Back to Journals » OncoTargets and Therapy » Volume 11
Curcumin suppresses the progression of laryngeal squamous cell carcinoma through the upregulation of miR-145 and inhibition of the PI3K/Akt/mTOR pathway
Received 7 December 2017
Accepted for publication 25 April 2018
Published 19 June 2018 Volume 2018:11 Pages 3521—3531
DOI https://doi.org/10.2147/OTT.S159236
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Samir Farghaly
Xiaofeng Zhu,1,* Ronghua Zhu2,*
1Department of Otorhinolaryngology, Zhengzhou Central Hospital Affiliated to Zhengzhou University, Zhengzhou 450000, People’s Republic of China; 2Department of Otorhinolaryngology, Jinan Central Hospital Affiliated to Shandong University, Jinan 250013, People’s Republic of China
*These authors contributed equally to this work
Background: Curcumin is a polyphenol extracted from the rhizomes of Curcuma longa with extensive biological and pharmacological effects. The present study aimed to investigate the mechanisms of curcumin in laryngeal squamous cell carcinoma (LSCC).
Methods: Quantitative real-time reverse transcriptase-polymerase chain reaction was performed to detect the expressions of miR-145 in LSCC tissues and cells. The effects of miR-145 and curcumin on cell proliferation, apoptosis, cell cycle, migration and invasion were explored by MTT assay, flow cytometry analysis, Transwell migration and invasion assay, respectively. The effects of miR-145 combined with curcumin on the phosphoinositol 1,3 kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) pathway were detected by Western blot analysis.
Results: miR-145 was significantly downregulated in LSCC tissues and cells. Curcumin administration upregulated miR-145 expression in LSCC cells in a dose-dependent manner. miR-145 overexpression and curcumin treatment both markedly suppressed cell proliferation, migration and invasion and induced cell cycle arrest and apoptosis in LSCC cells. Moreover, curcumin treatment reversed the enhanced effects on cell viability, migration and invasion and the inhibitory effects on apoptosis conferred by anti-miR-145 in LSCC cells. Curcumin treatment dramatically aggravated miR-145-induced inhibition of the PI3K/Akt/mTOR pathway and reversed anti-miR-145-mediated activation of the PI3K/Akt/mTOR pathway in LSCC cells.
Conclusion: Curcumin suppressed LSCC progression through the upregulation of miR-145 and inhibition of the PI3K/Akt/mTOR pathway.
Keywords: curcumin, miR-145, the PI3K/Akt/mTOR pathway, LSCC
Introduction
Laryngeal carcinoma (LC) is one of the most prevalent malignant tumors of the head and neck squamous cell carcinoma, and more than 90% of cases are pathologically diagnosed as laryngeal squamous cell carcinoma (LSCC).1,2 In 2016, there were an estimated 13,430 new diagnosed LSCC cases and about 3,620 LSCC-related deaths in the USA.3 Despite considerable progress in diagnostic and therapeutic modalities, including surgical resection, radiotherapy and chemotherapy, the clinical outcome of LSCC has not improved greatly due to tumor recurrence and distant metastasis, with the 5-year survival rates of 39%–44% in patients with advanced LC.4–6 Therefore, it is imperative to completely understand the molecular mechanisms that underlie the initiation and progression of LSCC.
MicroRNAs (miRNAs) have been considered as potential therapeutic targets for human diseases, including cancer, and have become a novel type of biomarker for cancer diagnosis and treatment.7 miRNAs are small, single-stranded, non-coding RNAs with 19–22 nucleotides in length that regulate gene expression at the post-transcriptional level through base pairing with the 3′ untranslated region of their target mRNAs.8 It is widely acknowledged that miRNAs serve significant roles in diverse cellular processes, including cell proliferation, development, cell cycle regulation, apoptosis, cell migration and invasion.9 Increasing studies have found that numerous miRNAs are abnormally expressed in various types of cancers, including LSCC.10,11 These miRNAs have been identified either as oncogenes or as tumor suppressors, which is dependent on the role of their targets.12 Among these miRNAs, miR-145, located in chromosome 5q32-33, is reported to be downregulated in multiple cancers, including LSCC, and exhibits anti-tumorigenic activity.13,14
Curcumin is a polyphenol extracted from the rhizomes of Curcuma longa with extensive biological and pharmacological effects, and has been widely used in People’s Republic of China for medicinal purposes for thousands of years.15 Mounting evidence has addressed the multiple anticancer properties of curcumin, including inhibition of proliferation, invasion, metastasis, angiogenesis and apoptosis induction, suggesting that curcumin has a strong therapeutic potential in multiple cancers through regulating tumor progression.16,17 Curcumin could also regulate several signaling pathways related to cell proliferation, apoptosis and progression, such as the phosphoinositol 1,3 kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) pathway.18–20 Additionally, accumulating evidence has indicated that curcumin plays an important role in cancer progression by altering specific miRNA expressions in a variety of cancers.17,21,22 Notably, curcumin was reported to inhibit cell proliferation and promote apoptosis of LC cells through Bcl-2 and PI3K/Akt, and by upregulating miR-15a.23 miR-145-5p was able to suppress cell proliferation, invasion and migration and induce apoptosis in melanoma cells by inhibiting the MAPK and PI3K/Akt pathways.24 However, whether curcumin could regulate the expression of miR-145 via the PI3K/Akt/mTOR pathway in LSCC remains largely unknown.
In the present study, the anticancer effect of curcumin on the development of LSCC was determined.
Materials and methods
Tissue samples
This study was approved by the Ethics Committee of Zhengzhou Central Hospital Affiliated to Zhengzhou University, and written informed consent was obtained from all the participants prior to tissue collection. LSCC tissue samples and adjacent normal tissue samples were obtained from 32 patients who underwent total or partial laryngectomy at the Department of Otorhinolaryngology, Zhengzhou Central Hospital Affiliated to Zhengzhou University between February 2015 and November 2016. LSCC patients were diagnosed according to the latest World Health Organization (WHO) criteria and TNM stage classification (UICC 2002). None of the enrolled patients received any cancer therapy including chemotherapy or radiotherapy before the operation. All tissues were immediately frozen in liquid nitrogen within 5 min of excision and then stored at −80°C until processed.
Cell lines and treatment
LSCC cell lines TU-177, TU212, AMC-HN-8 and TU686 and normal human oral keratinocytes (NHOKs) were used in this study. TU-177, TU212, AMC-HN-8 and TU686 cells were purchased from American Type Culture Collection (Manassas, VA, USA). NHOKs cells were purchased from ScienCell Research Laboratories (Carlsbad, CA, USA). Cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS; HyClone, Logan, UT, USA), 100 units/mL penicillin (Sigma-Aldrich Co., St Louis, MO, USA) and 100 μg/mL streptomycin (Sigma-Aldrich Co.) in a humidified incubator with 5% CO2 at 37°C. Curcumin (CAS number 458-37-7, 99.5% purity; Sigma-Aldrich Co.) was dissolved in dimethyl sulfoxide (DMSO) to make a stock concentration of 30 mM and stored at −20°C. For curcumin treatment, TU212 and AMC-HN-8 cells were cultured in 96-well plates and incubated with curcumin at a final concentration of 5, 10 or 20 μM for 48 h. Cells treated with DMSO alone were used as controls.
Cell transfection
TU212 and AMC-HN-8 cells were seeded into 6-well plates and cultured for overnight. Then, TU212 and AMC-HN-8 cells grown at 80% confluence were transfected with 30 nM miR-145 mimics (miR-145), miRNA scrambled control (miR-con), miR-145 inhibitor (anti-miR-145) or inhibitor control (anti-miR-con) using Lipofectamine 2000 reagent (Thermo Fisher Scientific, Waltham, MA, USA). Cells were harvested at 48 h posttransfection for further analysis.
Quantitative real-time reverse transcriptase-polymerase chain reaction (qRT-PCR)
Total RNA was extracted from cultured cells through TRIzol® reagent (Sangon Biotech, Shanghai, People’s Republic of China). For miR-145 expression detection, cDNA synthesis was performed from 50 ng total RNA using the TaqMan miRNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA). The expression level of miR-145 was quantified using TaqMan miRNA assays (Applied Biosystems) on a StepOnePlus™ real-time PCR instruments (Applied Biosystems). U6 small nuclear RNA was used as an internal control. For the detection of miRNA expression, cDNA was synthesized using PrimeScript RT reagent Kit (Thermo Fisher Scientific, Waltham, MA, USA) and subsequently amplified by PCR using a SYBR Premix Ex Taq II (Takara Biotechnology, Dalian, People’s Republic of China) on a StepOnePlus™ real-time PCR instrument (Applied Biosystems). PCR reaction conditions were performed as follows: 95°C for 2 min, followed by 40 cycles of 95°C for 15 s, 60°C 30 s and 72°C for 30 s. The expression levels of miRNA were calculated by the 2−ΔΔCt method.
Cell proliferation assay
MTT assay was performed to evaluate cell growth. TU212 and AMC-HN-8 cells were seeded into 96-well plates and incubated for overnight. Subsequently, cells were transfected with miR-145 or miR-NC and incubated for 24 h, 48 h and 72 h. Then, 20 μL MTT (5 mg/mL, Sigma-Aldrich Co.) was added and incubated for 4 h at 37°C. The medium was removed and 150 μL DMSO was added. Following shaking at room temperature for 15 min, the optical density was measured at 490 nm using a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Additionally, cell viability was also determined after TU212 and AMC-HN-8 cells were treated with different concentrations of curcumin (0, 5, 10 or 20 μM) for 48 h or treated with anti-miR-145, anti-miR-NC and anti-miR-145 + 20 μM curcumin for 48 h. Cell viability was also assessed after TU212 and AMC-HN-8 cells were transfected with anti-miR-145 or anti-miR-con, followed by treatment with various concentrations of curcumin (0, 20, 40, 60 or 80 μM) for 48 h.
Cell cycle analysis
TU212 and AMC-HN-8 cells were collected after treatment with different concentrations of curcumin (0, 5, 10 or 20 μM) for 48 h and fixed with 70% ethanol on ice for 2 h. After washing with ice-cold PBS twice, cells were stained with 0.05 mg/mL propidium iodide (PI; Sigma-Aldrich Co.) and 0.1 mg/mL RNAse (Sigma-Aldrich Co.) for 30 min, and cell cycle distribution was analyzed using a FACSVerse flow cytometer (BD Biosciences, San Jose, CA, USA).
Western blot
TU212 and AMC-HN-8 cells were collected after treatment with anti-miR-145, anti-miR-NC and anti-miR-145 + 20 μM curcumin for 48 h and lysed using a cell lysis buffer (Beyotime, Shanghai, People’s Republic of China) containing protease inhibitor phenylmethylsulfonyl fluoride (PMSF) (Beyotime). Cell lysates containing equal amounts of protein were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA). After being blocked overnight in 5% skimmed milk in Tris-buffered saline with Tween®-20, the membranes were incubated overnight at 4°C with specific antibodies against PI3K, Akt, p-Akt, mTOR and p-mTOR (all from Cell Signaling Technology, Beverly, MA, USA). The membranes were washed three times and then incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibody (Abcam, Cambridge, UK) for 1 h at room temperature. The protein bands were detected by the SuperSignal West Pico Chemiluminescent Substrate kit (Pierce Biotechnology, Inc., Rockford, IL, USA).
Cell migration and invasion assay
Cell migration and invasion assays were examined using Transwell chamber inserts (Millipore) with or without coated Matrigel (BD Biosciences). TU212 and AMC-HN-8 cells were treated with different concentrations of curcumin (0, 5, 10 or 20 μM) for 48 h or treated with miR-145, miR-NC, anti-miR-145, anti-miR-NC and anti-miR-145 + 20 μM curcumin for 48 h. The treated TU212 and AMC-HN-8 cells (1 × 105/mL) in serum-free medium were seeded into upper chambers of the inserts (Millipore), while 600 μL complete medium containing 10% FBS was added to the lower chambers as a chemoattractant. After incubation for 24 h, non-invading cells were wiped off by cotton swabs and cells on the underside of the inserts were fixed in methanol and stained with 0.1% crystal violet. Cells that invaded to the lower surface were imaged and counted in five randomly selected visual fields using a Nikon TE2000 microscope (Nikon, Tokyo, Japan) with 100× magnification.
Apoptosis analysis by flow cytometry
Cell apoptosis was examined using the Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit (BD Pharmingen, San Diego, CA, USA). To measure cell apoptosis, the treated cells were collected, washed with cold PBS twice and re-suspended in 1× binding buffer solution. Then, cells were double stained with Annexin V-FITC and PI for 15 min in the dark. Analyses of cell cycle and cellular apoptosis were performed using a FACS Calibur Flow Cytometer (Beckman Coulter, Atlanta, GA, USA).
Statistical analysis
All experimental results were expressed as mean ± standard deviation (SD). GraphPad Prism 5 (GraphPad, San Diego, CA, USA) with two-tailed unpaired Student’s t-test was used for statistical analysis. Differences were considered to indicate a statistically significance when P < 0.05.
Results
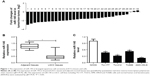
miR-145 was downregulated in LSCC tissues and cells
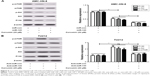
To assess the role of miR-145 in LSCC, the expression of miR-145 in 32 paired LSCC tissues and adjacent normal tissues was initially detected by qRT-PCR. The results showed that miR-145 was markedly downregulated in 32 paired LSCC tissues when compared with adjacent normal tissues (Figure 1A and B). The expression of miR-145 in LSCC cell lines were also determined and miR-145 was aberrantly downregulated in TU-177, TU212, AMC-HN-8 and TU686 cells in comparison with that in NHOKs, especially in TU212 and AMC-HN-8 cells (Figure 1C). Accordingly, TU212 and AMC-HN-8 cells were used for the further studies.
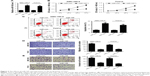
miR-145 overexpression inhibited the progression of LSCC cells
To confirm the functional role of miR-145 in LSCC, gain-of-function analyses were performed in TU212 and AMC-HN-8 cells by transfecting with miR-145 or miR-con. The results of qRT-PCR demonstrated that miR-145-introduced TU212 and AMC-HN-8 cells showed an obvious increase of miR-145 expression compared with that of miR-con-transfected cells (Figure 2A). MTT assay indicated that cell proliferation was dramatically blocked by ectopic expression of miR-145 compared to the corresponding controls (Figure 2B). Moreover, transfection with miR-145 mimics led to a remarkable increase of apoptotic rates in TU212 and AMC-HN-8 cells when compared with miR-con group (Figure 2C), as demonstrated by flow cytometry analysis. Furthermore, Transwell migration and invasion assays suggested that miR-145 overexpression greatly hindered the migration (Figure 2D) and invasion (Figure 2E) ability of TU212 and AMC-HN-8 cells relative to miR-con-transfected cells. Collectively, these results indicated that miR-145 plays a critical role in the development of LSCC.
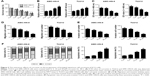
Curcumin suppressed the progression of LSCC cells
To assess the anticancer effects of curcumin on the development of LSCC cells, TU212 and AMC-HN-8 cells were treated with 5, 10 and 20 μM curcumin for 48 h. MTT assay demonstrated that IC50 value of curcumin in TU212 and AMC-HN-8 cells was about 20 μM (Figure 3A). qRT-PCR results showed that miR-145 expression was significantly elevated by 10 and 20 μM curcumin in TU212 and AMC-HN-8 cells compared with that in untreated control cells (Figure 3B). MTT assay showed that cell viability was effectively decreased in TU212 and AMC-HN-8 cells exposed to 10 or 20 μM curcumin in a dose-dependent manner (Figure 3C). Moreover, Transwell migration and invasion assays showed that curcumin administration exerted the similar dose-dependent inhibitory effects on the migration and invasion ability of TU212 and AMC-HN-8 cells (Figure 3D and E). Additionally, curcumin administration significantly elevated the proportion of cells in the G1 phase and concurrently reduced the proportion of cells in the S phase in a dose-dependent manner in TU212 and AMC-HN-8 cells (Figure 3F). In accordance with this, after TU212 and AMC-HN-8 cells were administrated with 10 or 20 μM curcumin for 48 h, the apoptotic rate was significantly increased compared with that in untreated control cells (Figure 3F). Taken together, it is concluded that curcumin exerted the anticancer effects on LSCC cells.
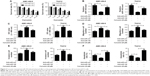
Curcumin reversed anti-miR-145-induced enhanced effect on the progression of LSCC cells
MTT assay showed that cell viability of anti-miR-145-transfected TU212 and AMC-HN-8 cells was dose dependently diminished with the increase of curcumin concentration (Figure 4A). IC50 value of curcumin in the anti-miR-145 group was ~20 μM, which was thereby selected for further experiments. To explore whether curcumin could alter the expression and functions of miR-145 in LSCC cells, TU212 and AMC-HN-8 cells were transfected with anti-miR-145 or anti-miR-con, followed by 20 μM curcumin treatment. Transfection of anti-miR-145 led to a marked reduction of miR-145 expression in TU212 and AMC-HN-8 cells, while curcumin treatment strikingly alleviated anti-miR-145-mediated decrease of miR-145 expression (Figure 4B), as illustrated by qRT-PCR. The results of MTT assay proved that cell viability was dramatically increased by introduction of anti-miR-145 in TU212 and AMC-HN-8 cells, which was apparently attenuated by curcumin treatment (Figure 4C). As compared to anti-miR-con group, anti-miR-145-introduced TU212 and AMC-HN-8 cells showed remarkably improved migration and invasion abilities, while co-treatment with anti-miR-145 and curcumin significantly attenuated these effects (Figure 4D and E). Furthermore, flow cytometry analysis demonstrated that miR-145 inhibition conspicuously retarded apoptosis of TU212 and AMC-HN-8 cells compared with control group, while curcumin treatment remarkably recuperated the inhibitory effect of miR-145 inhibition on apoptosis of TU212 and AMC-HN-8 cells (Figure 4F). Therefore, it is concluded that curcumin reversed anti-miR-145-induced enhanced effect on the progression of LSCC cells.
Curcumin exacerbated miR-145-induced inhibition of the PI3K/Akt/mTOR pathway in LSCC cells
To address whether curcumin could regulate the expression of miR-145 via the PI3K/Akt/mTOR pathway in LSCC cells, qRT-PCR and Western blot analysis were conducted to detect the protein levels of PI3K, p-Akt and p-mTOR in TU212 and AMC-HN-8 cells after treatment with miR-145, miR-con, miR-145 + 20 μM curcumin, anti-miR-145, anti-miR-con or anti-miR-145 + 20 μM curcumin. As shown in Figure 5A and B, the protein levels of PI3K, p-Akt and p-mTOR were significantly reduced in miR-145-transfected TU212 and AMC-HN-8 cells but markedly enhanced in anti-miR-145-introduced TU212 and AMC-HN-8 cells. Nevertheless, curcumin treatment dramatically aggravated miR-145-induced inhibition of the PI3K/Akt/mTOR pathway and reversed anti-miR-145-mediated activation of the PI3K/Akt/mTOR pathway in TU212 and AMC-HN-8 cells.
Discussion
LSCC still remains a seriothreat to the life and safety of LSCC patients and thus there is an urgent need to identify effective and safe therapeutic approaches for LSCC.25 In the present study, it is found that curcumin could significantly inhibit cell proliferation, migration and invasion and promote apoptosis of LSCC cells by upregulation of miR-145 and inhibition of the PI3K/Akt/mTOR pathway.
As a natural active ingredient, extensive biological properties have been identified for curcumin, such as anti-inflammatory, anti-oxidative, anti-infection, anti-liver fibrosis and atherosclerosis.26–28 More importantly, curcumin has been found to possess low toxicity but a remarkable anticancer effect on multiple malignancies, such as breast cancer,29 pancreatic cancer,30 glioma cancer31 and gastric cancer,32 which is in accordance with the results of our study. In the present study, it is demonstrated that curcumin treatment significantly inhibited the progression of LSCC cells by suppressing cell proliferation, migration and invasion and inducing cell cycle arrest and apoptosis, implicating curcumin as a clinically crucial drug for the treatment of LSCC. Consistently, it was previously demonstrated that curcumin could inhibit cell proliferation and promote apoptosis of LC cells. Moreover, a recent study also reported that curcumin could inhibit invasion and vasculogenic mimicry of LSCC in vitro through the inhibition of JAK-2/STAT-3 signaling pathway.33
Recently, emerging evidence suggests that deregulated miRNAs are frequently demonstrated as biomarkers or therapeutic targets in LSCC, and play a significant role in the pathogenesis of LSCC.34 miR-145 is well known to be one of the well-characterized tumor suppressor miRNAs whose expression is frequently decreased in distinct types of tumors, such as colorectal cancer35 breast cancer,36 ovarian cancer37 and prostate cancer.38 It has been also confirmed that miR-145 was downregulated and functioned as a tumor suppressor in LSCC.14 In line with the previous study, it is verified that miR-145 expression was lower in LSCC tissues and cells, and forced expression of miR-145 inhibited cell proliferation, migration and invasion and promoted apoptosis of LSCC cells, while anti-miR-145 exerted the opposite effects on LSCC cells, suggesting the tumor suppressive role of miR-145 in LSCC cells. Additionally, this present study also proved that curcumin reversed anti-miR-145-mediated enhanced effect on the progression of LSCC cells, suggesting that curcumin suppressed the progression of LSCC through upregulation of miR-145, which was similar with the previous study. For example, dendrosomal curcumin was reported to effectively suppress glioblastoma cell proliferation via activation of miR-145.39 Curcumin was shown to suppress proliferation, in vitro invasion and tumorigenicity of prostate cancer stem cells through ceRNA effect of miR-145 and lncRNA-ROR.40 However, the probable mechanisms on how curcumin regulate the expression and function of miR-145 are unknown.
The PI3K/AKT/mTOR signaling pathway, a major intracellular signaling pathway, has been extensively studied due to its critical role in cancer progression; it plays indispensable roles in multiple cellular processes, including metabolism, cell growth, survival, migration and differentiation.41 Overactivation of the PI3K/AKT/mTOR signaling pathway is frequently observed in various types of tumors including LSCC, which are linked to the development and progression of many tumors.42,43 Therefore, the PI3K/Akt/mTOR signaling pathway is recognized as the primary pathway for the survival of cancer cells and might be a useful clinical target for the treatment of cancer.44,45 It has been shown that curcumin could suppress the activation of the PI3K/Akt/mTOR signaling pathway in several malignancies, such as gastric cancer46 and renal cancer.19 Additionally, curcumin was reported to inhibit cell proliferation and induce apoptosis of human non-small cell lung cancer cells through the upregulation of miR-192-5p and suppression of the PI3K/Akt signaling pathway.47 Furthermore, curcumin was found to inhibit cell proliferation and promote apoptosis of LC cells through inhibiting Bcl-2 expression and the PI3K/Akt pathway, and by upregulating miR-15a.23 Similarly, the present study showed that curcumin treatment dramatically aggravated miR-145-induced inhibition of the PI3K/Akt/mTOR pathway, and reversed anti-miR-145-mediated activation of the PI3K/Akt/mTOR pathway in LSCC cells. Collectively, it is concluded that curcumin suppressed the progression of LSCC through upregulation of miR-145 and inhibition of the PI3K/Akt/mTOR pathway.
Conclusion
The present study focused the effect of curcumin against LSCC cells and demonstrated that curcumin suppressed the progression of LSCC through upregulation of miR-145 and inhibition of the PI3K/Akt/mTOR pathway, providing new insights into the effects and molecular mechanism of curcumin on LSCC. Further in vivo studies are needed to assess the anticancer effects of curcumin on LSCC to confirm the results.
Disclosure
The authors report no conflicts of interest in this work.
References
Ferlito A, Haigentz M Jr, Bradley PJ, et al. Causes of death of patients with laryngeal cancer. Eur Arch Otorhinolaryngol. 2014;271(3):425–434. | ||
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. | ||
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. | ||
Wang W, Lin P, Han C, Cai W, Zhao X, Sun B. Vasculogenic mimicry contributes to lymph node metastasis of laryngeal squamous cell carcinoma. J Exp Clin Cancer Res. 2010;29:60. | ||
Connor KL, Pattle S, Kerr GR, Junor E. Treatment, comorbidity and survival in stage III laryngeal cancer. Head Neck. 2015;37(5):698–706. | ||
Zhao XD, Zhang W, Liang HJ, Ji WY. Overexpression of miR-155 promotes proliferation and invasion of human laryngeal squamous cell carcinoma via targeting SOCS1 and STAT3. PLoS One. 2013;8(2):e56395. | ||
Li J, Tan S, Kooger R, Zhang C, Zhang Y. MicroRNAs as novel biological targets for detection and regulation. Chem Soc Rev. 2014;43(2):506–517. | ||
Ruvkun G. Clarifications on miRNA and cancer. Science. 2006;311(5757):36–37. | ||
Sassen S, Miska EA, Caldas C. MicroRNA: implications for cancer. Virchows Arch. 2008;452(1):1–10. | ||
Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6(11):857–866. | ||
Tian L, Li M, Ge J, et al. MiR-203 is downregulated in laryngeal squamous cell carcinoma and can suppress proliferation and induce apoptosis of tumours. Tumour Biol. 2014;35(6):5953–5963. | ||
Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302(1):1–12. | ||
Cao P, Zhou L, Zhang J, et al. Comprehensive expression profiling of microRNAs in laryngeal squamous cell carcinoma. Head Neck. 2013;35(5):720–728. | ||
Karatas OF, Yuceturk B, Suer I, et al. Role of miR-145 in human laryngeal squamous cell carcinoma. Head Neck. 2016;38(2):260–266. | ||
Devassy JG, Nwachukwu ID, Jones PJ. Curcumin and cancer: barriers to obtaining a health claim. Nutr Rev. 2015;73(3):155–165. | ||
Liao S, Xia J, Chen Z, et al. Inhibitory effect of curcumin on oral carcinoma CAL-27 cells via suppression of Notch-1 and NF-κB signaling pathways. J Cell Biochem. 2011;112(4):1055–1065. | ||
Zhou S, Zhang S, Shen H, et al. Curcumin inhibits cancer progression through regulating expression of microRNAs. Tumour Biol. 2017;39(2):1010428317691680. | ||
Tian B, Zhao Y, Liang T, et al. Curcumin inhibits urothelial tumor development by suppressing IGF2 and IGF2-mediated PI3K/AKT/mTOR signaling pathway. J Drug Target. 2017;25(7):626–636. | ||
Zhang H, Xu W, Li B, et al. Curcumin promotes cell cycle arrest and inhibits survival of human renal cancer cells by negative modulation of the PI3K/AKT signaling pathway. Cell Biochem Biophys. 2015;73(3):681–686. | ||
Wang C, Zhang X, Teng Z, Zhang T, Li Y. Downregulation of PI3K/Akt/mTOR signaling pathway in curcumin-induced autophagy in APP/PS1 double transgenic mice. Eur J Pharmacol. 2014;740:312–320. | ||
Mudduluru G, George-William JN, Muppala S, et al. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci Rep. 2011;31(3):185–197. | ||
Saini S, Arora S, Majid S, et al. Curcumin modulates microRNA-203-mediated regulation of the Src-Akt axis in bladder cancer. Cancer Prev Res (Phila). 2011;4(10):1698–1709. | ||
Mou S, Zhou Z, He Y, Liu F, Gong L. Curcumin inhibits cell proliferation and promotes apoptosis of laryngeal cancer cells through Bcl-2 and PI3K/Akt, and by upregulating miR-15a. Oncol Lett. 2017;14(4):4937–4942. | ||
Liu S, Gao G, Yan D, et al. Effects of miR-145-5p through NRAS on the cell proliferation, apoptosis, migration, and invasion in melanoma by inhibiting MAPK and PI3K/AKT pathways. Cancer Med. 2017;6(4):819–833. | ||
Hamilton DW, de Salis I, Donovan JL, Birchall M. The recruitment of patients to trials in head and neck cancer: a qualitative study of the EaStER trial of treatments for early laryngeal cancer. Eur Arch Otorhinolaryngol. 2013;270(8):2333–2337. | ||
Sreedhar R, Arumugam S, Thandavarayan RA, Karuppagounder V, Watanabe K. Curcumin as a therapeutic agent in the chemoprevention of inflammatory bowel disease. Drug Discov Today. 2016;21(5):843–849. | ||
Ali MS, Pandit V, Jain M, Dhar KL. Mucoadhesive microparticulate drug delivery system of curcumin against Helicobacter pylori infection: design, development and optimization. J Adv Pharm Technol Res. 2014;5(1):48–56. | ||
Borra SK, Mahendra J, Gurumurthy P, Jayamathi, Iqbal SS, Mahendra L. Effect of curcumin against oxidation of biomolecules by hydroxyl radicals. J Clin Diagn Res. 2014;8(10):CC01–CC05. | ||
Chen B, Zhang Y, Wang Y, Rao J, Jiang X, Xu Z. Curcumin inhibits proliferation of breast cancer cells through Nrf2-mediated down-regulation of Fen1 expression. J Steroid Biochem Mol Biol. 2014;143:11–18. | ||
Zhu Y, Bu S. Curcumin induces autophagy, apoptosis, and cell cycle arrest in human pancreatic cancer cells. Evid Based Complement Alternat Med. 2017;2017:5787218. | ||
Wang X, Deng J, Yuan J, et al. Curcumin exerts its tumor suppressive function via inhibition of NEDD4 oncoprotein in glioma cancer cells. Int J Oncol. 2017;51(2):467–477. | ||
Fu H, Wang C, Yang D, et al. Curcumin regulates proliferation, autophagy, and apoptosis in gastric cancer cells by affecting PI3K and P53 signaling. J Cell Physiol. 2018;233(6):4634–4642. | ||
Hu A, Huang JJ, Jin XJ, et al. Curcumin suppresses invasiveness and vasculogenic mimicry of squamous cell carcinoma of the larynx through the inhibition of JAK-2/STAT-3 signaling pathway. Am J Cancer Res. 2014;5(1):278–288. eCollection 2015. | ||
Li P, Liu H, Wang Z, et al. MicroRNAs in laryngeal cancer: implications for diagnosis, prognosis and therapy. Am J Transl Res. 2016;8(5):1935–1944. eCollection 2016. | ||
Wang W, Ji G, Xiao X, et al. Epigenetically regulated miR-145 suppresses colon cancer invasion and metastasis by targeting LASP1. Oncotarget. 2016;7(42):68674–68687. | ||
Ding Y, Zhang C, Zhang J, et al. miR-145 inhibits proliferation and migration of breast cancer cells by directly or indirectly regulating TGF-β1 expression. Int J Oncol. 2017;50(5):1701–1710. | ||
Wang L, Wu X, Wang B, Wang Q, Han L. Mechanisms of miR-145 regulating invasion and metastasis of ovarian carcinoma. Am J Transl Res. 2017;9(7):3443–3451. eCollection 2017. | ||
Chen X, Gong J, Zeng H, et al. MicroRNA145 targets BNIP3 and suppresses prostate cancer progression. Cancer Res. 2010;70(7):2728–2738. | ||
Tahmasebi Mirgani M, Isacchi B, Sadeghizadeh M, et al. Dendrosomal curcumin nanoformulation downregulates pluripotency genes via miR-145 activation in U87MG glioblastoma cells. Int J Nanomedicine. 2014;9:403–417. | ||
Liu T, Chi H, Chen J, et al. Curcumin suppresses proliferation and in vitro invasion of human prostate cancer stem cells by ceRNA effect of miR-145 and lncRNA-ROR. Gene. 2017;631:29–38. | ||
Khan KH, Yap TA, Yan L, Cunningham D. Targeting the PI3K-AKT-mTOR signaling network in cancer. Chin J Cancer. 2013;32(5):253–265. | ||
Bitting RL, Armstrong AJ. Targeting the PI3K/Akt/mTOR pathway in castration-resistant prostate cancer. Endocr Relat Cancer. 2013;20(3):R83–R99. | ||
Wang B, Qin H, Wang Y, et al. Effect of DJ-1 overexpression on the proliferation, apoptosis, invasion and migration of laryngeal squamous cell carcinoma SNU-46 cells through PI3K/AKT/mTOR. Oncol Rep. 2014;32(3):1108–1116. | ||
Martelli AM, Evangelisti C, Chiarini F, McCubrey JA. The phosphatidylinositol 3-kinase/Akt/mTOR signaling network as a therapeutic target in acute myelogenous leukemia patients. Oncotarget. 2010;1(2):89–103. | ||
Simpson DR, Mell LK, Cohen EE. Targeting the PI3K/AKT/mTOR pathway in squamous cell carcinoma of the head and neck. Oral Oncol. 2015;51(4):291–298. | ||
Li W, Zhou Y, Yang J, Li H, Zhang H, Zheng P. Curcumin induces apoptotic cell death and protective autophagy in human gastric cancer cells. Oncol Rep. 2017;37(6):3459–3466. | ||
Jin H, Qiao F, Wang Y, Xu Y, Shang Y. Curcumin inhibits cell proliferation and induces apoptosis of human non-small cell lung cancer cells through the upregulation of miR-192-5p and suppression of PI3K/Akt signaling pathway. Oncol Rep. 2015;34(5):2782–2789. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.