Back to Journals » International Journal of Women's Health » Volume 12
Current Perspectives of Prenatal Sonographic Diagnosis and Clinical Management Challenges of True Knot of the Umbilical Cord
Authors Sherer DM , Amoabeng O, Dryer AM, Dalloul M
Received 10 October 2019
Accepted for publication 23 February 2020
Published 27 March 2020 Volume 2020:12 Pages 221—233
DOI https://doi.org/10.2147/IJWH.S192260
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Everett Magann
David M Sherer, Opokua Amoabeng, Alexandra M Dryer, Mudar Dalloul
Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, State University of New York (SUNY), Downstate Health Sciences University, Brooklyn, NY, USA
Correspondence: David M Sherer
Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, State University of New York (SUNY), Downstate Health Sciences University, 450 Clarkson Avenue, Box 24, Brooklyn, NY, USA
Tel +1 718 270-2081
Fax +1 718 270-4122
Email [email protected]
Abstract: Umbilical cord accidents preceding labor are rare. Single and multiple nuchal cords, and true knot(s) of the umbilical cord, are often incidental findings noted at delivery of non-hypoxic non-acidotic newborns without any evidence of subsequent adverse neonatal outcome. In contrast to single nuchal cords, true knots of the umbilical cord, which occur in between 0.04% and 3% of all deliveries, have been associated with a reported 4 to 10 fold increased risk of stillbirth. First reported with real-time ultrasound, current widespread application of color Doppler, power Doppler and three-dimension sonography, has enabled increasingly more accurate prenatal sonographic diagnoses of true knot(s) of the umbilical cord. Reflecting the inability to visualize the entire umbilical cord at prenatal ultrasound assessment, despite detailed second and third-trimester scanning, many occurrences of incidental true knot of the umbilical cord remain undetected and are noted only at delivery. Although prenatal sonographic diagnostic accuracy is increasing, false positive sonographic diagnosis of true knot of the umbilical cord cannot be ruled out with certainty, and must continue to be considered clinically. Notwithstanding the inability to diagnose all true knots, currently there is a clear absence of clinical management guidelines by governing bodies regarding patients in whom prenatal sonographic diagnosis of true knot(s) of the umbilical cord is / are suspected. As a result, in many prenatal ultrasound units, suspected sonographic findings suggestive of or consistent with true knot of the umbilical cord are often disregarded, not documented, and patients are not uniformly informed of this potentially life-threatening condition, which carries an associated considerable risk of stillbirth. This commentary will address current perspectives of prenatal sonographic diagnostic and management challenges associated with true knot(s) of the umbilical cord in singleton pregnancies.
Keywords: prenatal ultrasound, true knot of the umbilical cord
Introduction
During early human embryogenesis, the umbilical cord develops from the yolk sac and allantois. At approximately 18 days post-conception, a duct-like extension of the yolk sac from the future caudal region of the embryo, develops into the connecting stalk – the transitory allantois.1 Subsequently, on post-conception day 22, both the allantois and extra-embryonic yolk sac extend into the mesenchyme of the connecting stalk. Between days 28 and 40, the expanding amniotic cavity surrounds the embryo and the allantois and yolk sac are compressed into a cord covered by amnion, thus forming the umbilical cord.1
The cord lengthens as the embryo prolapses backward into the amniotic sac. During the third post-conception week, two allantoic arteries (originating from the internal iliac arteries) and one allantoic vein (entering the hepatic vein) penetrate the placenta and become connected with the villous vessels. Wharton’s jelly, the subamniotic connective tissue of the umbilical cord, is derived from extraembryonic mesoblast (composed of hyaluronic acid with glycosyl and mannosyl groups distributed in a fine network of micro-fibrils and little collagen), accounting for the mucoid, compressible properties of the umbilical cord. Concurrent with elongation of the umbilical cord during the first trimester, this structure becomes coiled. The average length of the umbilical cord is approximately 55 cm, with lengths <35–40 cm and >80 cm, being considered short and excessively long, respectively. The latter has been associated with increased coiling and increased risk of entanglement and knotting.1
Thus, the length of the umbilical cord, Wharton’s jelly, the presence of two arteries, coiling, and suspension in amniotic fluid, all contribute to protective buffering of the cord from twisting, shearing and compression forces throughout gestation and specifically, labor.
Finally, an additional safety mechanism is the presence of a 1.5–2 cm shunt between the umbilical arteries within 3 cm of the placental cord insertion, the Hyrtl anastomosis, which is present in approximately 96% of umbilical cords.1 This arterial anastomosis equalizes pressures between the respective umbilical arteries before entering the placenta and functions as a safety valve in the event of placental compression or blockage of an umbilical artery.1 Prenatal sonographic assessment of the Hyrtl anastomosis, has depicted pulsatile unidirectional flow within the Hyrtl anastomosis toward the umbilical artery with lower resistance index, supporting the hypothesis that the Hyrtl anastomosis plays an important function when the placental areas supplied by the umbilical arteries, differ in size.2,3
An array of umbilical cord abnormalities exists including among others, single umbilical artery, fused umbilical arteries, umbilical cord cysts, umbilical artery aneurysm, four-vessel cords (resulting from the persistence of the right umbilical vein), umbilical vein varix, umbilical vein thrombosis, umbilical cord hemangioma, umbilical cord stricture, and abnormalities of the intra-abdominal fetal umbilical vein.1,4,5
Despite the previously described protective mechanisms of the umbilical cord, this structure – critical for fetal development, is prone to potential compression and entanglement problems, such as nuchal loops (single or multiple) and the formation of true (and compound) knots (Figure 1).6,7
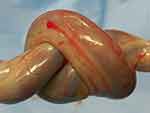 |
Figure 1 A true knot of the umbilical cord. |
An esoteric potential umbilical cord entanglement/knotting is that of monochorionic monoamniotic twins who often will incur this complication, leading to the recommendation of early delivery by Cesarean, as high perinatal mortality rates of between 28% and 47% have been associated with this occurrence.8–12 Interestingly, prenatal sonographic diagnosis of umbilical cord entanglement of monochorionic monoamniotic twins has been reported as early as 10 weeks’ gestation (Figure 2).13,14 Similarly interesting yet not surprising, is the high frequency of this observation among monochorionic monoamniotic twins, as high as 100% as reported by Dias et al in a study of eighteen such pregnancies.10 Perinatal loss rates reported in this study were 11.1% and 5.9% after 16 and 20 weeks’ gestation, respectively.10 A systematic review in 2013 of nine studies published between January 2000 and December 2011 of a total of 114 monoamniotic twin pregnancies (228 fetuses) with cord entanglement, reported an overall survival rate of 88.6% (201/228). Perinatal mortality was reported in 11.4% fetuses (26/228), of these 65% (17/26) were stillbirth and 35% (9/26) died at birth.15 Overall, a positive predictive value of 89% in the sonographic diagnosis of cord entanglement of monoamniotic twins has been attributed to color Doppler imaging and Doppler velocimetry.16
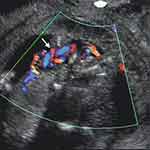 |
Figure 2 Transvaginal color Doppler imaging of entangled/entwined umbilical cords of monochorionic monoamniotic twins at 10 weeks’ gestation. Arrow points to “branching” of the umbilical artery indicative of cord entanglement. One week later both fetuses had succumbed following the entanglement.Note: Reproduced with permission from Sherer DM, Sokolovski M, Haratz-Rubinstein N. Diagnosis of umbilical cord entanglement of monoamniotic twins by first-trimester color doppler imaging. J Ultrasound Med. 2002;21(11):1307. © 2016 by the American Institute of Ultrasound in Medicine.13 |
That prenatal sonographic diagnosis of umbilical cord entanglement of monochorionic monoamniotic twins antedated prenatal sonographic diagnosis of true knot(s) of the umbilical cord in singleton pregnancies is not surprising. This likely reflects a number of reasons. First, as mentioned such umbilical cord entanglement is highly likely/commonplace in these cases and thus can be anticipated/suspected, sought and confirmed at sonographic assessment. Second, the presence of twins and the associated over-distended uterus likely enhances the likelihood of the umbilical cord entanglement being detectable, ie, with the clumped entanglement of the respective umbilical cords often located between the twins, surrounded by amniotic fluid enhancing/enabling sonographic detection.
A more rare event involving umbilical cord entanglement/knotting of twins is that of diamniotic twins, which may occur following spontaneous (or rarely intentional) septostomy and has been correctly identified by prenatal ultrasound in a limited number of occurrences (Figure 3).17–19
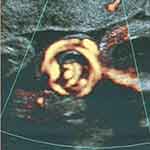 |
Figure 3 Umbilical cord entanglement of monochorionic diamniotic twins following spontaneous antepartum septostomy of the intervening membrane, sonographically mimicking a true knot of the umbilical cord.Note: Reproduced with permission from Sherer DM, Bitton C, Stimphil R, et al. Cord entanglement of monochorionic diamniotic twins following spontaneous antepartum septostomy sonographically simulating a true knot of the umbilical cord. Ultrasound Obstet Gynecol. 2005;26(6):676–678.17. Copyright © 2005 ISUOG. Published by John Wiley & Sons, Ltd.17 |
This commentary will address current perspectives of prenatal sonographic diagnostic and management challenges associated with true knot(s) of the umbilical cord in singleton pregnancies.
True Knot(s) of the Umbilical Cord
It is generally considered that true knots of the umbilical cord are formed/occur in early gestation (between 9–12 weeks),20 when the overall amniotic fluid volume is considerably larger than the fetus. The umbilical cord “tracks” the fetus, which may at times subsequently pass through the “following” loop of umbilical cord, which trails behind a fetus executing a (forward or backward) “somersault” movement thus creating a true knot, which later gradually tightens.
Although not considered a recurring event, Semchyshyn and later Polis et al, and Linde et al, reported the occurrence of a true knot of the umbilical cord in consecutive pregnancies.21–23 Rarely, multiple (four and five) true knots of the umbilical cord have been reported.24–27 Predisposing factors in the formation of true knots of the umbilical cord include: long umbilical cords, polyhydramnios, excessive fetal movements, gestational diabetes, multiparity, male fetuses, chronic hypertension,28–30 and interestingly, patients who have undergone genetic amniocentesis.1,20,23,31 In 2018, a population-based study of 856,300 singleton births at >22 weeks’ gestation in Norway confirmed the previously mentioned predisposing factor of a long umbilical cord. Interestingly, these authors found a more than doubled risk of recurrence of a long (or short) cord, knot and entanglement in the same patient.23
Chasnoff and Flecher measured ex–vivo venous perfusion pressures of 50 umbilical cords, with and without a true knot.32 This study confirmed that loose umbilical cord knot did not affect venous perfusion pressure. However, with tightened knots, the smaller the umbilical cord diameter, the greater was the pressure required to perfuse past the knot. Umbilical vessels, protected by the myxomatous structure of the Wharton’s jelly, were rarely completely occluded. These findings were considered to correlate clinically with the relatively high incidence, yet the low stillbirth rate, associated with a true knot of the umbilical cord.32
Pathology assessment of true knots of the umbilical cord reveals that true knots of the umbilical cord cause compression of Wharton’s jelly. Microscopy often reveals mural thrombosis in the umbilical vein at the site of knotting.1 Venous distention distal to the knot is a characteristic finding in knots with clinical significance, as is the tendency of the unknotted cord to curl if the knot has been present for some time.1
In a report of a single case, Gembruch and Baschat with the aid of Doppler sonography in 1996, confirmed the earlier study of Chasnoff and Fletcher in vivo.33 Following the sonographic diagnosis of a true knot of the umbilical cord at 23 weeks’ gestation, utilizing color-coded Doppler ultrasound followed by pulsed wave Doppler spectral analysis, depicted a stenotic effect on the umbilical venous blood flow, with normal arterial blood flow. Marked acceleration of umbilical venous blood flow velocities from 15 cm/s pre-stenotically to 100 cm/s post-stenotically were demonstrated. This occurrence was transient, and was not noted at follow-up assessments.33 A healthy newborn was delivered by Cesarean at 33 and 1/7 weeks’ gestation with the presence of a true knot confirmed at delivery.32
In contrast to single nuchal cords, which occur in between 15.8% and 30% of singleton fetuses at term, and have not been unequivocally associated with significant adverse perinatal outcome,6,20,34-53 true knot(s) of the umbilical cord occur in 0.04% to 3% of deliveries, and have been associated with perinatal morbidity in 11% of cases, and a notable 4 to10 fold increase in stillbirth.28,29,53-62
Although (multiple) true knots of the umbilical cord have been infrequently implicated with fetal growth restriction, a causal association remains unproven.23,25,26,60 Recently, Chien et al reported that six prenatal/perinatal factors including umbilical cord knot among others (preeclampsia, polyhydramnios, oligohydramnios, placenta previa and gestational diabetes), may increase the risk of autism spectral disorder.63 Of note, these factors, including true knot of the umbilical cord were also associated with the severity of autistic symptoms, specifically stereotyped behaviors and socio-communication deficits.63,23,25
Prenatal Sonographic Diagnosis
Prenatal sonographic diagnosis of true knot(s) of the umbilical cord may be performed with real-time sonography, and with increasing accuracy with application of color or power Doppler imaging, and/or three-dimensional ultrasound.
Initial reports of prenatal sonographic diagnosis of a true knot of the umbilical cord involved 2D (real-time) ultrasound (albeit with considerably less than convincing images).58,64 These authors reported that sonographic findings similar to a “four-leaf clover” were suggestive of a true knot of the umbilical cord.
In 1995, Sepulveda in a study of 5,575 deliveries, retrospectively reviewed previous prenatal ultrasound assessments of 18 cases in which a true knot of the umbilical cord was identified at delivery.65 All 18 patients assessed had undergone earlier mid-trimester studies in which no abnormality of the umbilical cord was noted. Thirteen of the eighteen patients (72%) had undergone an additional third-trimester color Doppler sonographic assessment, which also did not disclose the presence of a true knot.65 Interestingly, two cases in which a true knot of the umbilical cord was suspected were not confirmed as such at delivery. These authors concluded (incorrectly) that true knots of the umbilical cord do not exhibit characteristic prenatal sonographic findings and are therefore easily missed at routine sonography.65
In the interim, almost 25 years later, much has changed with regard to the prenatal sonographic findings of true knot(s) of the umbilical cord. This mainly reflects availability and widespread application of higher resolution technology, color Doppler, power Doppler, three-dimensional sonographic imaging modalities and clearly, increased awareness of sonographers.
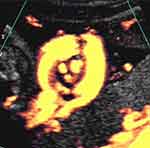
In 2004, Ramon y Cajal and Martinez, with the aid of color Doppler imaging in five cases, reported a new sonographic sign, which they termed the “hanging noose” sign in which an almost, yet incomplete circle of umbilical cord surrounds the centric, axial/transverse section through the umbilical cord (essentially interrogating the umbilical cord in an axial/transverse plane), “en face” in which the umbilical vessels (larger umbilical vein and two umbilical arteries) are clearly depicted within the aforementioned almost complete loop of cord.66 Of note, these authors also demonstrated intermittent narrowing of the umbilical vein (as depicted by power Doppler sonography) associated with pressure exerted by the transabdominal transducer.
In our unit, rather than referring to the “hanging noose sign” (with all of the associated negative connotations ), we prefer the far more patient-friendly term – the “smiley sign”, with the almost complete circle of umbilical cord representing the outline of the “face”, encircling the en-face umbilical arteries and umbilical vein, representing the “eyes” and “mouth/smile”, respectively, emanating from the knot (Figure 4).
Subsequently, three-dimensional ultrasound has enabled precise depiction of a true knot of the umbilical cord (Figures 5, 6, 7).67–76 Notwithstanding the potential clarity available with 3D ultrasound depiction of true knots(s) of the umbilical cord, we prefer to utilize color or power Doppler imaging initially, and only following notation of a likely true knot of the umbilical cord (“smiley sign”), will depict the presence of the already suspected true knot, with three-dimensional ultrasound.
 |
Figure 6 Three dimensional (3D) power Doppler image depicting true knot. Note the remarkable depiction of the umbilical vein and arteries, respectively. |
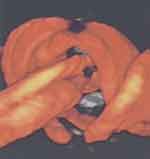 |
Figure 5 Three dimensional (3D) power Doppler image depicting true knot.Note: Reproduced with permission from Sherer DM, Dalloul M, Zigalo A, Bitton C, Dabiri L, Abulafia O. Power doppler and 3-dimensional sonographic diagnosis of multiple separate true knots of the umbilical cord. J Ultrasound Med. 2005;24:1321–1323. © 2016 by the American Institute of Ultrasound in Medicine.67 |
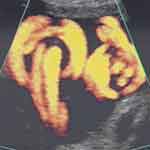 |
Figure 7 Power Doppler image depicting two adjacent, but separate true knots of the umbilical cord (note two “smiley faces”). Note: Reproduced with permission from Sherer DM, Dalloul M, Zigalo A, Bitton C, Dabiri L, Abulafia O. Power doppler and 3-dimensional sonographic diagnosis of multiple separate true knots of the umbilical cord. J Ultrasound Med. 2005;24:1321–1323. © 2016 by the American Institute of Ultrasound in Medicine.67 |
Sensitivity
It should be stated clearly that not all cases in which a true knot of the umbilical cord is suspected at prenatal sonography (even in the best of hands or advanced/enhanced technology), are confirmed as such at delivery. In contrast to single or multiple nuchal cords which may unravel and not be present at delivery, once a true knot occurs and is considered to have been depicted with prenatal sonography, it is highly unlikely that the fetus at advanced gestational ages (if ever) can or will, disentangle the already formed true knot. Thus clearly, false positive diagnoses occur. This raises valid questions regarding the overall sensitivity of the prenatal sonographic diagnosis of a true knot of the umbilical cord.
Indeed, in 2007 Hasbun et al in a limited study of eight consecutive cases assessed the precision of prenatal sonographic diagnosis of a true knot of the umbilical cord utilizing 3-dimensional power Doppler technology. Prenatal diagnosis was confirmed in only 5 of the 8 cases (62%).69
Recently, Bohîltea et al reported that of 18,500 deliveries during five years (between 2011 and 2105), 133 (0.71%) had true knots of the umbilical cord at delivery with only 16 cases diagnosed by prenatal ultrasound (0.08%).73
Hasbun et al attributed the false positive reports to multiple loops of the umbilical cord in the third-trimester. Our experience is similar in that indeed clumping/clustering of the umbilical cord can be incorrectly identified as a true knot of the umbilical cord, although it should be stated that with color Doppler, power Doppler and 3D sonography, false positive diagnoses should be fewer than earlier reported.69
Overall, the accuracy of sonographic prenatal diagnosis of the umbilical cord clearly depends on the presence of the knot in a visible area at sonography, awareness and experience of the sonographer, and the policy of the sonography unit in reporting this diagnosis.
In any event in our assessment, a sensitivity rate of approximately 2/3 of cases (or possibly higher) of a potential life-threatening condition (third-trimester stillbirth), in our assessment, is not to be taken lightly.
False Knot of the Umbilical Cord
False knot of the umbilical cord represents local vascular redundancies of umbilical vessels, and as such should not be listed as knots of the umbilical cord.1 In fact a less misleading, alternative term – “nodus spurious vasculosis” has been suggested for this condition of umbilical vessel redundancy.1 Notwithstanding, the sonographic appearance of exaggerated looping resulting in the spurious impression of greater than three umbilical cord vessels was reported associated with a false knot of the umbilical cord by Hertzberg et al in 1988.77 Currently, such sonographic findings in the clear absence of the described characteristic sonographic findings of a true knot in conjunction with application of three-dimensional sonography (and visualization of the umbilical cord from a different angle), as detailed by Merz and Pashj, enable clear prenatal sonographic differentiation of these two distinctly different anatomical entities.74
True Knot of the Umbilical Cord with Coexisting Nuchal Cord
Two recent reports have addressed prenatal sonographic diagnosis of coexisting true knot of the umbilical cord and nuchal cord.75,76 We reported a series of three such cases in the third-trimester, in which the true knot of the umbilical cord was located within the loop of nuchal cord itself (Figure 8).75 Interestingly, following extensive patient counseling, admission for continuous fetal monitoring and administration of intramuscular antenatal steroids to decrease overall prematurity-associated neonatal morbidities, in each of these three cases (in 36–37 weeks’ gestation) fetal heart rate monitoring disclosed prolonged fetal bradycardia which led to Cesarean delivery of uncompromised infants (in which all three cases had coexisting nuchal cords with a true knot of the umbilical cord located precisely as depicted by prenatal ultrasound within the nuchal cord loop) (Figure 8).75 While clearly one cannot assume that in these cases stillbirth, or other adverse neonatal outcomes would have occurred without intervention (electronic fetal heart rate monitoring following prenatal sonographic diagnosis), in light of the reported 11% of perinatal morbidity and 4 to 10 fold increase in stillbirth associated with this pathology, although unproven, this possibility should be considered.
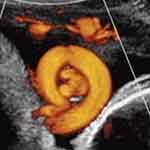 |
Figure 8 Power Doppler ultrasound image at 36 weeks’ gestation, depicting a co-existing nuchal and true knot of the umbilical cord. Prolonged fetal bradycardia necessitated Cesarean delivery, of a nonhypoxic nonacidotic infant who did well. Note: Reproduced with permission from Sherer DM, Dalloul M, Ward K, et al. Coexisting true umbilical cord knot and nuchal cord: possible cumulative increased risk of adverse perinatal outcome. Ultrasound Obstet Gynecol. 2017;50(3):404–405. Copyright © 2016 ISUOG. Published by John Wiley & Sons Ltd.75 |
In contrast, in a case reported by Gurau et al a fetus exhibiting a figure-eight umbilical cord complex true knot and triple nuchal cord at sonographic assessment at 34 weeks’ gestation was delivered by Cesarean without labor at 37 weeks’ gestation, without awaiting labor or fetal heart rate changes at fetal monitoring.76
Interestingly, while a true umbilical cord knot was found in the previously mentioned large population-based study of Linde et al to have a 4-fold risk of perinatal death, coexisting true knot of the umbilical cord and umbilical cord entanglement were noted to have more than additive effect to the association with perinatal death, clearly supporting our earlier reported clinical suspicion of this cumulative effect.31,75
Thus, we are presented/confronted with potential sonographic diagnosis of a true knot of the umbilical cord in which one cannot predict a favorable outcome (more likely) or conversely, stillbirth or adverse neonatal outcome (a damaged infant at birth). What therefore is expected of sonographers and clinicians?
Review of current literature supports that:
- Despite advances in prenatal sonographic diagnosis, the majority of cases of true knot of the umbilical cord remain incidental findings at delivery, most without adverse perinatal outcomes. Worded differently, despite prenatal sonographic assessment, many true knots may remain undetected and thus one must conclude that true knots do not always lend themselves to prenatal diagnosis, nor are they always associated with adverse perinatal outcome.
- An undetermined percentage of cases of true knot of the umbilical cord are associated with an increased rate of perinatal morbidity and stillbirth.
- Prenatal sonography cannot predict which cases of true knots of the umbilical cord will subsequently tighten or alternatively be associated with adverse perinatal outcome, namely stillbirth.
- Vaginal birth at term is not contraindicated in the presence of a diagnosis of true knot of the umbilical cord. Cesarean delivery for the diagnosis of a true knot of the umbilical cord is unwarranted in the absence of non-reassuring fetal status, or Doppler velocimetry changes indicating increased downstream resistance to flow in the umbilical artery (proximal to the knot) possibly suggesting umbilical cord compression.
Prenatal sonographic diagnosis of a true knot of the umbilical cord, is hampered by the following:
- The umbilical cord is seldom if ever, depicted throughout its entire length.Although Ugurlucan and Yuksel in 2015, in a study of 549 singleton pregnancies, suggested that sonographically tracing the entire length of the umbilical cord (“from the fetal insertion site to the placental insertion”) is feasible (with only one unsuccessful case [0.2%]), during the second trimester, this study has yet to be replicated by others, and regretfully is not our experience.78Otherwise, rare reports of sonographic depiction of the entire length of the umbilical cord have been limited either to the first trimester of pregnancy or cases of markedly shortened umbilical cord length.79,80 Interestingly, it appears that these cases of short umbilical cord length, are also those in which the formation of true knots of the umbilical cord would be highly unlikely.
- Obscured true knot of the umbilical cord.Irrespective of placental location (anterior or posterior), as gestational age advances, especially in the third trimester, a considerable length of the umbilical cord (throughout which umbilical cord knots may be present) may be obscured by the fetus and thus inaccessible to sonographic interrogation and prenatal sonographic diagnosis.
- False positive diagnosis.As mentioned previously, false diagnoses of true knot of the umbilical cord occur. Clustering or clumping of the umbilical cord (as noted at Cesarean delivery in cases of occult prolapse of the umbilical cord) appear to be associated with the potential for false positive diagnoses of true knot of the umbilical cord.
- Lack of an applicable screening tool.Given the low incidence of true knot of the umbilical cord and the inability of current imaging technology to depict the entire length of the umbilical cord, a robust uniform screening technology for true knots of the umbilical cord is clearly untenable at this point. Of note, notwithstanding the lack of clear screening capability for this condition our sonographers do scan visible portions of the umbilical cord and are attentive to the potential sonographic findings associated with true knot of the umbilical cord, and proceed to apply color Doppler and 3D imaging modalities when this diagnosis is suspected.
- Lack of well-defined clinical management guidelines following prenatal sonographic diagnosis.
Clearly there is a lack of evidence-based outcome data of patients in whom prenatal sonography has suggested the presence of a true knot of the umbilical cord.
The current (2018) combined American College of Radiology (ACR), American College of Obstetricians and Gynecologists (ACOG), Society of Maternal Fetal Medicine (SMFM), Society of Radiology of Ultrasound (SRU), ACR-ACOG-AIUM-SMFM-SRU PRACTICE PARAMETER for the Performance of Standard Diagnostic Obstetrical Ultrasound, regarding the umbilical cord, simply suffices with the statement: “The umbilical cord should be imaged and the number of vessels in the cord documented. The placental cord insertion site should be documented when technically possible”.81 The International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) similarly does not address the topic of nuchal or true knots of the umbilical cord.82
In the effective void resulting from the absence of evidence-based data and clear guidelines of recommended clinical management following prenatal sonographic diagnosis of true knot of the umbilical cord – some authors have suggested/inferred that such prenatal diagnoses potentially may compromise patients or even clinicians.83,84
Vasilj et al recently published a brief communication in which knowledge of suspected true knot of the umbilical cord at 27 weeks’ gestation was withheld from a patient (a personal friend of his) at 27 weeks’ gestation.83 “As the only treatment would be early delivery”, Vasilj, states “I opted to say nothing”. “Each time we saw each other, I hid my concern, but the anxiety I felt was not pleasant and the routine question do you feel your baby moving was very stressful”. The true knot remained visible at two later sonographic examinations with “normal umbilical Doppler flow”. A “knot, which was not tight” was confirmed following spontaneous delivery at 39 weeks’ gestation. The author notes that a day after delivery he “told her about the knot and explained to her all the doubts and concerns that he had experienced”. The patient “thanked him for not telling her earlier”. The author, defending his inaction, states that this was an incidental finding with no-evidence based approach that would alter obstetrical management.83
Although truly an incidental finding without established guidelines, respectfully, we differ with regard to this possibly unethical approach and respectfully wonder what Vasilj’s thoughts might have been in the event of an unexpected stillbirth? Would he then have confided similarly with the patient that he had suspected the existence of the true knot of the umbilical cord earlier, and similarly, what would the patient have thought of her not being informed in real-time, only to have sustained a later stillbirth? In our assessment, this approach clearly reflects outdated paternalistic medicine, unbecoming for the 21st century.
Earlier in 2006, Stempel concurrent with the Ramon y Cajal and Martinez's publication regarding 4D ultrasound depiction of a true knot of the umbilical cord, in the same issue of the American Journal of Obstetrics and Gynecology, opined regarding this diagnosis in a commentary entitled “Beyond the pretty pictures: Giving obstetricians just enough (umbilical) cord to hang themselves”.68,84 In this commentary, Stempel discussed the unknown accuracy and the lack of defined appropriate management of patients with the prenatal sonographic diagnosis of a true knot of the umbilical cord, the probability that antepartum biophysical testing might not avoid unpredictable, sudden cord occlusion and cautioned that obstetricians might cause “considerable mischief” by aggressively treating these patients.84 Stempel concluded with the statement “We must be careful lest we give obstetricians just enough information about the umbilical cord to hang themselves”. Implicit (although not stated), one can only infer from this statement that Stempel considered that it is the obstetrician/sonographer diagnosing a potential true knot of the umbilical cord, placing him or herself in harm's way (“to hang themselves”, ie, malpractice), rather than expressing concern for the patient who has a (seen/suspected, yet unreported) true knot of the umbilical cord – who later may sustain a stillbirth (purportedly, from an unexpected, undocumented, undisclosed true knot of the umbilical cord).84 Again, we respectfully differ. The option of “don’t ask (suspect/verify/confirm the presence of a true knot of the umbilical cord), don’t tell (inform and counsel the patient)” or suggest/imply to avoid such (uncertain) diagnoses and avoid timely (and likely anxiety creating) patient counseling in our view, is outdated and likely unethical.
Following that the previously mentioned governing bodies (and others) do not recommend reporting of either nuchal cord(s) or true knot(s) if/when observed, the question regarding what clinical implications if any, might be appropriate when the prenatal sonographic diagnosis of a true knot of the umbilical cord is considered, remains unresolved. Given the potential for adverse perinatal outcome in the event of true knots of the umbilical cord (neonatal morbidity or stillbirth), it appears that documentation of such and an open discussion with the patient at gestational ages above viability, regarding daily fetal movement assessment, and interval fetal testing until delivery, should be considered/conducted, although regretfully clearly stillbirth cannot be prevented in the interim between diagnosis and delivery, in all cases.
Continued expectant management with interval fetal testing, while awaiting the onset of spontaneous labor, clearly is a viable clinical management option.
Notwithstanding in our assessment, consideration should be given to dicussing the option of delivery with patients with prenatal sonographic diagnosis of a true knot of the umbilical cord and vertex-presenting fetuses at / or >37 weeks’ gestation, with the intent to avoid potential stillbirth.
Although Cesarean delivery is clearly not indicated for patients with prenatal sonographic diagnosis of true knot of the umbilical cord, this clearly remains the patient’s decision,67,76 otherwise, continuous intrapartum electronic fetal monitoring is imperative.
Intrapartum adverse outcome associated with a true knot of the umbilical cord cannot be prevented with intermittent (fetal monitoring every 30 mins and 5 mins in the first and second stages of labor, respectively, has been reported resulted in the delivery of an appropriate-for-gestation age neonate with Apgar scores of 2 in 1 min of life, who succumbed. Although not depicted at prenatal ultrasound earlier at 36 weeks’ gestation, true knot of the (excessively long) umbilical cord was noted 7 cm distal to the fetal insertion.85
In the foreseeable future, sonographic resolution will undoubtedly continue to improve, followed by increasing diagnostic accuracy. Potential widespread application of color Doppler, power Doppler, 3D ultrasound in conjunction with other imaging diagnostic tools (CT and/or MR imaging with currently available 3D computer-assisted reconstruction techniques) may further advance prenatal diagnosis of true knots of the umbilical cord in the foreseeable future and increase the frequency of this prenatal diagnosis. Three-dimensional reconstruction CT or MR are of special interest in that these imaging techniques may enable depiction of true knots of the umbilical cord located behind the fetal body, which are currently inaccessible to transabdominal sonography.
It appears that currently we are unprepared for these eventualities, reflecting the previously mentioned absence of clinical management guidelines following the prenatal sonographic suspicion of true knot of the umbilical cord.
In 1999, Sherer and Manning in an Editorial in Ultrasound in Obstetrics and Gynecology suggested that following prenatal sonographic diagnosis of nuchal cords (and this is considerably more applicable/correct regarding true knot(s) of the umbilical cord with their inherent increase in perinatal morbidity and 4–10 fold increase risk of stillbirth), options available to the sonographer/clinician include: disregard, inform, monitor and/or intervene.86 It appears to us that given the considerable increased risk of adverse outcome and especially stillbirth in association with true knots of the umbilical cord, in contrast to the lesser likelihood of adverse perinatal outcome (stillbirth) of fetuses with nuchal cord(s), similar if not more strict guidelines, should apply regarding the former.
Subsequent clinical management will clearly depend on the gestational age at diagnosis. It is our current belief that sonographic findings suggestive of/or consistent with true knot of the umbilical cord should not be disregarded or withheld from the patient above the gestational age of viability. Although clearly time consuming (and anxiety creating), disclosure and detailed counseling emphasizing the importance of daily fetal movement/kick counts, intermittent (preferably twice weekly) fetal testing, and intervention (delivery) when warranted, in our assessment are basic tenents of clinical management. In contrast to the “don’t ask don’t tell” approach recommended by Stempel and conducted later by Vasilij, we believe it is imperative that the patient be informed of this suspected diagnosis in detail, preferably in real-time during the initial ultrasound assessment.83,84 The possibility of false positive diagnosis clearly should be discussed although this would be on a case by case basis, according to the sonographic findings of each case, and associated imaging quality. Anything less than a detailed discussion in our belief is simply unethical. At / or above 37 weeks’ gestation the appearance of any evidence of potential (even transient) umbilical cord compression (variable or lambda decelerations of the fetal heart rate), changes in umbilical venous or arterial blood flow as depicted by Doppler sonography, oligohydramnios, fetal growth restriction (in which case placental reserve may already be compromised), delivery should be recommended. As mentioned above, although not directly recommending delivery, consideration should be given in the absence of fetal compromise at gestational ages at / or above 37 weeks' gestation to accomodate patients who request elective delivery following the sonographic diagnosis of true knot of the umbilical cord.
Considerably more complex are management guidelines involving gestational ages <37 weeks’ gestation. Notwithstanding, evidence of even transient umbilical cord compression should be met with administration of intramuscular antenatal steroids to decrease overall prematurity-associated neonatal morbidities and consideration for admission for continuous fetal heart rate monitoring, and delivery performed if warranted (see our management of cases with co-existing true knot and nuchal cord).75
Summary
True knot(s) of the umbilical cord often incidentally noted at delivery and associated with a clear increased risk of stillbirth, may be detected by prenatal sonography with increasing accuracy. Due to the myriad of reasons discussed, screening for true knot of the umbilical cord is not currently tenable, false positive diagnoses do exist and potential adverse outcomes cannot be predicted. Notwithstanding, we believe that given the association of true knot of the umbilical cord and 4–10 fold increased risk of stillbirth, following sonographic notation of findings consistent with a true knot of the umbilical cord, full disclosure of the potential diagnosis (including depiction of images), detailed counseling and guidance with well-outlined fetal testing and delivery plans tapered according to gestational age (as outlined above), and patient preference, are in order. We owe our patients and their unborn fetuses no less.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Benirschke K, Kaufman P. Anatomy and pathology of the umbilical cord and major fetal vessels. In: Benirschke K, Kaufman P, editors. Pathology of the Human Placenta.
2. Raio L, Ghezzi F, Di Naro E, Franchi M, Bruhwiler H. Prenatal assessment of the Hyrtl anastomosis and evaluation of its function: case report. Hum Reprod. 1999;14(7):1890–1893. doi:10.1093/humrep/14.7.1890
3. Raio L, Ghezzi F, Di Naro E, Balestrei D, Duria P, Scheinder H. In-utero characterization of the blood flow in Hyrtl anastomosis. Placenta. 2001;22(6):597–601. doi:10.1053/plac.2001.0685
4. Sherer DM, Anyaegbunam A. Prenatal ultrasonographic assessment of the umbilical cord: a review Part I. Obstet Gynecol Surv. 1997;52:515–523. doi:10.1097/00006254-199708000-00023
5. Sherer DM, Anyaegbunam A. Prenatal ultrasonographic assessment of the umbilical cord: a review Part II. Obstet Gynecol Surv. 1997;52:506–514. doi:10.1097/00006254-199708000-00022
6. Sherer DM, Manning FA. Prenatal ultrasonographic diagnosis of conditions associated with potential umbilical cord compression. Am J Perinatol. 1999;16(9):445–458. doi:10.1055/s-1999-6807
7. Camann W, Marquardt J. Complex umbilical-cord knot. N Engl J Med. 2003;349:159. doi:10.1056/NEJMicm020847
8. Hugon-Rodin J, Guilbert JB, Baron X, Camus E. Notching of the umbilical artery waveform associated with cord entanglement in a monoamniotic twin pregnancy. J Matern Fetal Neonatal Med. 2013;26(15):1559–1561. doi:10.3109/14767058.2013.794204
9. Hanaoka U, Tenkumo C, Ito M, Mori N, Tanaka H, Hata T. Three-dimensional surface-rendered imaging of cord entanglement in monoamniotic twins. Arch Gynecol Obstet. 2012;286(4):1091–1092. doi:10.1007/s00404-012-2406-3
10. Dias T, Mahsud-Dornan S, Bhide A, Papageorghiou AT, Thilaganathan B. Cord entanglement and perinatal outcome in monoamniotic twin pregnancies. Ultrasound Obstet Gynecol. 2010;35(2):201–204. doi:10.1002/uog.7501
11. Kuwata T, Matsubara S, Suzuki M. 3D color doppler of monoamniotic fluid cord entanglement. Arch Gynecol Obstet. 2010;281(5):973–974. doi:10.1007/s00404-009-1304-9.
12. Deutsch AB, Miller E, Spellacy WN, Mabry R. Ultrasound to identify cord knotting in monoamniotic monochorionic twins. Twin Res Hum Genet. 2007;10(1):216–218. doi:10.1375/twin.10.1.216
13. Sherer DM, Sokolovski M, Haratz-Rubinstein N. Diagnosis of umbilical cord entanglement of monoamniotic twins by first-trimester color doppler imaging. J Ultrasound Med. 2002;21(11):1307. doi:10.7863/jum.2002.21.11.1307
14. Arabin B, Laurini RN, van Eyck J. Early prenatal diagnosis of cord entanglement in monoamniotic multiple pregnancies. Ultrasound Obstet Gynecol. 1999;13(3):181–186. doi:10.1046/j.1469-0705.1999.13030181.x
15. Rossi AC, Prefumo F. Impact of cord entanglement on perinatal outcome of monoamniotic twins: a systematic review of the literature. Ultrasound Obstet Gynecol. 2013;41(2):131–135. (). doi:10.1002/uog.12345
16. Ertan AK, Schmidt W. Umbilical cord entanglement and color doppler ultrasound. Geburtshilfe Frauenheilkd. 1994;54:196–203. doi:10.1055/s-2007-1023582
17. Sherer DM, Bitton C, Stimphil R, et al. Cord entanglement of monochorionic diamniotic twins following spontaneous antepartum septostomy sonographically simulating a true knot of the umbilical cord. Ultrasound Obstet Gynecol. 2005;26(6):676–678. doi:10.1002/uog.2612
18. Ito A, Nakata M, Takano M, et al. Diagnosis of umbilical cord entanglement in a monochorionic diamniotic twin pregnancy with spontaneous septostomy of the dividing membranes using dual-gate Doppler imaging. J Med Ultrasound. 2018;45(1):189. doi:10.1007/s10396-017-0793-6.Epub
19. Fleming T, Miller T. Spontaneous septostomy in monochorionic diamniotic twins resulting in cord entanglement and fetal demise. Australas J Ultrasound Med. 2012;15(3):103–106. doi:10.1002/j.2205-0140.2012.tb00014.x
20. Hershkovitz R, Siberstein T, Sheiner E, et al. Risk factors associated with true knots of the umbilical cord. Eur J Obstet Gynecol Reprod Biol. 2001;98(1):36–39. doi:10.1016/S0301-2115(01)00312-8
21. Semchyshyn S. True knot of the umbilical cord in two consecutive pregnancies. Can Med Assoc J. 1973;109(4):269.
22. Polis RL, Santolaya-Forgas J, Tong C, et al. Personalized medicine in a patient with the antenatal diagnosis of an umbilical cord knot and a previous adverse outcome for this reason. J Ultrasound Med. 2014;33:735–740. doi:10.7863/ultra.33.4.735
23. Linde LE, Rasmussen S, Kessler J, Ebbing C. Extreme umbilical cord lengths, cord knot and entanglement: risk factors and risk of adverse outcomes, a population based study. PLoS One. 2018;13(3):e0194814. doi:10.1371/journal.pone.eCollection
24. Clerici G, Koutras I, Lizietti R, Di Renzo GC. Multiple true umbilical knots: a silent risk for intrauterine growth restriction with anomalous hemodynamic pattern. Fetal Diagn Ther. 2007;22(6):440–443. doi:10.1159/000106351
25. Ugianskiene A, Bor P. A rare case with multiple true knots together with single artery and four umbilical cord nuchal loops. Eur J Obstet Gynecol Reprod Biol. 2013;168(1):117–118. doi:10.1016/j.ejogrb.2012.12.020.
26. Srinivasan A, Graves L. Four true umbilical cord knots. J Obstet Gynaecol Can. 2006;28(1):32–35. doi:10.1016/S1701-2163(16)32053-9
27. Mehta S, Singla A, Sinha S, Grover A. Long cord: a knotty affair. J Clin Diagn Res. 2017;11(5):1. doi:10.7860/JCDR/2017/24748.9780
28. Blickstein I, Shoham-Schwartz Z, Lancet M. Predisposing factors in the formation of true knots of the umbilical cord—analysis of morphometric and perinatal data. Int J Obstet Gynecol. 1987;25(5):395–398. doi:10.1016/0020-7292(87)90346-8
29. Arias U, Heinonen S. Clinical significance of true umbilical cord knots: a population-based analysis. Am J Perinatol. 2002;19:127–132. doi:10.1055/s-2002-25311
30. Suzuki S. Excessively long umbilical cord: a preventive factor of miserable outcomes of pregnancies with true umbilical cord knots. J Matern Fetal Neonate Med. 2019;1:1–4. doi:10.1080/14767058.20191584177
31. Rogers MS, Ip YW, Qin Y, Rogers SM, Sahota D. Relationship between umbilical cord morphology and nuchal cord entanglement. Acta Obstet Gynecol Scand. 2003;82:32–37. doi:10.1034/j.1600-0412.2003.820106.x
32. Chasnoff IJ, Fletcher MA. True knot of the umbilical cord. Am J Obstet Gynecol. 1977;127(4):425–427. doi:10.1016/0002-9378(77)90501-4
33. Gembruch U, Baschat AA. True knot of the umbilical cord: transient constrictive effect to umbilical venous blood flow demonstrated by Doppler sonography. Ultrasound Obstet Gynecol. 1996;8:53–56. doi:10.1046/j.1469-0705.1996.08010053.x
34. Sherer DM, Sokolovski M, Dalloul M, Khoury-Collado F, Abulafia O. Is fetal cerebral vascular resistance affected by the presence of nuchal cord(s) in the third trimester of pregnancy? Ultrasound Obstet Gynecol. 2005;25(5):454–458. doi:10.1002/uog.1874
35. Larson JD, Rayburn WF, Crosby S, Thurnau GR. Multiple nuchal cord entanglements and intrapartum complications. Am J Obstet Gynecol. 1995;173:1228–1231. doi:10.1016/0002-9378(95)91359-9
36. Mian DB, Konan J, Kouakou KC, Angoi V, Gbary E, Itoua C. Severe antenatal strangulation and sudden fetal death occurs in term: case report. Clin Exp Obstet Gynecol. 2016;43:161–164.
37. Kesrouani A, Daher A, Maoula A, Attieh E, Richa S. Impact of a prenatally diagnosed nuchal cord on obstetrical outcome in an unselected population. J Matern Fetal Neonatal Med. 2017;30:434–436. doi:10.1080/14767058.2016.1174993
38. Narang Y, Vaid NB, Jain S, et al. Is nuchal cord justified as a cause of obstetrician anxiety? Arch Gynecol Obstet. 2014;289:795–801. doi:10.1007/s00404-013-3072-9
39. Hoh JK, Sung YM, Park MI. Fetal heart rate parameters and perinatal outcome in fetuses with nuchal cords. J Obstet Gynaecol Res. 2012;38:358–363. doi:10.1111/j.1447-0756.2011.01707.x
40. Schaffer L, Burkhardt T, Zimmermann R, Kurmanavicius J. Nuchal cords in term and postterm deliveries – do we need to know? Obstet Gynecol. 2005;106:23–28. doi:10.1097/01.AOG.0000165322.42051.0f
41. Dursun P, Salman MC, Ozyuncu O, Aksu T. Nuchal cord type B associated with an excessively long umbilical cord as a cause of stillbirth: a case report. Clin Exp Obstet Gynecol. 2004;31:158–159.
42. Carey JC, Rayburn WF. Nuchal cord encirclements and birth weight. J Reprod Med. 2003;48:460–462.
43. Clapp JF
44. Larson JD, Rayburn WF, Harlan VL. Nuchal cord entanglements and gestational age. Am J Perinatol. 1997;14:555–557. doi:10.1055/s-2007-994333
45. Jauniaux E, Ramsay B, Peellaerts C, Scholler Y. Perinatal features of pregnancies complicated by nuchal cord. Am J Perinatol. 1995;12:255–258. doi:10.1055/s-2007-994467
46. Jauniaux E, Mawissa C, Peellaerts C, Rodesch F. Nuchal cord in normal third-trimester: a color Doppler imaging study. Ultrasound Obstet Gynecol. 1992;1:. doi:10.1046/j.1469-0705.1992.02060417.x
47. Wang L, Kuromaki K, Kawabe A, Kikugawa A, Matsunaga S, Takagi A. Nuchal cord complication in male small for gestational age increases fetal distress risk during labor. Taiwan J Obstet Gynecol. 2016;554:568–574. doi:10.1016/j.tjog.2016.03.002
48. Kobayashi N, Aoki S, Oba MD, Takahashi T, Hirahara F. Effect of umbilical cord entanglement and position on pregnancy outcomes. Obstet Gynecol Int. 2015. Epub 2015 Jul 9.
49. Perlitz Y, Ben-Shlomo I, Ben Ami M. Acute polyhydramnios in term pregnancy may be caused by multiple nuchal cord loops. Ultrasound Obstet Gynecol. 2010;35:253–254. doi:10.1002/uog.7543
50. Wang Y, Le Ray C, Audibert F, Wagner MS. Management of nuchal cord with multiple loops. Obstet Gynecol. 2008;112:460–461. doi:10.1097/AOG.0b013e31816fd75c
51. Sherer DM, Dalloul M, Sabir S, London V, Haughton M, Abulafia O. Persistent quadruple nuchal cord throughout the third trimester associated with decelerating fetal growth. Ultrasound Obstet Gynecol. 2017;49(3):409–410. doi:10.1002/uog.15940.
52. Akkaya H, Büke B, Pekan MK, et al. Nuchal cord: is it really the silent risk of pregnancy? J Matern Fetal Neonatal Med. 2016:4. [Epub ahead of print].
53. Weiner E, Fainstein N, Schreiber L, Sagiv R, Bar J, Kovo M. The association between umbilical cord abnormalities and the development of non-reassuring fetal heart rate leading to emergent cesarean deliveries. J Perinatol. 2015;35(11):919–923. doi:10.1038/jp.2015.102.
54. Joura EA, Zeisler H, Sator MO. Epidemiology and clinical value of true umbilical cord knots. Wien Klin Wochehenschr. 1998;27:232–235.
55. Maher JT, Conti JA. A comparison of umbilical cord gas values between newborns with and without true knots. Obstet Gynecol. 1996;88:863–866. doi:10.1016/0029-7844(96)00313-4
56. Sornes T. Umbilical cord knots. Acta Obstet Gynecol Scand. 2000;79:157–159. doi:10.1080/j.1600-0412.2000.079003157.x
57. Ohana O, Holcberg G, Sergienko R, Sheiner E. Risk factors for intrauterine fetal death (1988–2009). J Matern Fetal Neonatal Med. 2011;24(9):1079–1083. doi:10.3109/14767058.2010.545918.
58. Collins JH. Two cases of multiple umbilical cord abnormalities resulting in stillbirth: prenatal observation with ultrasonography and fetal heart rates. Am J Obstet Gynecol. 1993;168:125–128. doi:10.1016/S0002-9378(12)90899-6
59. Räisänen S, Georiadis L, Harju M, Keski-Nisula L, Heinonen S. True umbilical cord knot and obstetric outcome. Int J Gynaecol Obstet. 2013;122:18–21. doi:10.1016/j.ijgo.2013.02.012
60. Ulm MR, Obswegeser R, Ulm B, Deutinger J. An undetected reason for severe fetal growth restriction. Eur J Ultrasound 1998;8:215–219.
61. Szczepanik ME, Wittich AC. True knot of the umbilical cord: a report of 13 cases. Mil Med. 2007;172(8):892–894. doi:10.7205/MILMED.172.8.892
62. Goldstein I, Timor‐Tritsch IE, Zaidise I, Divon M, Paloi E. Sinusoidal pattern together with signs of moderate fetal hypoxia associated with a true knot of cord. Eur J Obstet Gynecol Reprod Biol. 1981;11:221–225. doi:10.1016/0028-2243(81)90002-2
63. Chien YL, Chou MC, Chou WJ, et al. Prenatal and perinatal risk factors and the clinical implications on autism spectral disorder. Autism. 2019;23(3):783–791. doi:10.1177/1362361318772813
64. Collins JC, Muller RJ, Collins CL. Prenatal observation umbilical cord abnormalities: a triple knot and torsion of the umbilical cord. Am J Obstet Gynecol. 1993;169:102–104. doi:10.1016/0002-9378(93)90139-A
65. Sepulveda W, Shennan AH, Bower S, Nicolaidis P, Fisk NM. True knot of the umbilical cord: a difficult prenatal sonographic diagnosis. Ultrasound Obstet Gynecol. 1995;5:106–108. doi:10.1046/j.1469-0705.1995.05020106.x
66. Ramòn y Cajal CL, Martinez RO. Prenatal diagnosis of a true knot of the umbilical cord. Ultrasound Obstet Gynecol. 2004;23:99–100. doi:10.1002/uog.900
67. Sherer DM, Dalloul M, Zigalo A, Bitton C, Dabiri L, Abulafia O. Power doppler and 3-dimensional sonographic diagnosis of multiple separate true knots of the umbilical cord. J Ultrasound Med. 2005;24:1321–1323. doi:10.7863/jum.2005.24.9.1321
68. Ramòn y Cajal CL, RO M. Four-dimensional ultrasonography of a true knot of the umbilical cord. Am J Obstet Gynecol. 2006;195:1898. doi:10.1016/j.ajog.2006.05.044
69. Hasbun J, Alacalde JL, Sepulveda W. Three-dimensional power doppler sonography in the prenatal diagnosis of a true knot of the umbilical cord: values and limitations. J Ultrasound Med. 2007;26:1215–1220. doi:10.7863/jum.2007.26.9.1215
70. Scioscia M, Fornale M, Brunir F, Peretti D, Trivella G. Four-dimensional and doppler sonography in the diagnosis and surveillance of a true knot. J Clin Ultrasound. 2011;39(3):157–159. doi:10.1002/jcu.20757.
71. Rodriguez N, Angrita AM, Casabuenas A, Sarmeinto A. Three-dimensional high definition flow imaging in prenatal diagnosis of a true umbilical cord knot. Ultrasound Obstet Gynecol. 2012;39:245–246. doi:10.1002/uog.11075
72. Abuhammad A. Three-dimensional ultrasound with color doppler imaging of an umbilical cord true knot. Ultrasound Obstet Gynecol. 2014;43:360. doi:10.1002/uog.13297
73. Bohîltea RE, Turcan N, Cîrstoiu M. Prenatal ultrasound diagnosis and pregnancy outcome of umbilical cord knot – debate regarding ethical aspects of a series of cases. J Med Life. 2016;9:297–301.
74. Merz E, Pashaj S. True or false umbilical cord knot? Differentiation via 3D/4D color doppler ultrasound. Ultraschall Med. 2018;39(2):127–128. doi:10.1055/a-0571-8608.
75. Sherer DM, Dalloul M, Ward K, et al. Coexisting true umbilical cord knot and nuchal cord: possible cumulative increased risk of adverse perinatal outcome. Ultrasound Obstet Gynecol. 2017;50(3):404–405. doi:10.1002/uog.17389
76. Gurau D, Zaltz A, Yoo WK, Rahmani MR. All tied up and nowhere to go: report of a figure-eight umbilical cord complex true knot and triple nuchal cord detected on antenatal sonography. J Ultrasound Med. 2016;35:1361–1363. doi:10.7863/ultra.15.09044
77. Hertzberg BS, Bowie JD, Bradford WD, Bolick D. False knot of the umbilical cord: sonographic appearance and differential diagnosis. J Clin Ultrasound. 1988;16:599–602. doi:10.1002/(ISSN)1097-0096
78. Ugurlucan FG, Yuskel A. Is complete umbilical cord scanning possible at the second-trimester ultrasound scan? J Clin Ultrasound. 2015;43(4):249–253. doi:10.1002/jcu.22242.
79. Collins JH. Ultrasound measurement of umbilical cord length. Am J Obstet Gynecol. 1994;13(11):854.
80. Sherer DM, Dalloul M, Ajayi O, Kheyman M, Sokolovski M, Abulafia O. Prenatal sonographic diagnosis of a short umbilical cord in a dichorionic twin with normal anatomy. J Clin Ultrasound. 2010;38:91–93. doi:10.1002/jcu.20639
81. AIUM-ACR-ACOG-SMFM-SRU practice parameter for the performance of standard diagnostic obstetrical ultrasound examinations. J Ultrasound Med. 2018;9999:12. doi:10.1002/jum.14831
82. Salomon LJ, Alfirevic Z, Berghella V, et al. on behalf of the ISUOG Clinical Standards Committee. Practice guidelines for performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obstet Gynecol. 2012;37(1):116–126 doi:10/1002/uog.8831
83. Vasilj O, Matijevic R, Blagaic V, Mislovic B. Do we sometimes see too much? Prenatal diagnosis of a true umbilical cord knot. Eur J Obstet Gynecol Reprod Biol. 2015;187:73–74. doi:10.1016/j.ejogrb.2015.02.023
84. Stempel LE. Beyond the pretty pictures” Giving obstetricians just enough (umbilical) cord to hang themselves. Am J Obstet Gynecol. 2006;195:888–890. doi:10.1016/j.ajog.2006.06.001
85. Ikechebelu JI, Eleje GU, Ofojebe CJ. True umbilical cord knot leading to fetal demise. Ann Med Health Sci Res. 2014;4(Suppl2):S155–S158. doi:10.4103/2141-9248.138044
86. Sherer DM, Manning FA. Prenatal ultrasonographic diagnosis of nuchal cords(s): disregard, inform, monitor, intervene? Ultrasound Obstet Gynecol. 1999;16(3):103–120.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.