, Di Naro E, Franxhi M, Brühwiler H, Lüscher KP. Prenatal assessment of Wharton’s jelly in umbilical cords with single artery. Ultrasound Obstet Gynecol. 1999;14(1):42–46. doi:10.1046/j.1469-0705.1999.14010042.x
75. Strong TH
76. Strong TH
77. Strong TH
78. Sherer DM, Sokolovski M, Dalloul M, Khoury-Collado F, Abulafia O. Is fetal cerebral vascular resistance affected by the presence of nuchal cord(s) in the third-trimester of pregnancy? Ultrasound Obstet Gynecol. 2005;25(5):454–458. doi:10.1002/uog.1874
79. Strong TH
80. Lipitz S, Seidman DS, Gale R, et al. Is fetal growth affected by cord entanglement? J Perinatol. 1993;13(5):385–388.
81. Carey JC, Rayburn WF. Nuchal cord encirclements and birth weight. J Reprod Med. 2003;48(6):460–462.
82. Mastrobattista JM, Hollier LM, Yeomans ER, et al. Effects of nuchal cord on birthweight and immediate neonatal outcomes. Am J Perinatol. 2005;22(2):83–85. doi:10.1055/s-2005-837737
83. Osak R, Webster KM, Bocking AD, Campbell MK, Richardson BS. Nuchal cord evident at birth impacts on fetal size relative to that of the placenta. Early Hum Dev. 1997;49(3):193–202. doi:10.1016/S0378-3782(97)00030-3
84. Sornes T. Umbilical cord encirclements and fetal growth restriction. Obstet Gynecol. 1995;86(5):725–728. doi:10.1016/0029-7844(95)00258-S
85. Bord A, Yagel S, Valsky DV. Overburdened and under nourished. Am J Obstet Gynecol. 2007;197:
86. Hoh JK, Sung YM, Park MI. Fetal heart rate parameters and perinatal outcomes in fetuses with nuchal cords. J Obstet Gynaecol Res. 2012;38(2):358–363. doi:10.1111/j.1447-0756.2011.01707.x
87. Sherer DM, Dalloul M, Sabir S, London V, Haughton M, Abulafia O. Persistent quadruple nuchal cord throughout the third trimester associated with decelerating fetal growth. Ultrasound Obstet Gynecol. 2017;49(3):409–410. doi:10.1002/uog.5940
88. Shepherd AJ, Richardson CJ, Brown JP. Nuchal cord as a cause of fetal anemia. Am J Child Dis. 1985;139:71–73.
89. Vanhaesbrouck P, Vanneste K, de Paeter C, Van Trappen Y, Thiery M. Tight nuchal cord and neonatal hypovolemic shock. Arch Dis Child. 1987;62:1276–1277. doi:10.1136/adc.62.12.1276
90. Martinez-Biarge M, Cheong JL, Diez-Sebastian J, Mercuri E, Dubowitz LM, Cowan FM. Risk factors for neonatal arterial ischemic stroke: the importance of the intrapartum period. J Pediatr. 2016;173:62–68.e1. doi:10.1016/j.jpeds.2016.02.064
91. Zhao F, Geng Q, Knog F, Ning Y. Quantitative analysis of tightness of nuchal cord and its relationship with fetal intrauterine distress. Int J Clin Exp Med. 2015;8(10):17507–17514.
92. Pilu G, Falco P, Guazzarini M, Sandri F, Bovicelli L. Sonographic demonstration of nuchal cord and abnormal umbilical artery waveform heralding fetal distress. Ultrasound Obstet Gynecol. 1998;12(2):125–127. doi:10.1046/j.1469-0705.1998.12020125.x
93. Ghi T, D’Emidio L, Morandi R, Casadio P, Pilu G, Pelusi G. Nuchal cord entanglement and outcome of labor induction. J Prenat Med. 2007;1(4):57–60.
94. Flamm BL. Tight nuchal cord and shoulder dystocia: a potentially catastrophic complication. Obstet Gynecol. 1999;94(5 Pt 2):853.
95. Perlitz Y, Ben-Sholomo I, Ben-Ami M. Acute polyhydramnios in term pregnancy may be caused by multiple nuchal cord loops. Ultrasound Obstet Gynecol. 2010;35(2):253–254. doi:10.1002/uog.7543
96. Al-Kouatly HB, Schuster SS, Skupski DW. Double nuchal umbilical cord and breech presentation. The value of close follow up. Gynecol Obstet Invest. 2003;56(3):121–123. doi:10.1159/000073370
97. Boujenah J, Fleury C, Pharisien I, et al. Cord accident after external cephalic version: reality or mostly myth? Gynecol Obstet Fertil Senol. 2017;45(1):9–14. doi:10.1016/j.gofs.2016.12.001
98. Miser WF. Outcome of infants born with nuchal cords. J Family Pract. 1992;34(4):44–45.
99. Sheiner E, Abramowicz JS, Levy A, Silberstein T, Mazor M, Hershkovitz R. Nuchal cord is not associated with adverse perinatal outcome. Arch Gynecol Obstet. 2006;274(2):81–83. doi:10.1007/s00404-005-0110-2
100. Hankins GD, Synder RR, Hauth JC, Gilstrap LC, Hammond T. Nuchal cord and neonatal outcome. Obstet Gynecol. 1987;70:687–691.
101. Ghosh GS, Gudmundsson S. Nuchal cord in post-term pregnancy – relationship to suspected intrapartum fetal distress indicating operative intervention. J Perinat Med. 2008;36(2):142–144. doi:10.1515/JPM.2008.012
102. Larson JD, Rayburn WF, Crosby S, Thirnau GR. Multiple cord entanglements and intrapartum complications. Am J Obstet Gynecol. 1995;173:1228–1231. doi:10.1016/0002-9378(95)91359-9
103. Jauniaux E, Ramsay B, Peellaerts C, Scholler Y. Perinatal features of pregnancies complicated by nuchal cord. Am J Perinatol. 1995;12(4):225–228. doi:10.1055/s-2007-994467
104. Schreiber H, Daykan Y, Arbib N, Markovitch O, Berkovitz A, Biron-Shental T. Adverse pregnancy outcomes and multiple nuchal cord loops. Arch Gynecol Obstet. 2019;300(2):279–283. doi:10.10017s000404-019-05178
105. Mariya T, Fujibe Y, Shinkai S, et al. Multiple part cord entanglement and neonatal outcomes. Taiwan J Obstet Gynecol. 2018;57(5):672–676. doi:10.1016/j.tjog.2018.09.001
106. Önderglu LS, Dursun P, Durukan T. Perinatal features and umbilical cord blood gases in newborns complicated with nuchal cord. Turk J Pediatr. 2008;50:466–470.
107. Sepulveda W. Antenatal course and perinatal outcome after ultrasound detection of triple nuchal cord: a case series. J Matern Fetal Neonatal Med. 2019;16:1–6.
108. Clapp JF
109. MacLennan AH, Thompson SC, Gecz J. Cerebral palsy: causes, pathways, and the role of genetic variants. Am J Obstet Gynecol. 2015;213(6):779–788. doi:10.1016/j.ajog.2015.05.034
110. Nelson KB, Grether JK. Potentially asphyxiating conditions and spastic cerebral palsy in infants of normal birth weight. Am J Obstet Gynecol. 1998;179:507–513. doi:10.1016/S0002-9378(98)70387-4
111. Nielsen LE, Schendel D, Grove J, et al. Asphyxia-related risk factors and their timing in spastic cerebral palsy. BJOG. 2008;115(12):1518–1528. doi:10.1111/j.1471-0528.2008.0896.x
112. Greenwood C, Impey L. The association of nuchal cord with cerebral palsy is influenced by recording bias. Early Hum Dev. 2002;68(1):15–19. doi:10.1016/S0378-3782(02)00005-1
113. Gutvirtz G, Wainstock T, Massad R, Landau D, Sheiner E. Does nuchal cord at birth increase the risk for cerebral palsy? Early Hum Dev. 2019;33:1–4. doi:10.1016/j.earlhumdev.2019.04.006
114. Katz ME, Bass WT, White LE. Dural sinus ectasia after prolonged nuchal cord. J Ultrasound Med. 1992;11:289–292. doi:10.7863/jum.1992.11.6.289
115. Aziz AA, Strizke A. Giant transient dural sinus dilatation with neonatal cord. Pediatr Neonatol. 2019;60:346–347. doi:10.1016/j.pedneo.2018.08.001
116. Butenandt O, Vogl T, Stemmler J. Magnetic resonance tomography studies of the hypophysis in children born with umbilical cord entanglement. Clin Pediatr. 1996;208:26–28.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.