Back to Journals » Journal of Asthma and Allergy » Volume 15
Current and Future Strategies for the Diagnosis and Treatment of the Alpha-Gal Syndrome (AGS)
Authors Vaz-Rodrigues R, Mazuecos L, de la Fuente J
Received 21 May 2022
Accepted for publication 12 July 2022
Published 18 July 2022 Volume 2022:15 Pages 957—970
DOI https://doi.org/10.2147/JAA.S265660
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amrita Dosanjh
Rita Vaz-Rodrigues,1 Lorena Mazuecos,1 José de la Fuente1,2
1SaBio (Health and Biotechnology), Instituto de Investigación en Recursos Cinegéticos IREC (CSIC-UCLM-JCCM), Ciudad Real, 13005, Spain; 2Department of Veterinary Pathobiology, Center for Veterinary Health Sciences, Oklahoma State University, Stillwater, OK, 74078, USA
Correspondence: José de la Fuente, SaBio (Health and Biotechnology), Instituto de Investigación en Recursos Cinegéticos IREC (CSIC-UCLM-JCCM), Ronda de Toledo s/n, Ciudad Real, 13005, Spain, Tel +34 926 295 151, Email [email protected]
Abstract: The α-Gal syndrome (AGS) is a pathognomonic immunoglobulin E (IgE)-mediated delayed anaphylaxis in foods containing the oligosaccharide galactose-α-1,3-galactose (α-Gal) such as mammalian meat or dairy products. Clinical presentation of AGS can also comprise immediate hypersensitivity due to anticancer therapy, gelatin-containing vaccines or mammalian serum-based antivenom. The IgE initial sensitization is caused by hard-bodied tick bites and symptomatic individuals typically develop delayed pruritus, urticaria, angioedema, anaphylaxis, malaise or gut-related symptoms. Due to inapparent presentation, delayed reactions and a wide variety of patients´ clinical history, the AGS diagnosis and treatment remain challenging. This review covers not only current diagnostic methods used for AGS such as the skin prick test (SPT), the oral food challenge (OFC), anti-α-Gal IgE levels measurement and the basophil activation test (BAT), but also potentially relevant next-generation diagnostic tools like the mast cell activation test (MAT), the histamine-release (HR) assay, omics technologies and model-based reasoning (MBR). Moreover, it focuses on the therapeutical medical and non-medical methods available and current research methods that are being applied in order to elucidate the molecular, physiological and immune mechanisms underlying this allergic disorder. Lastly, future treatment and preventive tools are also discussed, being of utmost importance for the identification of tick salivary molecules, with or without α-Gal modifications, that trigger IgE sensitivity as they could be the key for further vaccine development. Bearing in mind climate change, the tick-host paradigm will shift towards an increasing number of AGS cases in new regions worldwide, which will pose new challenges for clinicians in the future.
Keywords: alpha-Gal syndrome, AGS, food allergy, alpha-Gal, glycan, tick, IgE
Introduction
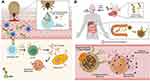
Galactose-α-1,3-galactose (α-Gal), an oligosaccharide that structurally resembles blood group B antigen, is present in both glycoproteins and glycolipids from non-catarrhine mammalian muscle cells and secretions.1,2 Old World monkeys, apes and humans evolved with the inability to synthesize α-Gal epitopes and, therefore, produce natural anti-α-Gal antibodies to control pathogen infection.3 This carbohydrate epitope is the causal agent of the α-Gal syndrome (AGS), a pathognomonic immunoglobulin E (IgE)-mediated delayed anaphylaxis in mammalian meat (eg pork, beef or lamb) or dairy products 3 to 6 hours post-consumption.4–7 Recently, an allergic cross reaction to flounder roe in patients suffering from AGS has been reported.8 The other clinical presentations of AGS comprise immediate hypersensitivity to α-Gal-containing drugs, firstly discovered using the monoclonal antibody cetuximab in anticancer therapy.6,9 There is growing evidence of allergic reactions caused by the α-Gal present in mammalian substances such as gelatin, glycerin, lactic acid and magnesium stearate used in the preparation process of several medications,9,10 such as gelatin-containing products (vaccines and volume colloids), mammalian serum-based antivenom and even various analgesics and nonsteroidal anti-inflammatory drugs (NSAIDs).2,6,11,12 The IgE initial sensitization is caused by hard-bodied tick bites from different species according to geographic location and is attributed to α-Gal-containing tick salivary glycoproteins, but also other tick salivary biomolecules without α-Gal modifications such as prostaglandin E2 (PGE2).13–17 The mechanisms behind tick α-Gal induction of sensitization are still unknown, but besides the α-Gal moiety, tick sialome components may play an important role in the chained immune reaction activation (Figure 1).18,19 Tick species such as Ixodes ricinus in Europe, Amblyomma americanum in North America, Haemaphysalis longicornis in Asia and Ixodes holocyclus in Australia are linked to AGS,20 currently considered an emergent life-threatening allergy in tick endemic areas worldwide.21–23 However, not all individuals bitten by ticks or those that carry elevated specific IgE (sIgE) against α-Gal develop AGS, in fact, the majority only produce sIgE against it.19 Symptomatic individuals typically show delayed pruritus, urticaria (acute or recurrent), angioedema, anaphylaxis, malaise or gut-related symptoms such as abdominal pain, vomits and diarrhea.22,24,25 Anaphylaxis has been triggered in up to 60% of AGS cases and can be fatal if it is not treated promptly.26–28 Clinical features reported tend to be restricted to gastrointestinal complaints, hampering the suspicion of an allergic etiology.29 Nevertheless, clinical observations in AGS patients are widely variable, showing proof of individual sensitivity.5 Augmenting factors, also called cofactors, such as exercise and alcohol intake, have been reported to play an important role in modulation of this individual susceptibility between patients.30 The medical history is of importance in these cases and details like meat-associated delayed allergic reactions and tick bite-exposure represent crucial factors for uncovering AGS, which otherwise can be misdiagnosed as idiopathic anaphylaxis or chronic spontaneous angioedema.7,30,31 Risk factors for developing sIgE to α-Gal are related to the probability of individual tick bite-exposure in certain environmental conditions, including practice of outdoor activities (eg, hunting or hiking), living in rural areas, pet-ownership, and certain jobs such as forest service employees.32–35 The sIgE values tend to increase according to the number of tick bites per year and on how recent those bites are.34,36 Moreover, individuals that do not have type B or AB blood group may have a higher risk of developing AGS, as blood group B antigen, similar to α-Gal, creates tolerance to this epitope.37
On the other hand, immune response to α-Gal has been studied for the control and prevention of diseases, exhibiting a protective role in human evolution catastrophic selection. The incidence of several infectious diseases caused by α-Gal containing-pathogens such as malaria or tuberculosis might be positively correlated with the frequency of the specific blood type B, and then, with a reduced immune response to α-Gal. However, this fact has been associated with a lower prevalence of food allergies related to anti-α-Gal IgE antibodies.38 In addition, it has been recently reported a positive correlation between anti-α-Gal IgM antibodies and the incidence of Plasmodium falciparum infection, decreasing its transmission.39 Despite the fact that uncontrolled levels of anti-α-Gal antibodies could compromise health in AGS patients, these findings suggest that anti-α-Gal antibodies (IgE or IgM) might protect against parasites containing α-Gal on their surface. Interestingly, anti-α-Gal antibodies have also been studied not only in vector-borne diseases but also in emerging virus infections. Recently, it has been reported that anti-α-Gal antibody levels negatively correlate not only with SARS-CoV-2 infection but also with COVID-19 symptomatology severity.40
Over 10 years have passed since the discovery of AGS,41 but many questions remain unclear that still need to be elucidated, especially those related to the diagnostic and therapeutical approaches used for this syndrome. The aim of the present review article is to outline current diagnostic methods used for AGS and potentially future diagnostic tools, combined with the most recent forms of treatment/management of this syndrome. Furthermore, innovative topics such as current research methods and future treatment and preventive strategies are also discussed.
Diagnostic Methods
The AGS is an allergic disorder that challenges clinical diagnosis due to inapparent presentation and delayed reactions.19,42 Like any other allergic disease, diagnosis relies on a well-detailed medical history in order to reach an accurate evaluation and prognostic of individual signs and symptoms.25 Diagnostic techniques for this syndrome are not specific and/or represent a risk for the patient’s health, whereas more precise methods still show limitations to its use.43 As discussed here, it is important to address the diagnostic tests more commonly used and methods that could potentially be employed in the future (Figure 2) for the challenging and complex allergy that involves the pathology of AGS.
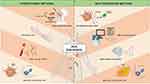 |
Figure 2 Conventional and next generation methods for the diagnosis of the alpha-Gal syndrome (AGS). |
Current Conventional Diagnostic Techniques
Skin Prick Test (SPT)
SPTs remains a useful diagnostic tool for several food allergies.44 Conversely, conducting this test for AGS diagnosis using conventional and commercially available mammalian meat extracts (beef, pork or lamb) lacks sensitivity, yielding low-reactive results (2–4 mm wheals), which may lead to misdiagnosis and incorrect patient management.45 Alternatively, cancer drug cetuximab can potentially be used as a sensitivity agent due to its high capacity to induce a strong skin reactivity in AGS patients, mainly caused by the larger amount of α-Gal epitopes exposed to the surface.46,47 Robust reactions also occur when mammalian meat extract is used, although this is not a feasible option for daily practice.16 Meat-derived gelatin from porcine or bovine, sometimes forming colloids, has also been used in allergic reaction diagnostics. Furthermore, it is important to consider that, although rare, the use of high-sensitivity components in SPT could potentially trigger a fatal anaphylactic shock reaction.48,49 Less commonly, intradermal testing (IDT) can also be used as a standardized methodology to evaluate skin reactions. As described by the SPT method, 20 minutes after allergen intradermal injection (around 0.1 mg/mL), swelling, redness and wheals are observed in the area of injection.50 Nevertheless, IDT is more likely to induce systemic anaphylactic reactions when compared to SPT.44 Overall, the clinical utility of SPT remains doubtful as no food allergen fits flawlessly in this diagnostic technique, conveying on several limitations and therefore making it not fit for a primary approach diagnostic tool.
Oral Food Challenge (OFC)
For the diagnosis of a food allergy (FA), OFC is the gold standard technique, offering further information regarding food tolerability and threshold of responsiveness.51 This method could be useful for discriminating AGS diagnosis from α-Gal sensitization if it did not convey to a risk of fatal or near-fatal delayed anaphylactic reactions.7,52 Therefore, this challenge can only be performed in specialized allergic centers, requiring long patient observation periods.7,53 Besides risk, OFC is not established as a standardized procedure for AGS and exposure reaction presents a high variability between patients. In fact, some patients may require the presence of cofactors in order to react, while others only respond to a particular type of red meat.54 Nevertheless, this method, tested also in combination with cofactors, is essential in patients in which drugs-containing α-Gal must be given for therapeutical reasons due to the clinically relevant information that it provides.55 Cofactors, such as acetylsalicylic acid (ASA) or alcohol, are well-known amplifiers of α-Gal reactions.6 Due to the wide difference between symptomatology of AGS patients, sensitivity to α-Gal can be truly variable.30 However, it has been observed that pork kidney intake, and no other product like muscle meat, even in the coadministration of cofactors, is a key element to raise AGS symptoms. This difference might be explained due to the higher number of α-Gal epitopes present in pork or beef kidney in comparison to other meats/innards.56,57
Anti-α-Gal IgE Titers
Currently, serum anti-α‐Gal IgE levels measured using an immune-enzymatic assay (bovine thyroglobulin-conjugated ImmunoCAP) is the confirmatory diagnostic method used for AGS diagnosis when medical history matches with this disease.58–60 Nonetheless, it remains unclear the clinical relevance of positive testing for anti-α‐Gal IgE using a cut-off value of 0.35 kU/L (where 1 kU/L = 1 IU/mL = 2.4 ng/mL).33,42,46,52,61 While Mabelane et al54 state that 5.5 kU/L is the cut-off point for clinically significant AGS, other studies reveal that there are no strict criteria regarding anti-α‐Gal IgE levels as an allergic symptomatology predictor.25,62 One thing is clear, though, is that levels of specific IgE are not a useful biomarker for predicting the severity of allergic reactions, as AGS patients experiencing anaphylactic reactions may maintain IgE levels overtime or even in rare occasions with anti-α‐Gal IgE negative results.25,63 Another issue with the anti-α‐Gal IgE diagnostic assay is that it can lead to false-positive results in those individuals where α‐Gal IgE sensitization may also be related to bee and wasp stings, parasitism, atopy or cat ownership, creating cases where these antibodies do not match the clinically pathognomonic history of AGS.25,42,62,64,65 For example, in a clinical study carried out in southern Germany, among 300 hunters with a 19.3% of IgE-α-Gal prevalence (58 individuals positive for cut-off value of 0.35 IU/mL), only 1.67% (5 individuals of the initial cohort) had allergic reactions to mammalian meat.33 Moreover, serum levels of IgE to α‐Gal tend to drop when patients do not experience recurrent tick bites, but again, the rate of declination between individuals is widely variable, being therefore recommended to repeat testing every 8 to 12 months.25,45 Nevertheless, due to AGS non-related therapeutical reasons, sometimes the measurement of anti-α‐Gal IgE levels may be needed to detect α‐Gal sensitization and therefore prevent drug-induced anaphylaxis.60 In summary, anti-α‐Gal IgE levels may be useful for AGS diagnosis, but clinical symptomatology and disease severity cannot be evaluated exclusively through this parameter, requiring complementation with other diagnostic techniques.
Basophil Activation Test (BAT)
Over the years, research studies allowed to recognize distinct improved biomarkers that provide a more accurate diagnosis of food allergies.66 This led the way to the use of the in vitro functional assay BAT, a flow-cytometry-based technique that quantifies the expression of activation membrane markers, namely, CD63 and CD203c, in order to analyze basophil degranulation when triggered by a specific allergen.66–68 In the research setting and specialized referral centers, BAT is being used as a diagnostic clarifier, allowing to distinguish between merely asymptomatic sensitized individuals and patients suffering from AGS.42,53,69 This newly emerging method offers good sensitivity and specificity, but presents several practical and logistical issues for implementation in clinical practice.43,70 First, blood must be processed within 24 hours after being collected in order to guarantee that basophil viability and reactivity are preserved.6,71 Second, questions regarding reproducibility and cost must be addressed before BAT implementation in practicing allergists.72 Unfortunately, methodology, concentration and markers are not standardized between laboratories in order to allow result comparison and test validation.73 Furthermore, standardization between systems and instruments required for accreditation (eg, EuroFlow™ Standard Operating Procedures) is missing, reducing BAT availability.74,75 This technique also lacks an established proficiency testing by regulatory entities. The current European Directive 98/79/EC on in vitro diagnostic medical devices76 will be replaced in 2022 by the new Regulation (EU) 2017/746 and introduce major changes in the sector, aiming for a smooth functioning of the internal market.77 In sum, efforts should be made to convey on transforming BAT into an on-hand tool for clinicians, due to the benefits it presents on risk allergy stratification, precise decision-making for α-Gal sensitized patients who lack medical evidence and selection of the correct doses for OFC in AGS-suffering individuals.53,72
Potentially Relevant Next Generation Diagnostic Techniques
Mast Cell Activation Test (MAT)
Another in vitro assay executed by flow cytometry is the MAT, a technique that measures CD63, a membrane activation marker that increases when mast cells (MCs) degranulate. This phenomenon occurs when MCs are triggered by allergen-sIgE antibodies.66,78 MAT presents high sensitivity and leads to a dose-dependent response to allergens,79 making it a potential and attractive complementary candidate for AGS diagnosis. Furthermore, MAT seems to overcome BAT’s major limitations. First, the use of MCs rather than basophils appears to be more suitable for allergy diagnosis due to the well-recognized effector function of MCs in comparison to the mere regulatory activity of basophils.80 Secondly, serum samples can be frozen prior to their use, as MAT does not require fresh viable cells, facilitating logistics and sample shipment if required.81 The MCs line can be activated directly through mas-related G protein-coupled receptor X2 (MGPRX2) with simultaneous analysis of positive and negative populations for this receptor. Herein, MC degranulation can be studied via upregulation of specific degranulation markers, such as CD63. Most common MRGPRX2-expressing cell lines used in combination with CD63 detection by flow cytometry are LAD-2 cells derived from human CD34+ cells.82,83 However, there is still a long way to go from standardization to validation to obtain a fully functional MAT assay, as currently this technique is particularly time-consuming and several key issues still persist regarding heterogeneity of MCs.70,84 Although MAT test is still under validation for clinical application, it represents a promising diagnostic approach, particularly as a confirmatory test when conventional methods generate ambiguous results.66,82
Histamine-Release (HR) Assay
Another emerging diagnostic test in the FA field is the HR assay, a standardized test based on fluorescence intensity that measures the amount of histamine released by activated basophils.85 Although even further studies are required to support standard results, this technique could potentially be used for AGS diagnosis since basophil reactivity was found to be higher in these patients when compared to α-Gal sensitized individuals.10 Indeed, this method displays similar high sensitivity and specificity values when compared to the BAT test,86 but further studies are required to support this result, especially involving AGS-suffering patients.
Omics Technologies
Collective characterization and quantitation of biomolecules known as “omics” technologies such as metabolomics, metagenomics, proteomics and transcriptomics could advance knowledge of the immune response in AGS and its molecular drivers, enabling the identification of biotargets for molecular diagnosis of this global impact disease.10,87 Not only the identification of host biomarkers and host-derived immune response mechanisms but also tick-derived biomolecules are important for the development of new diagnostic tools.52 Proteins present in tick sialome, especially highly conserved across tick species, could potentially serve as diagnostic antigens.47 A recent study by Villar et al88 identified by proteomics analysis of tick sialome and alphagalactome the 14-3-3 family chaperone that could possibly constitute a future diagnostic disease biomarker. The study identified that 14-3-3 family chaperone and other proteins were recognized by IgE in sera from AGS patients.88 Therefore, they proposed that these proteins may potentially be involved in the AGS and other disorders with the possibility of mediating protective immune mechanisms against tick infestations and pathogen infection.88 Nevertheless, there are also tick salivary gland proteins with non-α-Gal modifications that could probably be used to develop ELISA tests for antibody quantification as a complementary diagnostic method for AGS.47,52,89
Model-Based Reasoning (MBR)
Artificial intelligence (AI) and machine learning are considered to be powerful diagnostic assistance tools, which in the future could revolutionize the healthcare system providing an accurate and custom-based diagnosis.90 MBR algorithms aim to create a clinical diagnosis or decision-making model that utilize hybrid reasoning and data-driven AI in order to obtain high diagnostic yields by combining the integrative medicine concept.91 For puzzling and complex diseases, such as AGS, in which diagnostic tools lacking standardization and cofactors are also involved, the use of this integrative diagnostic technique could represent the best fitting method. The use of this methodology has been proposed by de la Fuente et al52 for improving AGS diagnosis, considering together clinical symptoms, risk factors and anti-α-Gal sIgE levels. They also proposed that the machine learning algorithm could be transformed into a code for a software creation with further implementation in clinical practice via mobile applications.52
Treatment/Management Methods
Ticks are today the most accepted evidence for sensitization to α-Gal, but other risk factors or co-factors are likely relevant.92 Daily diet counseling, tick bite avoidance or environmental education should be firstly considered in customizing an accurate treatment for the AGS.42,61 Subsequently, an expertise behind medical interventions is required for an adequate management of the disease over time.43 Although most cases are not emergency cases, invasive techniques are required for in shock patients treatment.6 Furthermore, established protocols, symptomatology, or information about the AGS characteristics are not available due to the wide natural history and variety of subjects all over the world. Herein we present some of the most common strategies and routinary methodology in AGS treatment/management when the disease is diagnosed.
Non-Medical Management
The pillar of the non-medical approach is based on avoidance. The prevention of tick bites is relevant because continuous exposure to tick bites may maintain or increase anti-α-Gal IgE titers and lead to allergic responses to previously tolerated foods.92 Despite limited evidence, patients who successfully avoid tick bites on a long term (1–2 years) have a higher chance of recovering tolerance to meat products, allowing the reintroduction of red meat into diet.10,42,93 The most common strategy for tick-bite prevention and management includes the use of lighter colored protective clothes treated with insect repellents or insecticides such as permethrin.21 Furthermore, prompt embedded tick removal using specialized fine-tipped forceps should be performed in order to reduce the amount of secreted salivary allergens.24,92
Secondly, avoidance of mammalian meat, by-products of meat (innards), fat (gelatin and lards) and other α-Gal-containing foods such as dairy products represent a crucial management strategy for AGS.10,92 To achieve this goal, dietary counseling is vital, and it can be combined with nutro clinical support to avoid nutritional deficiency, especially in highly sensitized individuals.6 Patients should receive a personalized dietary follow-up depending on which foods are allergy triggering and to routinely check for iron and vitamin B12 supplementation needs.21,61
Another major foundation for AGS management is education. Vulnerable patients should be taught on nutrition facts label reading, awareness of hidden exposures and be provided with a written plan on how to promptly operate in case of an allergic reaction.6,42,61 Clinicians must also inform patients of the risk of onset anaphylaxis not only due to cetuximab but also because of heparin, gelatin-containing vaccines and mammalian heart valves.94 The fact that numerous pharmaceutical products contain animal-derived excipients makes it harder to avoid all potentially immunogenic antigens.95 For this reason, it is recommended for AGS-suffering patients to wear warning bracelets about their condition so that physicians are aware and can prevent future life-threatening situations in emergency cases.61 In fact, due to the worldwide increase in individuals with high anti-α-Gal IgE titers and possibly undiagnosed AGS-patients, an allergy prescreening before administration of α-Gal containing medication might be recommended.96
Medical Management
Due to the AGS delay and unexpected symptomatology, emergency treatment is of utmost importance to correctly manage allergic reactions and potentially life-threatening anaphylaxis.6 Furthermore, the high variability regarding severity and timing of the symptoms represents a challenge for the medical management of this disease.21 Intramuscular epinephrine administration represents the initial recommendation. For patients in shock, intravenous epinephrine should be applied alongside with fluid resuscitation and occasional vasopressors. In case of airway obstruction, intubation may be necessary.97 Afterwards, in order to properly reduce the risk of a multisystem allergic reaction, it is imperative to always carry an epinephrine auto-injector.21 For tick-bite local reactions, symptomatic treatment with oral antihistamines, corticosteroids and cold compresses should be enough to reduce non-serious symptoms such as pruritus, urticaria and angioedema.98 As AGS symptomatology and severity are reported to have high individual variability and rely mostly on symptomatic treatment, information collected mostly from case reports is presented in Table 1, together with their clinical management apart from the anaphylaxis acute treatment-response already discussed. A recent study described a clinical case with abnormal neuro-psychiatric behavior (abulia, aphasia, abnormal gait, and reduction of limb movement) related to AGS and a possible α-Gal driven immune-related hypothalamic dysfunction that needs further investigation.99 Other symptomatology such as palpitations and tachycardia are self-limiting and therefore resolve spontaneously.100
 |
Table 1 Drugs and Associated Pharmacological Class Used for α-Gal Syndrome Medical Treatment |
Current Research Methods
As described above, humans evolved as non-capable organisms to produce the glycan α-Gal.114 Together with the fact that a wide variability exists between individuals suffering from this disease,5 AGS comprehension becomes a complex goal in which molecular and physiological mechanisms need to be elucidated. Several experimental model hosts are currently available for the study of AGS and the immune response to α-Gal. Zebrafish (Danio rerio) model has been established and validated under laboratory conditions. This animal model was developed by Contreras et al,115 in which allergic hemorrhagic anaphylactic-type reactions together with behavior changes and mortality were observed in response to tick salivary compounds and mammalian meat consumption. The reactions were associated with tissue-specific toll-like-receptor-mediated responses in Th1 and Th2 helper cells. These data support the use of zebrafish as an animal model for the study of the AGS and bring a new perspective for future strategies in the control of infectious diseases as reported for tuberculosis using vaccination with α-Gal.116
Murine models have been used for decades as validated experimental in vivo methodology for investigating both human and animal diseases due to the advantages that these models offer in terms of time, reproducibility and genetic characteristics.117,118 However, wild-type mice produce biologically active α1,3-galactosyltransferase (α1,3GT) for the synthesis of α-Gal and thus lack anti-Gal antibodies.119 Knocking out the α1,3GT gene results in the absence of α-Gal epitopes, not only in murine models but also in pigs,120,121 thus becoming “humanized” experimental animal models.
The mice C57BL/6 line is one of the most common strains used in research.122 The humanized murine model of this strain for studying AGS (GTKO, AGKO or α1,3-GalT-KO), has been used for the study of tick-induced IgE response-model for α-Gal reactions,123,124 but also for testing other immunological approaches as tick-borne allergies and Chagas disease investigation.125,126
Using these animal models, further research is needed to investigate AGS risk factors and epidemiology in order to propose an accurate treatment strategy for each patient.
Future Treatment Strategies
Mammalian meat desensitization by oral immunotherapy (OIT) has been proposed as a promising treatment for AGS as it would improve patient’s welfare and safer management.127 It consists of daily intake of small and generally increasing amounts of allergen, in order to reduce the immune response and consequently produce own allergen desensitization.128 To date, there are only three successful case reports (two adults and one pediatric case) of AGS with oral desensitization to beef meat.127,129 Although this type of treatment leads to a sustained unresponsiveness,93 it requires an individual effort from the patient to consume daily 120 grams of cooked mammalian meat in order to maintain desensitization,129 which also becomes an obligation and can have an impact on the patient’s routine, commodity and mental wellbeing. Indeed, daily mammalian meat intake could compromise the patients to develop other metabolic and cardiometabolic diseases such as hypertension, diabetes and obesity.130,131 Additionally, allergen-specific immunotherapy (AIT) using natural and recombinant α-Gal containing proteins from tick sialome is also being considered for AGS treatment.17 Nonetheless, this type of therapies comes with a risk of life-threatening anaphylactic adverse reactions and demand a thoughtful and balanced management of accurate dose efficacy versus side effect appearance.66,132
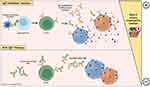
Given the potential risks associated with immunotherapy, the use of allergen non-specific treatments, such as anti-IgE therapeutic monoclonal antibodies (mAbs), has found application in the treatment of food allergy.133,134 In anti-IgE therapy, mAbs bind to free serum IgE and IgE-coated B cells, acting as a competitive substrate and reducing the availability and binding between these antibodies (natural IgE) and allergy mediators such as basophils and mast cells, which increases reaction threshold and consequently reduces the risk of mild and severe anaphylactic reactions (Figure 3).43,134 Combining anti-IgE therapy as a pre-treatment with immunotherapy techniques leads to a safer administration of OIT and allows to reach the maintenance dose more rapidly.128,133,135 The anti-IgE agent omalizumab has been sporadically used in specialized centers as monotherapy in AGS patients for successfully controlling continued reactivity, allowing the introduction of a small amount of mammalian meat in their diet.42 With such a positive preliminary outcome in these patients and promising data results in other FAs (peanut and cow milk), new clinical trials using biological therapies for AGS are needed, potentially representing a future effective treatment.42,43,133
Management of FAs is becoming less generic and more target oriented.66 Consequently, there is still a need to improve our understanding of the immunological mechanisms behind tick bite sensitization and therefore identify new and more specific targets for the development of new treatment interventions for AGS.136
Future Preventive Tools
Prevention from developing AGS stands on avoiding the initial α-Gal sensitization caused by tick bites, being particularly beneficial for at-risk population.21,35,36 Apart from the common strategies for tick-bite prevention mentioned above, the development of tick-antigen-based vaccines could not only protect against AGS but also against other tick-borne diseases.14
Therefore, to follow the vaccinomics approach, it is essential to identify tick bioactive molecules and consequent signaling pathways that mediate tick-host-pathogen interactions.137,138 For example, in a study by Mateos-Hernández et al,47 tick sialome proteins, with or without α-Gal modifications, that led to a protective immune response and were recognized by AGS patients but not control individuals could serve as potential target antigen candidates for vaccine development. The identification of the poorly understood molecular mechanisms behind the development of spontaneous acquired tick resistance (ATR) is also of key importance as it could help in the search for new vaccine formulations.139 Discovering which tick salivary antigens are natural targets of ATR will help to aim towards the inhibition of tick feeding, reproduction and further pathogen transmission.140
Conclusions
AGS is an atypical, underdiagnosed vector-borne allergy that presents clinical implications beyond expected due to the presence of α-Gal in various animal-derived medical products, hindering the treatment of several other pathologies.141 Since the discovery of AGS, many advancements have been made in order to obtain a better knowledge in terms of disease epidemiology, medical approach and molecular mechanisms. Nevertheless, current diagnostic methods lack specificity or are too risky for routinary appliance, creating the need to overcome these limitations with more precise methods. Also, a uniformization-based approach of diagnostic guidelines could be beneficial, creating comparable data and offering an opportunity to improve clinical decision-making accuracy. Further diagnostic, treatment and preventive advances will only be possible if the molecular and immune mechanisms behind AGS are uncovered. Furthermore, it is of utmost importance to identify tick salivary molecules, with or without α-Gal modifications, that trigger IgE sensitivity as they could be the key for further vaccine development. With climate change, the tick-host paradigm will shift towards an increasing number of AGS cases in new regions worldwide,22 which will pose new challenges for clinicians in the future.
Funding
Research on AGS was funded by Ministerio de Ciencia e Innovación/Agencia Estatal de Investigación MCIN/AEI/10.13039/501100011033, Spain and EU-FEDER (Grant BIOGAL PID2020-116761GB-I00). R. Vaz-Rodrigues was supported by a doctoral contract (2022/20675) from Universidad de Castilla-La Mancha (UCLM), Spain, co-financed by the European Social Fund (ESF). L. Mazuecos was supported by a post-doctoral grant (2021-POST-32002) from UCLM co-financed by ESF.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Hils M, Wölbing F, Hilger C, Fischer J, Hoffard N, Biedermann T. The history of carbohydrates in type I allergy. Front Immunol. 2020;11(10):1–14. doi:10.3389/fimmu.2020.586924
2. Hilger C, Fischer J, Wölbing F, Biedermann T. Role and mechanism of Galactose-Alpha-1,3-Galactose in the elicitation of delayed anaphylactic reactions to red meat. Curr Allergy and Asthma Rep. 2019;19(1):1–11. doi:10.1007/s11882-019-0835-9
3. Galili U. Evolution in primates by “Catastrophic-selection” interplay between enveloped virus epidemics, mutated genes of enzymes synthesizing carbohydrate antigens, and natural anti-carbohydrate antibodies. Am J Phys Anthropol. 2019;168(2):352–363. doi:10.1002/ajpa.23745
4. Commins SP, Satinover SM, Hosen J, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-α-1,3-galactose. J Allergy Clin Immunol. 2009;123(2):426–433.e2. doi:10.1016/j.jaci.2008.10.052
5. Fischer J, Yazdi AS, Biedermann T. Clinical spectrum of α-Gal syndrome: from immediate-type to delayed immediate-type reactions to mammalian innards and meat. Allergo J Int. 2016;25(2):55–62. doi:10.1007/s40629-016-0099-z
6. Çelebioğlu E, Akarsu A, Şahiner ÜM. IgE mediated food allergy throughout the life. Turk J Med Sci. 2021;51(1):49–60. doi:10.3906/sag-2006-95
7. Fischer J, Reepschläger T, Schricker T, Raap U. Alpha-gal syndrome: overview of clinical presentation and pathophysiology. Hautarzt. 2022;73(3):195–200. doi:10.1007/s00105-022-04943-4
8. Chinuki Y, Takahashi H, Morita E. IgE antibodies to galactose-α-1,3-galactose, an epitope of red meat allergen, cross-react with a novel flounder roe allergen. J Investig Allergol Clin Immunol. 2021;32(4):1–7. doi:10.18176/jiaci.0754
9. Dunkman WJ, Rycek W, Manning MW. What does a red meat allergy have to do with anesthesia? Perioperative management of alpha-gal syndrome. Anesth Analg. 2019;129(5):1242–1248. doi:10.1213/ANE.0000000000003460
10. Cabezas-Cruz A, Hodžić A, Román-Carrasco P, et al. Environmental and molecular drivers of the α-Gal syndrome. Front Immunol. 2019;10(5):1–12. doi:10.3389/fimmu.2019.01210
11. Cabezas-Cruz A, Valdés J, de la Fuente J. Cancer research meets tick vectors for infectious diseases. Lancet Infect Dis. 2014;14(10):916–917. doi:10.1016/S1473-3099(14)70902-8
12. Chung CH, Mirakhur B, Chan E, et al. Cetuximab-induced anaphylaxis and IgE specific for Galactose-α-1,3-Galactose. N Engl J Med. 2008;358(11):1109–1117. doi:10.1056/nejmoa074943
13. de la Fuente J, Pacheco I, Villar M, Cabezas-Cruz A. The alpha-Gal syndrome: new insights into the tick-host conflict and cooperation. Parasit Vectors. 2019;12(1):1–5. doi:10.1186/s13071-019-3413-z
14. Rodríguez Y, Rojas M, Gershwin ME, Anaya JM. Tick-borne diseases and autoimmunity: a comprehensive review. J Autoimmun. 2018;88(3):21–42. doi:10.1016/j.jaut.2017.11.007
15. Cabezas-Cruz A, Mateos-Hernández L, Chmelař J, Villar M, de la Fuente J. Salivary prostaglandin E2: role in tick-induced allergy to red meat. Trends Parasitol. 2017;33(7):495–498. doi:10.1016/j.pt.2017.03.004
16. Román-Carrasco P, Hemmer W, Cabezas-Cruz A, Hodžić A, de la Fuente J, Swoboda I. The α-Gal syndrome and potential mechanisms. Front Allergy. 2021;2(12):1–17. doi:10.3389/falgy.2021.783279
17. Ramasamy R. Mosquito vector proteins homologous to α1-3 galactosyl transferases of tick vectors in the context of protective immunity against malaria and hypersensitivity to vector bites. Parasit Vectors. 2021;14(1):1–6. doi:10.1186/s13071-021-04801-7
18. Wilson JM, Schuyler AJ, Schroeder N, Platts-Mills TAE. Galactose-α-1,3-Galactose: atypical food allergen or model IgE hypersensitivity? Curr Allergy Asthma Rep. 2017;17(1):3–9. doi:10.1007/s11882-017-0672-7
19. Cabezas-Cruz A, Hodžić A, Mateos-Hernandez L, Contreras M, De La Fuente J. Tick-human interactions: from allergic klendusity to the α-Gal syndrome. Biochem J. 2021;478(9):1783–1794. doi:10.1042/BCJ20200915
20. Carson AS, Gardner A, Iweala OI. Where’s the beef? Understanding allergic responses to red meat in alpha-gal syndrome. J Immunol. 2022;208(2):267–277. doi:10.4049/jimmunol.2100712
21. Wong XL, Sebaratnam DF. Mammalian meat allergy. Int J Dermatol. 2018;57(12):1433–1436. doi:10.1111/ijd.14208
22. Young I, Prematunge C, Pussegoda K, Corrin T, Waddell L. Tick exposures and alpha-gal syndrome: a systematic review of the evidence. Ticks Tick Borne Dis. 2021;12(3):101674. doi:10.1016/j.ttbdis.2021.101674
23. Diaz JH. Red meat allergies after Lone Star tick (Amblyomma americanum) bites. South Med J. 2020;113(6):267–274. doi:10.14423/SMJ.0000000000001102
24. van Nunen S. Tick-induced allergies: mammalian meat allergy, tick anaphylaxis and their significance. Asia Pac Allergy. 2015;5(1):3–16. doi:10.5415/apallergy.2015.5.1.3
25. Platts-Mills TAE, Commins SP, Biedermann T, et al. On the cause and consequences of IgE to galactose-α-1,3-galactose: a report from the National Institute of Allergy and Infectious Diseases Workshop on understanding IgE-mediated mammalian meat allergy. J Allergy Clin Immunol. 2020;145(4):1061–1071. doi:10.1016/j.jaci.2020.01.047
26. Joral A, Azketa N, Sanchez P, et al. The quantification of IgG specific to α-Gal could be used as a risk marker for suffering mammalian meat allergy. Foods. 2022;11(3):466. doi:10.3390/foods11030466
27. McGain F, Welton R, Solley GO, Winkel KD. First fatalities from tick bite anaphylaxis. J Allergy Clin Immunol Pract. 2016;2(7):309–314. doi:10.1080/21674086.1933.11925177
28. Mullins RJ, Wainstein BK, Barnes EH, Liew WK, Campbell DE. Increases in anaphylaxis fatalities in Australia from 1997 to 2013. Clin Exp Allergy. 2016;46(8):1099–1110. doi:10.1111/cea.12748
29. Commins SP. Invited commentary: alpha-gal allergy: tip of the iceberg to a pivotal immune response. Curr Allergy Asthma Rep. 2016;16(9):1–3. doi:10.1007/s11882-016-0641-6
30. Levin M, Apostolovic D, Biedermann T, et al. Galactose α-1,3-galactose phenotypes: lessons from various patient populations. Ann Allergy Asthma Immunol. 2019;122(6):598–602. doi:10.1016/j.anai.2019.03.021
31. Zurbano-Azqueta L, Antón-Casas E, Duque-Gómez S, Jiménez-Gómez I, Fernández-Pellón L, López-Gutiérrez J. Alpha-gal syndrome. Allergy to red meat and gelatin. Rev Clin Esp. 2021. doi:10.1016/j.rceng.2021.06.005
32. Mateo-Borrega MB, Garcia B, Larramendi CH, et al. Ige-mediated sensitization to galactose-α-1,3-galactose (α-gal) in urticaria and anaphylaxis in Spain: geographical variations and risk factors. J Investig Allergol Clin Immunol. 2019;29(6):436–443. doi:10.18176/jiaci.0373
33. Fischer J, Lupberger E, Hebsaker J, et al. Prevalence of type I sensitization to alpha-gal in forest service employees and hunters. Allergy. 2017;72(10):1540–1547. doi:10.1111/all.13156
34. Villalta D, Pantarotto L, Da Re M, et al. High prevalence of sIgE to Galactose-α1,3-galactose in rural pre-Alps area: a cross-sectional study. Clin Exp Allergy. 2016;46(2):377–380. doi:10.1111/cea.12655
35. Mitchell CL, Lin FC, Vaughn M, Apperson CS, Meshnick SR, Commins SP. Association between Lone Star tick bites and increased alpha-gal sensitization: evidence from a prospective cohort of outdoor workers. Parasit Vectors. 2020;13(1):1–4. doi:10.1186/s13071-020-04343-4
36. Venturini M, Lobera T, Sebastián A, Portillo A, Oteo JA. IgE to α-Gal in foresters and forest workers from La Rioja, North of Spain. J Investig Allergol Clin Immunol. 2018;28(2):106–112. doi:10.18176/jiaci.0218
37. Cabezas-Cruz A, de la Fuente J, Fischer J. Prevalence of type I sensitization to alpha-gal in forest service employees and hunters: is the blood type an overlooked risk factor in epidemiological studies of the α-Gal syndrome? Allergy. 2017;72(12):2044–2047. doi:10.1111/all.13206
38. Cabezas-Cruz A, Mateos-Hernández L, Alberdi P, et al. Effect of blood type on anti-a-Gal immunity and the incidence of infectious diseases. Exp Mol Med. 2017;49(3):e301–e301. doi:10.1038/emm.2016.164
39. Yilmaz B, Portugal S, Tran TM, et al. Gut microbiota elicits a protective immune response against malaria transmission. Cell. 2014;159(6):1277–1289. doi:10.1016/j.cell.2014.10.053
40. Urra JM, Ferreras-Colino E, Contreras M, et al. The antibody response to the glycan α-Gal correlates with COVID-19 disease symptoms. J Med Virol. 2021;93(4):2065–2075. doi:10.1002/jmv.26575
41. Steinke JW, Platts-Mills TAE, Commins SP. The alpha-gal story: lessons learned from connecting the dots. J Allergy Clin Immunol. 2015;135(3):589–596. doi:10.1016/j.jaci.2014.12.1947
42. Commins SP. Diagnosis & management of alpha-gal syndrome: lessons from 2500 patients. Expert Rev Clin Immunol. 2020;16(7):667–677. doi:10.1080/1744666X.2020.1782745
43. Lopes JP, Sicherer S. Food allergy: epidemiology, pathogenesis, diagnosis, prevention, and treatment. Curr Opin Immunol. 2020;66:57–64. doi:10.1016/j.coi.2020.03.014
44. Antunes J, Borrego L, Romeira A, Pinto P. Skin prick tests and allergy diagnosis. Allergol Immunopathol. 2009;37(3):155–164. doi:10.1016/S0301-0546(09)71728-8
45. Shroba J, Rath N, Barnes C. Possible role of environmental factors in the development of food allergies. Clin Rev Allergy Immunol. 2019;57(3):303–311. doi:10.1007/s12016-018-8703-2
46. Jacquenet S, Moneret-Vautrin DA, Bihain BE. Mammalian meat-induced anaphylaxis: clinical relevance of anti-galactose-α-1,3-galactose IgE confirmed by means of skin tests to cetuximab. J Allergy Clin Immunol. 2009;124(3):603–605. doi:10.1016/j.jaci.2009.06.014
47. Mateos-Hernández L, Villar M, Moral A, et al. Tick-host conflict: immunoglobulin E antibodies to tick proteins in patients with anaphylaxis to tick bite. Oncotarget. 2017;8(13):20630–20644. doi:10.18632/oncotarget.15243
48. Hocaoglu AB, Cipe F, Aydogmus C. Are skin prick tests really safe? A case of anaphylaxis caused by skin prick testing with inhalant allergens. Allergol Immunopathol. 2015;43(2):215–216. doi:10.1016/j.aller.2013.09.011
49. Alnæs M. Anaphylaxis following prick-by-prick testing with peanut. Clin Case Rep. 2020;8(12):2366–2368. doi:10.1002/ccr3.3154
50. Chiriac AM, Bousquet J, Demoly P. In vivo methods for the study and diagnosis of allergy. In: Burks AW, Holgate ST, O´Hehir RE, et al. editors. Middleton’s Allergy: Principles and Practice.
51. Calvani M, Bianchi A, Reginelli C, Peresso M, Testa A. Oral food challenge. Medicina. 2019;55(10):1–16. doi:10.3390/medicina55100651
52. de la Fuente J, Cabezas-Cruz A, Pacheco I. Alpha-gal syndrome: challenges to understanding sensitization and clinical reactions to alpha-gal. Expert Rev Mol Diagn. 2020;20(9):905–911. doi:10.1080/14737159.2020.1792781
53. Mehlich J, Fischer J, Hilger C, et al. The basophil activation test differentiates between patients with alpha-gal syndrome and asymptomatic alpha-gal sensitization. J Allergy Clin Immunol. 2019;143(1):182–189. doi:10.1016/j.jaci.2018.06.049
54. Mabelane T, Basera W, Botha M, Thomas HF, Ramjith J, Levin ME. Predictive values of alpha-gal IgE levels and alpha-gal IgE: total IgE ratio and oral food challenge proven meat allergy in a population with a high prevalence of reported red meat allergy. Pediatr Allergy Immunol. 2018;29(8):841–849. doi:10.1111/pai.12969
55. Eberlein B, Mehlich J, Reidenbach K, et al. Negative oral provocation test with porcine pancreatic enzyme plus cofactors despite confirmed α-gal syndrome. J Investig Allergol Clin Immunol. 2020;30(6):468–469. doi:10.18176/jiaci.0513
56. Morisset M, Richard C, Astier C, et al. Anaphylaxis to pork kidney is related to IgE antibodies specific for galactose-alpha-1,3-galactose. Allergy. 2012;67(5):699–704. doi:10.1111/j.1398-9995.2012.02799.x
57. Fischer J, Biedermann T. Delayed immediate-type hypersensitivity to red meat and innards: current insights into a novel disease entity. J Dtsch Dermatol Ges. 2016;14(1):38–43. doi:10.1111/ddg.12821
58. Jappe U, Minge S, Kreft B, et al. Meat allergy associated with galactosyl-α-(1,3)-galactose (α-Gal)-Closing diagnostic gaps by anti-α-Gal IgE immune profiling. Allergy. 2018;73(1):93–105. doi:10.1111/all.13238
59. Leung DYM, Szefler SJ. Tick bites: a common cause of IgE antibodies to alpha-gal. J Allergy Clin Immunol. 2011;127(5):1131–1132. doi:10.1016/j.jaci.2011.03.019
60. Chinuki Y, Morita E. Alpha-Gal-containing biologics and anaphylaxis. Allergol Int. 2019;68(3):296–300. doi:10.1016/j.alit.2019.04.001
61. Mabelane T, Ogunbanjo GA. Ingestion of mammalian meat and alpha-gal allergy: clinical relevance in primary care. Afr J Prim Health Care Fam Med. 2019;11(1):1–5. doi:10.4102/phcfm.v11i1.1901
62. Park Y, Kim D, Boorgula GD, et al. Alpha-gal and cross-reactive carbohydrate determinants in the N-Glycans of salivary glands in the Lone Star Tick, Amblyomma americanum. Vaccines. 2020;8(1):1–16. doi:10.3390/vaccines8010018
63. Apostolovic D, Grundström J, Perusko M, et al. Course of IgE to α-Gal in a Swedish population of α-Gal syndrome patients. Clin Transl Allergy. 2021;11(10):2–4. doi:10.1002/clt2.12087
64. Gonzalez-Quintela A, Dam Laursen AS, Vidal C, Skaaby T, Gude F, Linneberg A. IgE antibodies to alpha-gal in the general adult population: relationship with tick bites, atopy, and cat ownership. Clin Exp Allergy. 2014;44(8):1061–1068. doi:10.1111/cea.12326
65. Wilson JM, Keshavarz B, Retterer M, et al. A dynamic relationship between two regional causes of IgE-mediated anaphylaxis: α-Gal syndrome and imported fire ant. J Allergy Clin Immunol. 2021;147(2):643–652.e7. doi:10.1016/j.jaci.2020.05.034
66. Passanisi S, Lombardo F, Crisafulli G, Salzano G, Aversa T, Pajno GB. Novel diagnostic techniques and therapeutic strategies for IgE-mediated food allergy. Allergy Asthma Proc. 2021;42(2):124–130. doi:10.2500/AAP.2021.42.200129
67. Foong RX, Santos AF. Biomarkers of diagnosis and resolution of food allergy. Pediatr Allergy Immunol. 2021;32(2):223–233. doi:10.1111/pai.13389
68. Fernandez-Santamaria R, Bogas G, Salas M, et al. The role of basophil activation test in drug allergy. Curr Treat Options Allergy. 2021;8(4):298–313. doi:10.1007/s40521-021-00294-y
69. Schmidle P, Eberlein B, Darsow U, Kugler C, Biedermann T, Brockow K. α-Gal-Syndrom – nicht nur eine Fleischallergie. Allergologie. 2021;44(4):288–296. doi:10.5414/ALX02185
70. Santos AF, Kulis MD, Sampson HA. Bringing the next generation of food allergy diagnostics into the clinic. J Allergy Clin Immunol Pract. 2022;10(1):1–9. doi:10.1016/j.jaip.2021.09.009
71. Santos AF, Lack G. Basophil activation test: food challenge in a test tube or specialist research tool? Clin Transl Allergy. 2016;6(1):1–9. doi:10.1186/s13601-016-0098-7
72. Wilson JM, Platts-Mills TAE. IgE to galactose-α-1,3-galactose and the α-Gal syndrome: insights from basophil activation testing. J Allergy Clin Immunol. 2018;143(1):101–103. doi:10.1016/j.jaci.2018.10.029
73. Doña I, Ariza A, Fernández TD, Torres MJ. Basophil activation test for allergy diagnosis. J Vis Exp. 2021;171(5):e62600. doi:10.3791/62600
74. Ebo DG, Bridts CH, Mertens CH, et al. Principles, potential, and limitations of ex vivo basophil activation by flow cytometry in allergology: a narrative review. J Allergy Clin Immunol. 2021;147(4):1143–1153. doi:10.1016/j.jaci.2020.10.027
75. Santos AF, Alpan O, Hoffmann HJ. Basophil activation test: mechanisms and considerations for use in clinical trials and clinical practice. Allergy. 2021;76(11):2420–2432. doi:10.1111/all.14747
76. Directive 98/79/EC on in vitro diagnostic medical devices; 1998. Available from: http://data.europa.eu/eli/dir/1998/79/oj.
77. Regulation (EU) 2017/746 on in vitro diagnostic medical devices; 2022. Available from: http://data.europa.eu/eli/reg/2017/746/2022-01-28.
78. Bahri R, Bulfone-Paus S. Mast Cell Activation Test (MAT). Methods Mol Biol. 2020;2163:227–238. doi:10.1007/978-1-0716-0696-4_19
79. Bahri R, Custovic A, Korosec P, et al. Mast cell activation test in the diagnosis of allergic disease and anaphylaxis. J Allergy Clin Immunol. 2018;142(2):485–496.e16. doi:10.1016/j.jaci.2018.01.043
80. Chirumbolo S, Bjørklund G, Sboarina A, Vella A. The role of basophils as innate immune regulatory cells in allergy and immunotherapy. Hum Vaccines Immunother. 2018;14(4):815–831. doi:10.1080/21645515.2017.1417711
81. Elst J, Sabato V, van der Poorten MLM, et al. Basophil and mast cell activation tests by flow cytometry in immediate drug hypersensitivity: diagnosis and beyond. J Immunol Methods. 2021;495(4):113050. doi:10.1016/j.jim.2021.113050
82. Elst J, van der Poorten M-LM, van Gasse AL, et al. Mast cell activation tests by flow cytometry: a new diagnostic asset? Clin Exp Allergy. 2021;51(11):1482–1500. doi:10.1111/cea.13984
83. Kirshenbaum AS, Yin Y, Bruce Sundstrom J, Bandara G, Metcalfe DD. Description and characterization of a novel human mast cell line for scientific study. Int J Mol Sci. 2019;20(22):5520. doi:10.3390/ijms20225520
84. Chirumbolo S, Bjørklund G, Vella A. Mast cell activation test versus basophil activation test and related competing issues. J Allergy Clin Immunol. 2018;142(3):1018–1019. doi:10.1016/j.jaci.2018.06.020
85. Gray CL. Current controversies and future prospects for peanut allergy prevention, diagnosis and therapies. J Asthma Allergy. 2020;13:51–66. doi:10.2147/JAA.S196268
86. Larsen LF, Juel-Berg N, Hansen KS, et al. A comparative study on basophil activation test, histamine release assay, and passive sensitization histamine release assay in the diagnosis of peanut allergy. Allergy. 2018;73(1):137–144. doi:10.1111/all.13243
87. de la Fuente J, Pacheco I, Contreras M, Mateos-Hernández L, Villar M, Cabezas-Cruz A. Guillain-Barré and alpha-gal syndromes: saccharides-induced immune responses. Explor Res Hypothesis Med. 2019;4(4):87–89. doi:10.14218/erhm.2019.00027
88. Villar M, Pacheco I, Mateos-Hernández L, et al. Characterization of tick salivary gland and saliva alphagalactome reveals candidate alpha-gal syndrome disease biomarkers. Expert Rev Proteomics. 2021;18(12):1099–1116. doi:10.1080/14789450.2021.2018305
89. Cabezas-Cruz A, Mateos-Hernández L, Pérez-Cruz M, et al. Regulation of the immune response to α-Gal and vector-borne diseases. Trends Parasitol. 2015;31(10):470–476. doi:10.1016/j.pt.2015.06.016
90. Richens JG, Lee CM, Johri S. Improving the accuracy of medical diagnosis with causal machine learning. Nat Commun. 2020;11:3923. doi:10.1038/s41467-020-17419-7
91. Geng W, Qin X, Yang T, et al. Model-based reasoning of clinical diagnosis in integrative medicine: real-world methodological study of electronic medical records and natural language processing methods. JMIR Med Inform. 2020;8(12):e23082. doi:10.2196/23082
92. Saretta F, Giovannini M, Mori F, et al. Alpha-gal syndrome in children: peculiarities of a “Tick-Borne” allergic disease. Front Pediatr. 2021;9(12). doi:10.3389/fped.2021.801753
93. Wilson JM, Platts-Mills TAE. Red meat allergy in children and adults. Curr Opin Allergy Clin Immunol. 2019;19(3):229–235. doi:10.1097/ACI.0000000000000523
94. Platts-Mills TAE, Li R, Keshavarz B, Smith AR, Wilson JM. Diagnosis and management of patients with the α-Gal syndrome. J Allergy Clin Immunol Pract. 2020;8(1):15–23. doi:10.1016/j.jaip.2019.09.017
95. Nwamara U, Kaplan MC, Mason N, Ingemi AI. A retrospective evaluation of heparin product reactions in patients with alpha-gal allergies. Ticks Tick Borne Dis. 2022;13(1):101869. doi:10.1016/j.ttbdis.2021.101869
96. Wen S, Unuma K, Chinuki Y, Hikino H, Uemura K. Fatal anaphylaxis due to alpha-gal syndrome after initial cetuximab administration: the first forensic case report. Leg Med. 2021;51(3):101878. doi:10.1016/j.legalmed.2021.101878
97. McHugh K, Repanshek Z. Anaphylaxis: emergency Department Treatment. Emerg Med Clin North Am. 2022;40(1):19–32. doi:10.1016/j.emc.2021.08.004
98. Lee YH, Kasper LH. Immune responses of different mouse strains after challenge with equivalent lethal doses of Toxoplasma gondii. Parasite. 2004;11:89–97. doi:10.1051/parasite/200411189
99. Daripa B, Lucchese S. Novel case presentation of abulia after Lone Star tick bite as evidenced by raised titers of alpha-gal specific IgM immunoglobulin and a possibility of alpha-gal driven hypothalamic dysfunction as the pathomechanism. Cureus. 2022;14(4):e24551. doi:10.7759/cureus.24551
100. Wuerdeman MF, Harrison JM. A case of tick-bite-induced red meat allergy. Mil Med. 2014;179(4):e473–e475. doi:10.7205/MILMED-D-13-00369
101. Jackson WL. Mammalian meat allergy following a tick bite: a case report. Oxf Med Case Rep. 2018;2018(2):58–60. doi:10.1093/omcr/omx098
102. Kaplan AC, Carson MP. Diagnosing meat allergy after tick bite without delay. J Am Board Fam Med. 2018;31(4):650–652. doi:10.3122/jabfm.2018.04.170425
103. Khoury JK, Khoury NC, Schaefer D, Chitnis A, Hassen GW. A tick-acquired red meat allergy. Am J Emerg Med. 2018;36(2):
104. Brzozowska M, Mokrzycka N, Porębski G. Alpha-gal syndrome: the first report in Poland. Cent Eur J Immunol. 2021;46(3):398–400. doi:10.5114/ceji.2021.108766
105. Anemüller W, Mohr M, Brans R, Homann A, Jappe U. Alpha-Gal-assoziierte verzögerteAnaphylaxie gegen rotes Fleischals Berufskrankheit. Hautarzt. 2018;69(10):848–852. doi:10.1007/s00105-018-4224-4
106. Ghahramani GK, Temprano J. Tick bite-related meat allergy as a cause of chronic urticaria, angioedema, and anaphylaxis in endemic areas. Int J Dermatol. 2015;54(2):e64–e65. doi:10.1111/ijd.12672
107. Wen L, Zhou J, Yin J, et al. Delayed anaphylaxis to red meat associated with specific IgE antibodies to galactose. Allergy Asthma Immunol Res. 2014;7(1):92–94. doi:10.4168/aair.2015.7.1.92
108. Zhang B, Hauk M, Clyne J. Alpha-gal antibody due to Lone Star tick bite, a unique case of allergic reaction. IDCases. 2020;22:e00908. doi:10.1016/j.idcr.2020.e00908
109. Saleem M, Nilsson C. A pediatric case of tick-bite–Induced meat allergy and recall urticaria. Clin Case Rep. 2021;9(9):1–4. doi:10.1002/ccr3.4773
110. Guillier A, Fauconneau A, de Barruel F, Guez S, Doutre MS. Allergic hypersensitivity to red meat induced by tick bites: a French case report. Eur J Dermatol. 2015;25(3):275–276. doi:10.1684/ejd.2015.2533
111. Brenner DM, Lacy BE. Antispasmodics for chronic abdominal pain: analysis of North American treatment options. Am J Gastroenterol. 2021;116(8):1587–1600. doi:10.14309/ajg.0000000000001266
112. Bircher AJ, Hofmeier KS, Link S, Heijnen I. Food allergy to the carbohydrate galactose-alpha-1,3-galactose (alpha-gal): four case reports and a review. Eur J Dermatol. 2017;27(1):3–9. doi:10.1684/ejd.2016.2908
113. Emmanuel A, Quigley EMM, Simrén M, et al. Factors affecting satisfaction with treatment in European women with chronic constipation: an internet survey. United Eur Gastroenterol J. 2013;1(5):375–384. doi:10.1177/2050640613494200
114. Galili U. Significance of the evolutionary α1,3-Galactosyltransferase (GGTA1) gene inactivation in preventing extinction of apes and old world monkeys. J Mol Evol. 2015;80(1):1–9. doi:10.1007/s00239-014-9652-x
115. Contreras M, Pacheco I, Alberdi P, et al. Allergic reactions and immunity in response to tick salivary biogenic substances and red meat consumption in the Zebrafish Model. Front Cell Infect Microbiol. 2020;10(3). doi:10.3389/fcimb.2020.00078
116. Pacheco I, Contreras M, Villar M, et al. Vaccination with alpha-gal protects against mycobacterial infection in the zebrafish model of tuberculosis. Vaccines. 2020;8(2):195. doi:10.3390/vaccines8020195
117. Bryda EC. The mighty mouse: the impact of rodents on advances in biomedical research. Mo Med. 2013;110(3):207–211.
118. Fujiwara S. Humanized mice: a brief overview on their diverse applications in biomedical research. J Cell Physiol. 2018;233(4):2889–2901. doi:10.1002/jcp.26022
119. Koike C, Fung JJ, Geller DA, et al. Molecular basis of evolutionary loss of the α1,3-Galactosyltransferase gene in higher primates. J Biol Chem. 2002;277(12):10114–10120. doi:10.1074/jbc.M110527200
120. Dai Y, Vaught TD, Boone J, et al. Targeted disruption of the α1,3-galactosyltransferase gene in cloned pigs. Nat Biotechnol. 2002;20(3):251–255. doi:10.1038/nbt0302-251
121. Tearle RG, Tange MJ, Zannettino ZL, et al. The alpha-1,3-galactosyltransferase knockout mouse. Implications for xenotransplantation. Transplantation. 1996;61(1):13–19. doi:10.1097/00007890-199601150-00004
122. Bryant CD. The blessings and curses of C57BL/6 substrains in mouse genetic studies. Ann N Y Acad Sci. 2011;1245(1):31–33. doi:10.1111/j.1749-6632.2011.06325.x
123. Choudhary SK, Karim S, Iweala OI, et al. Tick salivary gland extract induces alpha-gal syndrome in alpha-gal deficient mice. Immun Inflamm Dis. 2021;9(3):984–990. doi:10.1002/iid3.457
124. Araujo RN, Franco PF, Rodrigues H, et al. Amblyomma sculptum tick saliva: α-Gal identification, antibody response and possible association with red meat allergy in Brazil. Int J Parasitol. 2016;46(3):213–220. doi:10.1016/j.ijpara.2015.12.005
125. Ayala EV, Rodrigues Da Cunha G, Azevedo MA, et al. C57BL/6 α-1,3-Galactosyltransferase knockout mouse as an animal model for experimental Chagas disease. ACS Infect Dis. 2020;6(7):1807–1815. doi:10.1021/acsinfecdis.0c00061
126. Chandrasekhar JL, Cox KM, Loo WM, Qiao H, Tung KS, Erickson LD. Cutaneous exposure to clinically relevant Lone Star ticks promotes IgE production and hypersensitivity through CD4 + T cell– and MyD88-dependent pathways in mice. J Immunol. 2019;203(4):813–824. doi:10.4049/jimmunol.1801156
127. Unal D, Coskun R, Demir S, Gelincik A, Colakoglu B, Buyukozturk S. Successful beef desensitization in 2 adult patients with a delayed-type reaction to red meat. J Allergy Clin Immunol Pract. 2017;5(2):502–503. doi:10.1016/j.jaip.2016.12.008
128. Michelet M, Balbino B, Guilleminault L, Reber LL. IgE in the pathophysiology and therapy of food allergy. Eur J Immunol. 2021;51(3):531–543. doi:10.1002/eji.202048833
129. Yucel E, Sipahi Cimen S, Varol S, Suleyman A, Ozdemir C, Tamay ZU. Red meat desensitization in a child with delayed anaphylaxis due to alpha-Gal allergy. Pediatr Allergy Immunol. 2019;30(7):771–773. doi:10.1111/pai.13092
130. Zhubi-Bakija F, Bajraktari G, Bytyçi I, et al. The impact of type of dietary protein, animal versus vegetable, in modifying cardiometabolic risk factors: a position paper from the International Lipid Expert Panel (ILEP). Clin Nutr. 2021;40(1):255–276. doi:10.1016/j.clnu.2020.05.017
131. Abenavoli L, Boccuto L, Federico A, et al. Diet and non-alcoholic fatty liver disease: the Mediterranean way. Int J Environ Res Public Health. 2019;16(17):3011. doi:10.3390/ijerph16173011
132. Badina L, Burlo F, Belluzzi B, Babich S, Berti I, Barbi E. Life-threatening anaphylaxis in children with cow’s milk allergy during oral immunotherapy and after treatment failure. Immun Inflamm Dis. 2022;10(2):e607. doi:10.1002/iid3.607
133. Guilleminault L, Michelet M, Reber LL. Combining Anti-IgE monoclonal antibodies and oral immunotherapy for the treatment of food allergy. Clin Rev Allergy Immunol. 2022;62(1):216–231. doi:10.1007/s12016-021-08902-0
134. Ramesh M, Karagic M. New modalities of allergen immunotherapy. Human Vaccin Immunother. 2018;14(12):2848–2863. doi:10.1080/21645515.2018.1502126
135. Long A, Borro M, Sampath V, Chinthrajah RS. New developments in non-allergen-specific therapy for the treatment of food allergy. Curr Allergy Asthma Rep. 2020;20(1). doi:10.1007/s11882-020-0897-8
136. Chandrasekhar JL, Cox KM, Erickson LD, Cell B. Responses in the development of mammalian meat allergy. Front Immunol. 2020;11(7):1–16. doi:10.3389/fimmu.2020.01532
137. de la Fuente J, Kopáček P, Lew-Tabor A, Maritz-Olivier C. Strategies for new and improved vaccines against ticks and tick-borne diseases. Parasite Immunol. 2016;38(12):754–769. doi:10.1111/pim.12339
138. Hromníková D, Furka D, Furka S, et al. Prevention of tick-borne diseases: challenge to recent medicine. Biologia. 2022;77(3):1533–1554. doi:10.1007/s11756-021-00966-9
139. Narasimhan S, Kurokawa C, DeBlasio M, et al. Acquired tick resistance: the trail is hot. Parasite Immunol. 2021;43(5):e12808. doi:10.1111/pim.12808
140. Karasuyama H, Miyake K, Yoshikawa S. Immunobiology of acquired resistance to ticks. Front Immunol. 2020;11(10):601504. doi:10.3389/fimmu.2020.601504
141. Kuravi KV, Sorrells LT, Nellis JR, et al. Allergic response to medical products in patients with alpha-gal syndrome. J Thorac Cardiovasc Surg. 2021;9(3). doi:10.1016/j.jtcvs.2021.03.100
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.