Back to Journals » Cancer Management and Research » Volume 12
Curcumin Inhibits the Tumorigenesis of Breast Cancer by Blocking Tafazzin/Yes-Associated Protein Axis
Authors Shen Y, Han Z, Liu S, Jiao Y, Li Y, Yuan H
Received 20 January 2020
Accepted for publication 14 February 2020
Published 27 February 2020 Volume 2020:12 Pages 1493—1502
DOI https://doi.org/10.2147/CMAR.S246691
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bilikere Dwarakanath
This paper has been retracted
Yuxiu Shen, Zaigang Han, Shuang Liu, Yang Jiao, Ying Li, Hongyan Yuan
Department of Pharmacology, Affiliated Hospital of Beihua University, Jilin City, Jilin Province 132000, People’s Republic of China
Correspondence: Yuxiu Shen
Department of Pharmacology, Affiliated Hospital of Beihua University, No. 12, Jiefang Middle Road, Jilin City, Jilin Province 132000, Tel +86- 13384401555
Email [email protected]
Purpose: This study was aimed to explore the anti-tumor effect of curcumin on breast cancer (BC) and the underlying mechanism involving Tafazzin (TAZ)/Yes-associated protein (YAP) axis.
Methods: Different concentrations of curcumin (0, 10, 20 and 30 μM) were used to treat BC cells (MCF-7 and MDA-MB-231 cells). The viability, colony formation, apoptosis, migration, and invasion of BC cells were detected by MTT, colony formation, flow cytometry, wound-healing and transwell assay, respectively. The protein expression of TAZ and YAP (effectors of Hippo signaling pathway) was detected by Western blot. MDA-MB-231 cells were injected into mice to verify the anti-tumor effect of curcumin in vivo.
Results: Curcumin (20 and 30 μM) inhibited the proliferation, migration and invasion, and promoted the apoptosis of MCF-7 and MDA-MB-231 cells. Curcumin decreased the protein expression of TAZ and YAP in MCF-7 and MDA-MB-231 cells. Overexpression of YAP reversed the anti-tumor effect of curcumin on MDA-MB-231 cells. In addition, curcumin (100, 200 and 300 mg/kg/d) inhibited the growth of tumor xenografts in mice, and down-regulated the protein expression of TAZ and YAP in tumor xenografts. However, curcumin at a concentration of 300 mg/kg/d slowed the increasing of body weight in mice.
Conclusion: Curcumin inhibited the tumorigenesis of BC by blocking TAZ/YAP axis.
Keywords: curcumin, breast cancer, Hippo signaling pathway, proliferation, metastasis
Introduction
Breast cancer (BC) is a common cancer in women with high morbidity and mortality in the world.1,2 It has been reported that the mortality of BC increases by 1.7% annually in Asian.3 Currently, BC is mainly treated by surgery, chemotherapy, and radiotherapy, but these therapies can only alleviate and delay this disease.4 There is a high risk of recurrence in the later period of BC, leading to poor outcomes.5 Therefore, exploring novel therapeutic strategies for BC is crucial.
Curcumin is an active phenolic pigment that is isolated from turmeric (Curcuma longa).6,7 Curcumin has diverse properties on tumor cells, including anti-proliferation, anti-inflammatory, and anti-oxidant.8–11 Previous studies have shown that curcumin can effectively suppress the invasion and proliferation of human cancers, such as wilms’ tumor (WT),12 BC,13–15 esophageal cancers,16 and pancreatic cancer.17 Jia et al have found that the proliferation, invasion and migration of WT cells are restrained by the treatment of curcumin.12 Dharmalingam et al have shown that curcumin treatment inhibits the proliferation and colony formation of esophageal cancer cells in a dose and time-dependent manner.16 Choudhuri et al have proved that curcumin induces the apoptosis of BC cells.13 In addition, Bang and Kim have confirmed that curcumin significantly inhibits the motility and invasion of BC cells.15 Although the anti-tumor role of curcumin has been identified by a large number of researches, the therapeutic mechanism of curcumin on BC is still not entirely understood.
Hippo signaling pathway is a conservative signal transduction pathway that is involved in the regulation of cell proliferation, apoptosis, and differentiation.18 Tafazzin (TAZ) and Yes-associated protein (YAP) are key effectors of Hippo signaling pathway that are involved in the homeostasis of the internal environment.19 Notably, TAZ/YAP axis plays an important regulatory role in BC cells. For example, overexpression of TAZ and YAP induces the epithelial mesenchymal transformation (EMT), inhibits the apoptosis, and promotes the proliferation of BC cells.20 The hyperactivation of TAZ/YAP leads to a variety of tumor-promoting functions in BC, such as EMT, cancer stem cell generation and therapeutic resistance.21 MiR-591 mimics inhibits the proliferation and invasion of BC cells through down-regulating YAP1 expression.22 In addition, a curcumin derivative CL-6 induces the apoptosis of gastric cancer cells, and inhibits both the protein and mRNA expression of YAP/YAP.23 However, whether the anti-tumor role of curcumin on BC is associated with TAZ/YAP axis is still unclear.
In this study, we explored the effects of curcumin on the proliferation, migration, invasion and apoptosis of BC cells, and on the growth of tumor xenografts in mice. The therapeutic mechanism of curcumin in BC involving TAZ/YAP axis was evaluated for the first time. Our findings reveal a novel therapeutic mechanism of curcumin on BC, which lay a theoretical foundation for clinical treatment of BC.
Materials and Methods
Cell Culture
BC cells (MCF-7 and MDA-MB-231) were purchased from Guyan Biotech Co., Ltd. (Shanghai, China). Cells were cultured in DMEM medium containing 10% fetal bovine serum and 1% penicillin-streptomycin (Procell life science & technology Co., Ltd., Wuhan, China), and maintained at a 37°C incubator with 5% CO2.
Cell Transfection and Treatment
Curcumin (Sigma-Aldrich, St Louis, USA) was dissolved in dimethyl sulfoxide (DMSO), and then diluted in DMEM at different concentrations (0, 10, 20 and 30 μM). BC cells were seeded in 12-well plates at a density of 1 × 105/well, and incubated with different concentrations of curcumin for 48 h (Curcumin group). YAP overexpression lentivirus vector (Len-YAP-OE) was constructed by OBiO Corp., Ltd. (Shanghai, China). MDA-MB-231 cells were transfected with Len-YAP-OE (Len-YAP-OE group) and empty vector (Mock group) using Lipofectamine 3000 (Thermo Fisher Scientific, Waltham, MA, USA). In addition, some Len-YAP-OE-transfected MDA-MB-231 cells were incubated with 30 μM curcumin for 48 h (Curcumin + Len-YAP-OE group). Cells without transfection and treatment were considered as the control (Control group).
Quantitative Real-Time PCR (qRT-PCR)
Total RNA was extracted from cells using TRIZOL regent (Thermo Fisher Scientific), and then reverse-transcribed into cDNA using cDNA Reverse Transcription Kit (Thermo Fisher Scientific). qRT-PCR was performed using the SYBR Green PCR kit (TaKaRa, China). The PCR conditions were as follows: 96°C for 5 min, 30 cycles of 96°C for 5 min, 54°C for 30 s, and 72°C for 50 s. Relative mRNA expression level was calculated by the 2−ΔΔCt method. GAPDH was used for normalization. The primer sequences (Shanghai Jierui Bioengineering, China) were shown in Table 1.
 |
Table 1 Primer Sequences Used in qRT-PCR |
Western Blot
Total proteins were isolated from cells and tissues using RIPA lysis buffer (Santa Cruz, Dallas, USA). The protein samples were run on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and transferred onto polyvinylidene fluoride (PVDF) membrane (Thermo Fisher Scientific). After blocking with 5% skim milk in TBST, the PVDF membrane was incubated with primary antibody (anti-YAP, 1:1000, 14,074; anti-TAZ, 1:1000, 83,669, Cell Signaling Technology, USA) for 12 h at 4°C. After three times of washing with TBST, the PVDF membrane was incubated with horseradish peroxidase-conjugated secondary antibody (anti-rabbit 1:5000, ZB-2301, Beijing Zhongshan Jinqiao Biotechnology, China) for 1 h at 25°C. The protein bands were visualized using an ECL system (Thermo Fisher Scientific) and analyzed by Image LabTM Software (Bio-Rad, USA). GAPDH was used as an internal reference.
MTT Assay
Cells were seeded into 96-well plates at a density of 2 × 104/well. After cultured for 24 and 48 h, cells were incubated with MTT solution (5 mg/mL, 20 μL, Gefan biotechnology Co., Ltd., Shanghai, China) for 4 h. The medium was then discarded and DMSO was added to dissolve the crystals. The absorbance at OD450 was measured using a Microplate reader (SpectraMax M5, Molecular Devices, CA, USA).
Colony Formation Assay
Cells were seeded into six-well plates at a density of 3 × 105/well, and cultured for 2 weeks. After washed twice with PBS, the cells were fixed with 4% formaldehyde (Bio-protocol, Beijing, China) for 15 min and stained with crystal violet (Sangon Biotech Co., Ltd., Shanghai, China) for 15 min. The colony numbers were counted under a microscope (Olympus, Tokyo, Japan), and the colony formation rate was calculated.
Wound Healing Assay
Cells were seeded into six-well plates at a density of 5 × 105/well, and cultured for 24 h. Scratch was then made using a pipette tip. After 48 h of culturing, the wounded area was observed under an optical microscope (Olympus), and the wound healing rate was calculated.
Transwell Assay
Cell invasion was detected using transwell chamber (Corning, NY, USA). Briefly, cells were seeded in the upper chamber pre-coated with matrigel at a density of 1 × 105/chamber. The medium containing 10% FBS was added in the lower chamber. After 24 h of culturing, cells on the upper chamber were removed. Cells in the lower chamber were fixed with 4% paraformaldehyde (Shanghai Maokang Biotechnology Co., Ltd, Shanghai, China) for 15 min, and stained with 0.1% crystal violet (Sangon Biotech Co., Ltd.) for 30 min. Positive stained cells were counted under a microscope (Olympus) at five randomly selected fields, and the invasion rate was calculated.
Flow Cytometry
Cell apoptosis was detected using an Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) kit (Thermo Fisher Scientific). Briefly, cells were seeded into six-well plates at a density of 1 × 105 cells/well. Cells were then incubated with Annexin V-FITC and PI for 15 min under darkness. The cell apoptosis was analyzed by a FACScan flow cytometer (Becton Dickinson Biosciences, San Jose, CA). The apoptosis rate (%) was calculated as the percentage of cells in the LR (early apoptosis cells) and UR (late apoptosis cells) regions in the scatter diagram.
Establishment of Subcutaneous Tumor Xenografts in Mice
Male BALB/c nude mice were obtained from Qianbi Biotechnology Co., Ltd (Shanghai, China). MDA-MB-231 cells were subcutaneous injected into the right flank of mice to establish tumor xenografts. One week later, these mice were intraperitoneally injected with different concentrations of curcumin (0, 100, 200, 300 mg/kg/d) for 3 weeks (n = 6 each group). The tumor volume was measured every week with calipers, and calculated as (a⋅b2)/2, where a is the largest diameter in millimeters and b is the smallest diameter in millimeters. After the last measurement (the 4th week), mice were anesthetized with pentobarbital sodium (60 mg/kg), and killed by cervical dislocation. The tumor xenografts were removed and weighed. All animal experiments were approved by the Animal Care and Use Committee of our hospital, and were performed in accordance with the Guide for the Care and Use of Laboratory Animals (eighth edition, 2011, National Institutes of Health, USA).
Statistical Analysis
All analyses were performed using SPSS 22.0 software (SPSS, Inc., Chicago, IL, USA). Data were presented as mean ± standard deviation (SD). One-way ANOVA followed by the Turkey’s post hoc test was used to analyze the differences among multi-groups. P < 0.05 was considered to be statistically significant.
Results
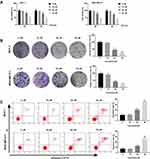
Curcumin Inhibits the Proliferation and Promotes the Apoptosis of BC Cells
The effects of curcumin on the cell viability and colony formation of BC cells were determined by MTT assay and cloning formation assay, respectively. The cell viability and colony formation rate of BC cells were significantly decreased with increasing concentrations of curcumin in a dose-dependent manner (beginning from 20 μM, P < 0.05) (Figure 1A and B). The apoptosis rate of BC cells was detected by flow cytometry. The apoptosis rate of BC cells was increased with increasing concentrations of curcumin in a dose-dependent manner (beginning from 20 μM, P < 0.05) (Figure 1C). Curcumin at a concentration of 10 μM did not influence the cell viability, colony formation and apoptosis of BC cells (Figure 1A–C).
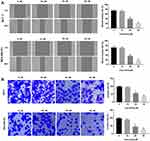
Curcumin Inhibits the Migration and Invasion of BC Cells
The effects of curcumin on the cell migration and invasion of BC cells were determined by wound healing and transwell assay, respectively. The wound healing rate of BC cells was significantly decreased with increasing concentrations of curcumin in a dose-dependent manner (beginning from 20 μM, P < 0.01) (Figure 2A). In consistent with the wound healing rate, the invasion rate was also significantly decreased with increasing concentrations of curcumin in a dose-dependent manner (beginning from 20 μM, P < 0.01) (Figure 2B). Curcumin at a concentration of 10 μM did not influence the migration and invasion of BC cells (Figure 2A and B).
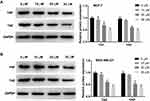
Curcumin Inhibits the Protein Expression of YAP and TAZ in BC Cells
Western blot was used to detect the protein expression of TAZ and YAP in BC cells. The protein expression of TAZ and YAP was significantly decreased with increasing concentrations of curcumin in a dose-dependent manner (beginning from 20 μM, P < 0.05). Curcumin at a concentration of 10 μM did not influence the protein expression of TAZ and YAP in BC cells (Figure 3A and B).
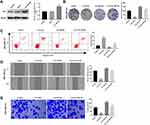
Overexpression of YAP Reverses the Anti-Tumor Effect of Curcumin on BC Cells
In order to investigate the therapeutic mechanism of curcumin involving TAZ/YAP axis, YAP was overexpressed in MDA-MB-231 cells by the transfection of Len-YAP-OE. Western blot showed that the protein expression of YAP in the Len-YAP-OE group was significantly up-regulated compared with the Control group (P < 0.01) (Figure 4A). Overexpression of YAP significantly increased the colony formation rate, invasion rate and wound healing rate, and decreased the apoptosis rate of MDA-MB-231 cells (P < 0.01) (Figure 4B–E). Notably, overexpression of YAP significantly reversed the anti-tumor effect of curcumin on MDA-MB-231 cells (P < 0.01) (Figure 4B–E).
Curcumin Inhibits the Growth of Subcutaneous Tumor Xenografts in Mice
To reveal the anti-tumor effect of curcumin in vivo, MDA-MB-231 cells were injected into mice to establish tumor xenografts. Different concentrations of curcumin (0, 100, 200, 300 mg/kg/d) were injected into mice. After the injection of MDA-MB-231 cells for 4 weeks, the tumor weight was significantly decreased with increasing concentrations of curcumin at a dose-dependent manner (P < 0.05) (Figure 5A). The injection of curcumin also significantly slowed the increasing of tumor volume in a dose-dependent manner (P < 0.05) (Figure 5B). In addition, the injection of curcumin at a concentration of 300 mg/kg/d significantly slowed the increasing of body weight of mice from the 2nd week (P < 0.01). The injection of curcumin at concentrations of 100 and 200 mg/kg/d did not influence the body weight of mice (Figure 5C). The protein expression of TAZ and YAP in tumor xenografts was further detected by Western blot. The protein expression of TAZ and YAP in tumor xenografts was significantly decreased with increasing concentrations of curcumin in a dose-dependent manner (P < 0.05) (Figure 5D).
Discussion
In recent years, traditional Chinese medicines have been widely used in the treatment of BC, such as Astragalus membranaceus,24 Taxus chinensis25 and Angelica sinensis.26 Curcumin is a natural component extracted from turmeric that exhibits obvious anti-tumor activity with less toxicity. Petiti et al have indicated that the curcumin inhibits the growth of HEL cells in a dose and time-dependent manner, reaching the maximum effect at a dose of 20 μM for 48 h, by reducing the proliferation of 93%.27 Ye et al have confirmed that the treatment of CL-6 (2.5, 5.0, and 7.5 μM) promotes the apoptosis of AGS cells.23 In this study, curcumin significantly decreased the cell viability and colony formation rate, and increased the apoptosis rate of BC cells in a dose-dependent manner (beginning from 20 μM). Our findings are consistent with previous studies, and further indicate that curcumin can inhibit the proliferation and induce the apoptosis of BC cells in vitro.
Cancer metastasis involves many cellular biological processes, including cell separation, adhesion, migration and invasion.28 The invasion and migration of tumor cells are the main causes of cancer metastasis.29,30 Ye et al have shown that a curcumin derivative CL-6 inhibits the migration and invasion of gastric cancer cells.23 Chiu et al have proved that curcumin inhibits the migratory activity of MDA-MB-231 cells.31 Sun et al have found that curcumin inhibits the migration and invasion of MCF7 cells.32 In consistent with previous studies, wound-healing and transwell assay in this study showed that the migration and invasion of BC cells were significantly decreased by curcumin in a dose-dependent manner (beginning from 20 μM). Our results indicate that the curcumin can inhibit the migration and invasion of BC cells in vitro.
Curcumin plays an anti-tumor role in cancer cells through regulating diverse pathways, such as JAK/STAT,33 RhoA/ROCK/MMPs,32 nuclear factor-kappaB,34 and SLUG/Hexokinase 2.35 Hippo pathway is involved in organ development and tissue stability via regulating cell differentiation, proliferation and apoptosis.36 Yang et al have found that curcumin down-regulates the expression of the key effectors of Hippo signaling pathway, YAP and TAZ in bladder cancer cells.37 In consistent with the above study, we found that curcumin inhibited the expression of YAP and TAZ in BC cells. These results indicate that curcumin blocks TAZ/YAP axis in BC. Yuan et al have proved that knockdown of YAP promotes the migration and invasion of BC cells.38 Lai et al have found that overexpression of YAP or TAZ induces the EMT and taxol resistance in MCF10A cells.39 In order to further verify the therapeutic mechanism of curcumin involving TAZ/YAP axis, YAP was overexpressed in MDA-MB-231 cells. Our findings showed that overexpression of YAP reversed the anti-tumor effect of curcumin on MDA-MB-231 cells. We speculate that curcumin may inhibit the tumorigenesis of BC through blocking TAZ/YAP axis.
Up to now, many scholars have proved the anti-tumor effect of curcumin in animal models in vivo. Kunnumakkara et al have indicated that curcumin sensitizes the colorectal cancer to radiation in a xenograft mouse model.40 Yang et al have proved that curcumin decreases the tumor volume and weight, and induces the apoptosis of PC-3 cells in mice.41 Curcumin inhibits the growth of gastric cancer xenografts in mice.42 In this study, we found that curcumin significantly decreased the tumor weight and volume in mice. Our findings are consistent with previous studies, and further illustrate that curcumin can inhibit the tumor growth in vivo. Notably, 300 mg/kg/d curcumin decreased the body weight of mice. This result indicates that 300 mg/kg/d curcumin may be toxic to mice. Curcumin should be used at specific doses in clinical practice. In addition, we also found that curcumin decreased the protein expression of TAZ and YAP in tumor xenografts. These results re-confirmed that the anti-tumor effect of curcumin is related to the blocking of TAZ/YAP axis.
Conclusions
In conclusion, curcumin (20 and 30 μM) inhibited the proliferation, migration and invasion, and promoted the apoptosis of BC cells. Curcumin (100, and 200 mg/kg/d) inhibited the growth of tumor xenografts in mice. The anti-tumor effect of curcumin on BC was closely associated with the blocking of TAZ/YAP axis. A certain concentration of curcumin may inhibit the tumorigenesis of BC through blocking TAZ/YAP axis.
Ethics Approval and Consent to Participate
This study was approved by the ethics committee of Affiliated Hospital of Beihua University. All animal experiments and programs were approved by the Animal Care and Use Committee of Affiliated Hospital of Beihua University, and were performed in accordance with the Guide for the Care and Use of Laboratory Animals (eighth edition, 2011, National Institutes of Health, USA).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel R, Desantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2014;64(4):252–271.
2. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi:10.1002/ijc.29210
3. Desantis CE, Ma J, Goding SA, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin. 2017;67(6):439. doi:10.3322/caac.21412
4. Rose MA, Henderson IC, Gelman R, et al. Premenopausal breast cancer patients treated with conservative surgery, radiotherapy and adjuvant chemotherapy have a low risk of local failure. Int J Radiat Oncol Biol Phys. 1989;17(4):711–717. doi:10.1016/0360-3016(89)90056-4
5. Marquette C, Nabell L. Chemotherapy-resistant metastatic breast cancer. Curr Treat Options Oncol. 2012;13(2):263–275. doi:10.1007/s11864-012-0184-6
6. Yegnanarayan R, Saraf A, Balwani J. Comparison of anti-inflammatory activity of various extracts of Curcuma longa (Linn). Indian J Med Res. 1976;64(4):601.
7. Kumar S, Kesharwani SS, Mathur H, Tyagi M, Bhat GJ, Tummala H. Molecular complexation of curcumin with pH sensitive cationic copolymer enhances the aqueous solubility, stability and bioavailability of curcumin. Eur J Pharm Sci. 2016;82:86–96. doi:10.1016/j.ejps.2015.11.010
8. Xu X, Zhu Y. Curcumin inhibits human non-small cell lung cancer xenografts by targeting STAT3 pathway. Am J Transl Res. 2017;9(8):3633.
9. Wang XP, Wang QX, Lin HP, Chang N. Anti-tumor bioactivities of curcumin on mice loaded with gastric carcinoma. Food Funct. 2017;8(9):3319. doi:10.1039/C7FO00555E
10. Li B, Shi C, Zhao JM, Wang L. The effects of Curcumin on HCT-116 cells proliferation and apoptosis via the miR-491/PEG10 pathway. J Cell Biochem. 2018;119(4). doi:10.1002/jcb.27261
11. Kesharwani SS, Ahmad R, Bakkari MA, et al. Site-directed non-covalent polymer-drug complexes for inflammatory bowel disease (IBD): formulation development, characterization and pharmacological evaluation. J Control Release. 2018;290:165–179. doi:10.1016/j.jconrel.2018.08.004
12. Jia W, Deng F, Fu W, et al. Curcumin suppresses wilms’ tumor metastasis by inhibiting RECK methylation. Biomed Pharmacother. 2019;111:1204–1212. doi:10.1016/j.biopha.2018.12.111
13. Choudhuri T, Pal S, Agwarwal ML, Das T, Sa G. Curcumin induces apoptosis in human breast cancer cells through p53‐dependent Bax induction. FEBS Lett. 2002;512(1):334–340. doi:10.1016/S0014-5793(02)02292-5
14. Barsela G, Epelbaum R, Schaffer M. Curcumin as an anti-cancer agent: review of the gap between basic and clinical applications. Curr Med Chem. 2010;17(3):190–197.
15. Bang MH, Kim WK. Effect of curcumin on cancer invasion and matrix metalloproteinase-9 Activity in MDA-MB-231 human breast cancer cell. Korean J Nutr. 2006;39(8):756–761.
16. Dharmalingam S, Sivapriya P, Prabhu R, et al. Curcumin induces cell death in esophageal cancer cells through modulating notch signaling. PLoS One. 2012;7(2):e30590. doi:10.1371/journal.pone.0030590
17. Glienke W, Maute LJ, Bergmann L. Curcumin inhibits constitutive STAT3 phosphorylation in human pancreatic cancer cell lines and downregulation of survivin/BIRC5 gene expression. Cancer Invest. 2010;28(2):166. doi:10.3109/07357900903287006
18. Fa-Xing Y, Kun-Liang G. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27(4):355–371. doi:10.1101/gad.210773.112
19. Hong W, Guan KL. The YAP and TAZ transcription co-activators: key downstream effectors of the mammalian Hippo pathway. Semin Cell Dev Biol. 2012;23(7):785–793. doi:10.1016/j.semcdb.2012.05.004
20. Varelas X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development. 2014;141(8):1614–1626. doi:10.1242/dev.102376
21. Maugeri-Sacca M, Barba M, Pizzuti L, et al. The Hippo transducers TAZ and YAP in breast cancer: oncogenic activities and clinical implications. Expert Rev Mol Med. 2015;17:e14. doi:10.1017/erm.2015.12
22. Huang X, Tang F, Weng Z, Zhou M, Zhang Q. MiR-591 functions as tumor suppressor in breast cancer by targeting TCF4 and inhibits Hippo-YAP/TAZ signaling pathway. Cancer Cell Int. 2019;19(1). doi:10.1186/s12935-019-0818-x
23. Ye C, Wang W, Xia G, et al. a novel curcumin derivative cl-6 exerts antitumor effect in human gastric cancer cells by inducing apoptosis through hippo–YaP signaling pathway. Onco Targets Ther. 2019;12:2259. doi:10.2147/OTT.S196914
24. Zhou R, Chen H, Chen J, Chen X, Wen Y, Xu L. Extract from Astragalus membranaceus inhibit breast cancer cells proliferation via PI3K/AKT/mTOR signaling pathway. BMC Complement Altern Med. 2018;18(1):83. doi:10.1186/s12906-018-2148-2
25. Zhao D, Qin K, Cao CM, Wang W. Effect of the compounds from Taxus chinensis var. mairei and T. cuspidata on the proliferation of human breast cancer cells. Nat Prod Res Dev. 2007;19(4):635–638.
26. Lau CB, Ho TC, Chan TW, Kim SC. Use of dong quai (Angelica sinensis) to treat peri- or postmenopausal symptoms in women with breast cancer: is it appropriate? Menopause. 2005;12(6):734–740. doi:10.1097/01.gme.0000184419.65943.01
27. Petiti J, Rosso V, Lo Iacono M, et al. Curcumin induces apoptosis in JAK2‐mutated cells by the inhibition of JAK2/STAT and mTORC1 pathways. J Cell Mol Med. 2019;23(6):4349–4357. doi:10.1111/jcmm.2019.23.issue-6
28. Keskinov AA, Shurin MR. Myeloid regulatory cells in tumor spreading and metastasis. Immunobiology. 2015;220(2):236–242. doi:10.1016/j.imbio.2014.07.017
29. Liu JJ, Liu JY, Chen J, et al. Scinderin promotes the invasion and metastasis of gastric cancer cells and predicts the outcome of patients. Cancer Lett. 2016;376(1):110–117. doi:10.1016/j.canlet.2016.03.035
30. Zhou S, He Y, Yang S, et al. The regulatory roles of lncRNAs in the process of breast cancer invasion and metastasis. Biosci Rep. 2018;38(5). doi:10.1042/BSR20180772
31. Chiu T, Su C. Curcumin inhibits proliferation and migration by increasing the Bax to Bcl-2 ratio and decreasing NF-kappaBp65 expression in breast cancer MDA-MB-231 cells. Int J Mol Med. 2009;23(4):469.
32. Sun K, Duan X, Cai H, et al. Curcumin inhibits LPA-induced invasion by attenuating RhoA/ROCK/MMPs pathway in MCF7 breast cancer cells. Int J Clin Exp Med. 2016;16(1):37–47.
33. Sun Y, Liu L, Wang Y, et al. Curcumin inhibits the proliferation and invasion of MG-63 cells through inactivation of the p-JAK2/p-STAT3 pathway. Onco Targets Ther. 2019;12:2011. doi:10.2147/OTT.S172909
34. Aggarwal BB, Shishir S, Yasunari T, et al. Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin Cancer Res. 2005;11(20):7490–7498. doi:10.1158/1078-0432.CCR-05-1192
35. Geng C, Li J, Ding F, et al. Curcumin suppresses 4-hydroxytamoxifen resistance in breast cancer cells by targeting SLUG/Hexokinase 2 pathway. Biochem Biophys Res Commun. 2016;473(1):147–153. doi:10.1016/j.bbrc.2016.03.067
36. Jianbin H, Shian W, Jose B, Krista M, Duojia P. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell. 2005;122(3):421–434. doi:10.1016/j.cell.2005.06.007
37. Yang G, Qi S, Shan X, et al. Curcumin promotes KLF5 proteasome degradation through downregulating YAP/TAZ in bladder cancer cells. Int J Mol Sci. 2014;15(9):15173–15187. doi:10.3390/ijms150915173
38. Yuan M, Tomlinson V, Lara R, et al. Yes-associated protein (YAP) functions as a tumor suppressor in breast. Cell Death Differ. 2008;15(11):1752–1759. doi:10.1038/cdd.2008.108
39. Lai D, Ho KC, Hao Y, Yang X. Taxol resistance in breast cancer cells is mediated by the hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Cancer Res. 2011;71(7):2728–2738. doi:10.1158/0008-5472.CAN-10-2711
40. Kunnumakkara AB, Parmeswaran D, Sushovan G, et al. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappaB-regulated gene products. Clin Cancer Res. 2008;14(7):2128. doi:10.1158/1078-0432.CCR-07-4722
41. Yang J, Ning J, Peng L, He D. Effect of curcumin on Bcl-2 and Bax expression in nude mice prostate cancer. Int J Clin Exp Pathol. 2015;8(8):9272.
42. Liu X, Sun K, Song A, Zhang X, He X. Curcumin inhibits proliferation of gastric cancer cells by impairing ATP-sensitive potassium channel opening. World J Surg Oncol. 2014;12:389. doi:10.1186/1477-7819-12-389
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.