Back to Journals » Drug Design, Development and Therapy » Volume 10
Curcumin enhances the cytogenotoxic effect of etoposide in leukemia cells through induction of reactive oxygen species
Authors Papiez M, Krzyściak W, Szade K, Bukowska-Straková K, Kozakowska M, Hajduk K, Bystrowska B, Dulak J, Jozkowicz A
Received 19 July 2015
Accepted for publication 25 November 2015
Published 4 February 2016 Volume 2016:10 Pages 557—570
DOI https://doi.org/10.2147/DDDT.S92687
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Wei Duan
Monika A Papież,1 Wirginia Krzyściak,2 Krzysztof Szade,3 Karolina Bukowska-Straková,3,4 Magdalena Kozakowska,3 Karolina Hajduk,3 Beata Bystrowska,5 Jozef Dulak,3,6 Alicja Jozkowicz3
1Department of Cytobiology, 2Department of Medical Diagnostic, Faculty of Pharmacy, Jagiellonian University Medical College, 3Department of Medical Biotechnology, Faculty of Biochemistry, Biophysics and Biotechnology, Jagiellonian University, 4Department of Clinical Immunology, Institute of Pediatrics, 5Department of Toxicology, Faculty of Pharmacy, Jagiellonian University Medical College, 6Malopolska Centre of Biotechnology, Jagiellonian University, Krakow, Poland
Abstract: Curcumin may exert a more selective cytotoxic effect in tumor cells with elevated levels of free radicals. Here, we investigated whether curcumin can modulate etoposide action in myeloid leukemia cells and in normal cells of hematopoietic origin. HL-60 cell line, normal myeloid progenitor cluster of differentiation (CD)-34+ cells, and granulocytes were incubated for 4 or 24 hours at different concentrations of curcumin and/or etoposide. Brown Norway rats with acute myeloid leukemia (BNML) were used to prove the influence of curcumin on etoposide action in vivo. Rats were treated with curcumin for 23 days and etoposide was administered for the final 3 days of the experiment. Curcumin synergistically potentiated the cytotoxic effect of etoposide, and it intensified apoptosis and phosphorylation of the histone H2AX induced by this cytostatic drug in leukemic HL-60 cells. In contrast, curcumin did not significantly modify etoposide-induced cytotoxicity and H2AX phosphorylation in normal CD34+ cells and granulocytes. Curcumin modified the cytotoxic action of etoposide in HL-60 cells through intensification of free radical production because preincubation with N-acetyl-l-cysteine (NAC) significantly reduced the cytotoxic effect of curcumin itself and a combination of two compounds. In contrast, NAC did not decrease the cytotoxic effect of etoposide. Thus, oxidative stress plays a greater role in the cytotoxic effect of curcumin than that of etoposide in HL-60 cells. In vitro results were confirmed in a BNML model. Pretreatment with curcumin enhanced the antileukemic activity of etoposide in BNML rats (1.57-fold tumor reduction versus etoposide alone; P<0.05) and induced apoptosis of BNML cells more efficiently than etoposide alone (1.54-fold change versus etoposide alone; P<0.05), but this treatment protected nonleukemic B-cells from apoptosis. Thus, curcumin can increase the antileukemic effect of etoposide through reactive oxygen species in sensitive myeloid leukemia cells, and it is harmless to normal human cells.
Keywords: acute myeloid leukemia, curcumin, etoposide, ROS, γ-H2AX, apoptosis
Introduction
Curcumin is a phytochemical compound isolated from the rhizomes of Curcuma longa L. having antitumor activity.1 Curcumin might be selectively cytotoxic to tumor cells and is less cytotoxic to normal cells.2 Similarly, curcumin could selectively modulate the cytotoxic effect of many chemotherapeutic agents in tumor cells.3 Such selective action of curcumin results from differences in metabolism between normal and malignant cells. Cancer cells have many mutations in proto-oncogenes, which can contribute to an increase in free radicals.4 Curcumin can exert selective pro-oxidant action in cancer cells with high levels of free radicals and, in this way, induce apoptosis.5,6 Curcumin may also inhibit the antiapoptotic genes that are often overexpressed in leukemia cells, which may be a cause of resistance to anticancer drugs.7 Among antiapoptotic genes, special attention has recently been paid to survivin, which is overexpressed in acute myeloid leukemia (AML) stem/progenitor cells and is associated with poor prognosis.8,9
The effect of curcumin on the activity of certain cytostatic drugs, including etoposide, is not yet well known. Etoposide is used in the treatment of a wide spectrum of solid tumors, lymphomas, and leukemias.10 Several studies have shown that curcumin can inhibit the activation, nuclear translocation, and binding to DNA of nuclear factor-κB (NFκB) in cancer cells.11 In contrast, etoposide enhances the activity of this nuclear factor, promoting cell survival.12 It is believed that inhibition of NFκB could increase the cytotoxic effects of etoposide in cancer cells. Therefore, it can be supposed that curcumin will potentiate the effect of etoposide, inter alia, by inhibiting NFκB.
A topoactive drug, etoposide inhibits the topoisomerase II–DNA cleavable complex, resulting in DNA damage, including double-stranded DNA breaks (DSBs), causing chromosomal aberration or apoptosis.13,14 The myelotoxic and leukemogenic action of etoposide makes it difficult to use a sufficiently high dose of this medication. The reason for the strong cytotoxicity of etoposide in hematopoietic precursors is the high activity of not only topoisomerase II, but also myeloperoxidase (MPO).15 MPO metabolizes etoposide to highly toxic phenoxyl radicals and ortho-quinones. These radicals lead to a decrease in glutathione (GSH) and enhancement of oxidative stress, which in turn contributes to an increase in DNA damage.16 Oxidative damage to phospholipids and the loss of mitochondrial membrane integrity result in cell apoptosis. MPO activity dominates cytochrome P450 expression in myeloid lineage cells.17
Etoposide also exerts particularly strong mutagenic effects in precursors of myeloid lineage. It does not cause any other type of leukemia, but treatment-related acute myeloid leukemia (t-AML) occurs in a large percentage of patients treated with this cytostatic drug.18 The results of studies indicate a role of etoposide phenoxyl radicals in the development of t-AML. It has been proved that the presence of phenoxyl radicals of etoposide correlates with the appearance of MLL gene translocation, characteristic of t-AML, in early myeloid precursors expressing cluster of differentiation (CD)-34 stem cell antigens.19
Compounds that could increase the cytotoxic effect of etoposide in cancer cells and, at the same time, not increase the side effects of etoposide in bone marrow cells are still being sought. One such compound possessing selective action in cancer cells is curcumin. So far, there has been no research on the effects of curcumin on the activity of etoposide in myeloid leukemia cells.
The aim of this study was to determine whether cotreatment with curcumin can modify the effect of etoposide in leukemic cells and their normal counterparts in vitro and in vivo.
Materials and methods
Cell culture conditions
An HL-60 cell line was purchased from the European branch of American Type Culture Collection (ATCC, UK). The cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 supplemented with 10% fetal bovine serum. Primary human cord blood CD34+ cells were purchased from Stemcell Technologies Inc (Vancouver, Canada). CD34+ cells were grown in StemSpan™ serum-free expansion medium containing pretested bovine serum albumin, insulin, transferrin, and supplements in Iscove’s Modified Dulbecco’s Medium. The medium was supplemented with StemSpan expansion supplement CC100 containing recombinant human (rh) Fms-related tyrosine kinase 3 ligand, rh stem cell factor, rh interleukin (IL)-3, and rh IL-6. These cells were isolated from the umbilical cord blood mononuclear cells of healthy donors using positive immunomagnetic separation techniques. The purity of CD34+ cells >90% was checked via flow cytometry (Becton Dickinson [BD] Biosciences Immunocytometry Systems, San Jose, CA, USA). Immediately after thawing, a viable cell count was done using the trypan blue exclusion method. HL-60 cells and CD34+ cells were cultured without antibiotics at 37°C in a 5% CO2 and 95% humidified atmosphere. CD34+ cells were cultured for no longer than 2 weeks.
Cells were incubated with different concentrations of curcumin and/or etoposide dissolved in dimethyl sulfoxide (DMSO) for 4 or 24 hours. Control cells were treated only with DMSO, the concentration of which was 0.06% in the culture medium. In accordance with the guidelines of the Bioethics Committee of the Jagiellonian University, ethics approval is not required on cells commercially purchased in the company. Therefore, research on HL-60 cell line (ATCC, UK) and on CD34 cells (Stem Cell Technology, Canada) did not require ethics approval.
Isolation of granulocytes from human venous/peripheral blood
Heparinized blood (lithium heparin – anticoagulant) was collected from healthy donors (female, 22–47 years of age) after approval was obtained from the Bioethics Committee of the Jagiellonian University and donors provided written informed consent. To isolate granulocytes, blood samples were centrifuged in a density gradient using Gradisol G. The granulocytes were counted and their viability was assessed using 0.4% trypan blue exclusion methods. The homogeneity of the population was evaluated with flow cytometry technique. Detailed information on the isolation of granulocytes is provided in the Supplementary materials.
Determination of cytotoxicity, apoptosis, and combination index
To determine the cytotoxicity after treatment, HL-60 cells in the logarithmic growth phase, primary CD34+ cells, and granulocytes were seeded in 24-well culture plates at a density of 0.4×106 cells per well and were incubated for 24 hours with different concentrations of etoposide and/or curcumin. After incubation, the cells were washed twice in phosphate-buffered saline (PBS) (230 × g centrifuged) for 5 minutes. Cytotoxicity was determined using propidium iodide (PI) staining. Apoptosis was detected using annexin-V-allophycocyanin and PI staining. The cells were immediately analyzed by flow cytometry. Analysis was performed on at least 20,000 single cells. Detailed information on the staining is provided in the Supplementary materials. CalcuSyn (Biosoft, Cambridge, UK) software was used in the calculation of half-maximal inhibitory concentration (IC50) and the combination index (CI) for HL-60 cells. The CI was evaluated based on the multiple drug effect equation of Chou-Talalay.20 CI values <1 indicate synergism, =1 indicate an additive effect, and >1 indicate antagonism.
Cell staining and analysis for H2AX, cell cycle, and NFκB
For the detection of DNA damage, mainly DSBs, phosphorylated histone H2AX (γ-H2AX) was detected as a marker.21,22 The cells were fixed on ice with 1% methanol-free formaldehyde solution in PBS for 15 minutes and permeabilized with ice-cold 70% ethanol in deionized water. Cells (0.4×106) were stained with primary rabbit monoclonal anti-γ-H2AX (Ser139) antibody. Next, they were incubated with the secondary anti-rabbit immunoglobulin (Ig) G (H+L) F(ab′)2 fragment of goat antibody conjugated with Alexa Fluor 647. DNA was stained with PI and RNase A in PBS.
To determine the level of nuclear NFκB in HL-60 cells, the cells were prepared using the Cycletest™ PLUS DNA reagent kit (Becton Dickinson). Then, nuclei were stained with mouse monoclonal anti-NFκBp65–phycoerythrin-conjugated IgG1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
The cells were analyzed with an LSR II flow cytometer, and quantitative analysis of γ-H2AX-positive cells or the nuclear level of NFκB was carried out using FACSDiva software (BD Biosciences Immunocytometry Systems).
The level of cellular NFκBp65 was also determined through Western blot analysis. Detailed information on the staining procedure is provided in the Supplementary materials.
Measurement of reactive oxygen species
Reactive oxygen species (ROS) were detected in the cells using 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) staining. After incubation with the investigated compounds for 24 hours, the cells were washed with Hank’s balanced salt solution and incubated with 10 μM CM-H2DCFDA for 30 minutes in the dark. The intensity of fluorescence was measured in an LSR II flow cytometer. The analysis was performed on at least 20,000 events. Detailed information on the staining procedure is provided in the Supplementary materials.
Determination of intracellular GSH in HL-60 cells
Total GSH in the HL-60 cells was measured spectrophotometrically by the Ellman method23 based on the reaction of thiols with the chromogenic 5,5′-dithiobis-2-nitrobenzoate, whereby formation of the yellow dianion of 5-thio-2-nitrobenzoic acid is measured. GSH concentrations were expressed as nanomoles per milligram of protein. Detailed information on the determination of GSH is provided in the Supplementary materials.
Liquid chromatography/mass spectrometry
The liquid chromatography (LC) separation was performed on an Agilent HPLC 1100 series system (Agilent, Waldbronn, Germany). The samples were separated on a Supelco Discovery C18 column (250 mm ×4.0 mm, 5 mm particle size). Mass spectrometry (MS) analyses were performed on an Applied Biosystems MDSciex (Concord, ON, Canada) API 2000 triple quadrupole mass spectrometer equipped with an electrospray ionization interface. Detailed information on the LC/MS analysis is provided in the Supplementary materials.
BNML model of myeloid leukemia
All experiments were performed in accordance with legal requirements, under a license granted by the Jagiellonian University Ethical Committee. Male Brown Norway (BN/CrlCmd) rats were obtained from the Polish Academy of Sciences Medical Research Center (Warsaw, Poland). Brown Norway rat myeloid leukemia (BNML) was induced by intravenous inoculation of BN rats with spleen-derived leukemia cells (1×106 cells in 0.5 mL of PBS injected into tail vein). Cells were kindly provided by Professor ACM Martens, Utrecht University, the Netherlands.24
The doses of curcumin (100 and 200 mg/kg) were chosen based on previous reports.2,25 Curcumin was dissolved in 100 μL of DMSO and then 400 μL of corn oil was added; the solution was administered by oral gavage once daily, starting 2 days after leukemic cell injection and continuing to the day 23. Etoposide (50 mg/kg) was administered intraperitoneally once daily for the final 3 days of the experiment. Control animals were treated with vehicle only.
Excision of tissues infiltrated by leukemic cells and cell isolation
Spleens were excised from rats, weighed, and macerated in a Petri dish filled with RPMI-1640 medium. Erythrocytes were lysed using red blood cell-lysing buffer containing 0.83% ammonium chloride in 0.01 M Tris buffer (pH 7.5).
Immunophenotyping and detection of cell apoptosis/necrosis
The suspensions of cells isolated from the spleens were stained with mouse primary monoclonal anti-RM124 antibody, which recognizes the specific epitope that is highly expressed on leukemic cells, is rarely found on neutrophils, and is absent on normal, nonleukemic blast cells and lymphocytes.24 Cells were stained with a secondary goat anti-mouse monoclonal antibody specific against the μ-chain conjugated to R-phycoerythrin. Apoptosis was detected by annexin V and 7-amino-actinomycin D (7AAD) staining and analyzed with an LSRII flow cytometer (Becton Dickinson). Detailed information on the staining procedure can be found in the Supplementary materials.
Survivin mRNA expression in sorted BNML cells, B lymphocytes, and HL-60 cells
Cells (1×107) were sorted using a MoFlo XDP cell sorter (Beckman Coulter, Los Angeles, CA, USA) (Supplementary materials). Expression of mRNA was investigated using real-time polymerase chain reaction (PCR). Gene expression was calculated based on the ΔCt method. Detailed information on the implementation of reverse transcription–PCR is provided in the Supplementary materials.
Statistical analysis
One-way analysis of variance (ANOVA) with the Newman–Keuls post hoc test was used to calculate the statistical significance between groups. All data are shown as mean ± standard error of the mean.
Results
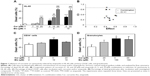
Curcumin enhances the cytotoxic effects of etoposide in leukemic cells more strongly than in normal cells of myeloid origin
Curcumin and etoposide exert cytotoxic activity in HL-60 cells in a concentration-dependent manner (Figure 1A). The investigated polyphenol exerted significant cytotoxic activity at a concentration of 20 μM after 24 hours in an HL-60 line. Therefore, this concentration of curcumin (20 μM) was used in experiments with CD34+ cells and granulocytes. Normal proliferating CD34+ cells and mature granulocytes were more resistant to the tested compounds (Figure 1). Curcumin concentrations of 20 μM did not result in significant cytotoxicity in these normal cells (Figure 1C and D). Etoposide at a concentration of 10 μM increased the number of dead CD34+ cells by approximately 30% compared to the controls (Figure 1C). In contrast, after incubation of HL-60 cells with 10 μM of this chemotherapeutic agent, there were three times more dead cells than was the case with CD34+ (Figure 1A).
Curcumin significantly intensified the cytotoxic effects of etoposide in HL-60 cells (Figure 1A). To determine the character of the interaction between the investigated compounds, we analyzed the CI. This analysis showed that most cases of combinations of curcumin and etoposide at different concentrations exhibited synergism (CI <0.6, Figure 1B). Only the combination of curcumin at a concentration of 20 μM and etoposide at a concentration of 10 μM showed an additive interaction (CI =1.016). Importantly, curcumin at a concentration of 20 μM did not significantly modify the cytotoxic effects of etoposide in CD34+ cells or mature granulocytes (Figure 1C and D). IC50 values for etoposide and curcumin were 7.29 and 31.17 μM, respectively.
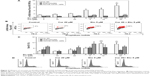
Curcumin increases genotoxic and apoptotic effects of etoposide in HL-60 cells
To determine whether curcumin can modify the level of DSBs induced by etoposide in leukemic and normal cells, the intensity of fluorescence of γ-H2AX was determined after 4-hour incubation with the tested compounds. Other studies have shown that etoposide caused intense phosphorylation of H2AX after this incubation period.26 Curcumin at a concentration of 20 μM significantly intensified the phosphorylation of H2AX induced by etoposide in HL-60 cells and did not exert such actions in normal CD34+ progenitor cells or granulocytes (Figure 2A and B). Intensification of etoposide genotoxicity by curcumin in HL-60 cells correlated with the apoptotic effect. The percentage of the sub-G1 fraction (subdiploidal fraction) increased significantly after incubation of HL-60 cells with a combination of curcumin at concentrations of 10 or 20 μM and etoposide (5 μM) in comparison to the use of the cytotoxic drug alone (Figure 2C and D). In normal cells, there was an observable tendency for an increase in sub-G1 after treatment with etoposide. A clear but insignificant decrease in the level of the subdiploidal fraction was observed after cotreatment of normal cells with curcumin 20 μM and etoposide compared to the cytotoxic drug alone, especially in the case of mature granulocytes (Figure 2C).
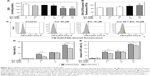
Curcumin enhances the cytotoxicity of etoposide in leukemia cells by increasing the oxidative stress
We investigated whether curcumin, as a factor modulating ROS-sensitive signaling pathways, has an impact on the cytotoxic effect of etoposide in HL-60 cells. Curcumin alone at a concentration of 20 μM, exerting a cytotoxic effect in HL-60 cells, enhanced the oxidative stress in these cells, because its action resulted in a significant decrease in the level of reduced GSH and an increase in levels of free radicals in comparison to the control (Figure 3A–C). A similar effect was exerted in HL-60 cells by etoposide at a concentration of 10 μM. The combination of curcumin and etoposide significantly enhanced the symptoms of oxidative stress, as reflected in a significant drop in GSH and increase in free radical levels over that of etoposide (Figure 3A–C). On the other hand, preincubation with N-acetyl-L-cysteine (NAC) significantly reduced the cytotoxic and apoptotic effects of the investigated polyphenol and combinations of curcumin (20 μM) and etoposide (10 μM), in comparison to the appropriate controls (Figure 3D and E). These results indicate that escalating oxidative stress is involved in the cytotoxic action of curcumin and in the modification by this polyphenol of etoposide cytotoxicity in HL-60 cells.
Curcumin does not significantly modify the expression of nuclear NFκB in HL-60 cells
In many studies, the cytotoxic effects of curcumin correlate with inhibition of NFκB10,27,28 and, therefore, we investigated whether the impact of the synergistic effect of curcumin and etoposide leads to a change in the expression of this transcription factor.
We did not find any significant effects of the investigated compounds, used separately or in combination, on the nuclear level of NFκB (Supplementary figures) or whole-cell level of phospho-p65 (data not shown).
Curcumin and tetrahydrocurcumin content in HL-60 cells
Quantitative analysis using LC/MS showed that the average content of curcumin is 7.19 μg/mL and that of its major metabolite tetrahydrocurcumin is 5.25 μg/mL, after 24 hours of incubation with 20 μM of the investigated polyphenol (Supplementary figures).
Curcumin increases the antileukemic activity of etoposide in a rat model of AML
To verify whether curcumin can modulate the antileukemic activity of etoposide in vivo, we used a BNML rat model of transplantable AML, which closely resembles human AML in growth characteristics and chemotherapeutic susceptibility.24,29 Rats were infused with leukemic cells and received curcumin daily from day 2 to day 23 (Figure 4A). Etoposide was administered for the final 3 days (Figure 4A).
Development of leukemia in rats was manifested by a decrease in body weight (from 196.6±3.3 to 170.6±7.3 g, P<0.05) (Figure 4B), whereas spleen weight was doubled, as measured on day 23 after infusion of leukemic cells (Figure 4C). Numerous nodules at the surface of the spleens additionally confirmed the presence of leukemia (Figure 4D).
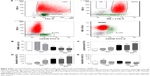
Etoposide alone effectively reduced the progression of the disease, as indicated by the decreased spleen weight (Figure 4E). Moreover, flow cytometric analysis (Figure 5A) showed that rats treated with etoposide displayed a lower proportion of leukemic cells (Figure 5B) and a higher proportion of host B-cells (Figure 5C) in the spleen, when compared with their untreated, control counterparts.
At a higher dose of 200 mg/kg, curcumin exerted an antileukemic potential comparable to that of etoposide at a dose of 50 mg/kg (Figure 4E). Importantly, pretreatment with curcumin (200 mg/kg) followed by administration of etoposide significantly enhanced the antileukemic effect of the drug, further reducing the spleen weight and correcting the proportion of leukemic cells and healthy B-cells (Figure 5B and C).
Curcumin increases etoposide-induced apoptosis of BNML cells
Given that etoposide is known for its proapoptotic action, we checked whether the pretreatment of rats with curcumin can modulate etoposide-stimulated apoptosis in leukemic cells. As shown in Figure 5D, etoposide administered alone was a potent proapoptotic agent, as evidenced by an increased proportion of Annexin V-positive leukemic BNML cells isolated from the rat spleen.
Curcumin also increased the apoptosis of leukemic cells in a dose-dependent manner and, again, the effectiveness of the higher dose (200 mg/kg) was similar to that of etoposide (Figure 5D). The highest apoptosis rate was found in leukemic cells isolated from rats pretreated with curcumin (200 mg/kg) and then injected with etoposide (Figure 5D; P<0.05 versus etoposide only). This shows that curcumin can increase the proapoptotic potential of etoposide in leukemic cells in vivo.
Importantly, this proapoptotic potential seems to be cell-type selective, as no induction of apoptosis in the nonleukemic B-cells was detected in response to either etoposide alone or curcumin (200 mg/kg)/etoposide treatment (Figure 5E). Moreover, the low rate of B-cell apoptosis in rat spleens was further decreased in the curcumin (200 mg/kg)/etoposide group. It appears, however, that the biological meaning of these statistically significant differences – decrease in B-cell apoptosis in curcumin/etoposide group and some increase in animals treated with a lower dose of curcumin (100 mg/kg) – is rather questionable, as in no sample did the apoptosis rate exceed 0.5% (Figure 5E).
Influence of curcumin on survivin expression after etoposide treatment of leukemic rats and HL-60 cells
Because leukemic cells are known to overexpress antiapoptotic proteins, we checked whether curcumin modulates the expression of genes involved in these processes. Etoposide showed a tendency to induce the expression of antiapoptotic survivin in leukemic cells, and this effect was partially prevented by pretreatment with curcumin (Figure 6A). The differences did not, however, reach statistical significance (P=0.07, one-way ANOVA). Etoposide and curcumin did not affect the expression of survivin in nonleukemic B-cells (Figure 6B). Similarly, a combination of curcumin and etoposide does not exert significant effect on the expression of survivin in comparison to etoposide alone in HL-60 cells. Therefore, the level of survivin expression does not play a significant role in the antileukemia activity of curcumin/etoposide (Figure 6C).
Discussion
A major problem in the treatment of AML is its frequent recurrence because of the relatively few effective conventional therapies and their high cytogenotoxicity in normal cells.30–33 Our in vitro studies show that curcumin has less potent cytogenotoxic activity in normal human CD34+ myeloid progenitor cells and human granulocytes compared with leukemic HL-60 cells.
Cotreatment with curcumin and etoposide exerts a synergistic cytotoxic effect in HL-60 cells. In another study, a similar synergistic effect of curcumin with daunorubicin was observed in myeloid leukemic cell lines.34 Intensifying the effect of curcumin on the cytotoxic action of etoposide has been demonstrated in brain tumor and retinoblastoma cell lines.35,36 In the present study, curcumin potentiated the cytotoxic effect of etoposide in HL-60 cells through intensification of its genotoxicity, which is indicated by the significant increase in the amount of γ-H2AX after treatment with a combination of curcumin and etoposide, accompanied by a corresponding increase in the percentage of the sub-G1 fraction in comparison to the cytostatic drug alone.
One method to increase the cytogenotoxic effect of etoposide by curcumin may be via the ability of this polyphenol to intensify oxidative stress in cancer cells. We have shown that curcumin significantly potentiated the pro-oxidative effect of etoposide in HL-60 cells by increasing the production of free radicals and reducing the level of GSH. The pro-oxidative effect of curcumin contributed to the escalation of the apoptotic and cytotoxic actions of etoposide, as antioxidant NAC significantly reduced the cytotoxic effect of the combination of curcumin and etoposide in HL-60 cells. The pro-oxidative effect of curcumin could also potentiate the DNA damage induced by etoposide because it is known that free radicals can directly lead to the formation of DSBs.4
Increased levels of free radicals are often observed in cancer cells. Pro-oxidants can augment the level of oxidative stress in these cells, and the resulting DNA damage and apoptosis, and may lead to cell growth arrest. Our results are consistent with those of Sánchez et al,37 who confirmed that curcumin induces apoptosis of myeloid leukemia cells and enhances the cytotoxic activity of arsenic trioxide by increasing oxidative stress. Other studies have shown that the beta-diketone structure of curcumin is responsible for its pro-oxidative effect.38 Analysis using LC/MS revealed that curcumin (7.19 μg/mL) quantitatively dominated over the main metabolite, tetrahydrocurcumin (THC, 5.25 μg/mL), in HL-60 cells. As demonstrated in other studies, THC has no pro-oxidative action in cells.39
The present results show that oxidative stress plays a greater role in the cytotoxic effect of curcumin than in the case of etoposide. Preincubation with NAC significantly reduced the cytotoxic effect of curcumin but did not exert such an effect on etoposide action. Oxidative stress is thus involved in the cytotoxic action of curcumin, and the cytotoxic activity of etoposide mainly corresponds to DNA damage.
Curcumin, neither alone nor in combination with etoposide, inhibited the nuclear and whole-cell levels of NFκBp65. These results are also consistent with the studies of Sánchez et al,37 who showed no effect of curcumin on the activation and DNA binding of NFκB in AML cells.
It is known that the leukemogenic and myelosuppressive effect of etoposide is caused by the cytogenotoxic activity in myeloid precursors.19,33 Limiting the cytogenotoxic action of etoposide in these cells could allow for an increase in the dose of this drug. Our research shows that curcumin at a concentration of 20 μM, which enhances the action of etoposide in HL-60 cells, leads to an insignificant decrease in the number of dead CD34+ cells induced by this cytostatic drug. One reason for the difference in the effects of curcumin on tumor and normal CD34+ cells predisposed to leukemogenesis under etoposide action may be endogenous differences in the redox state of the cells. It should be added that curcumin and etoposide can influence the progenitor cells in different ways. Other studies show that stem cells are characterized by low levels of the ROS necessary for the capacity for self-renewal. While the increase in ROS promotes the differentiation of these cells, curcumin and etoposide could, theoretically, significantly increase the level of ROS in CD34+ cells and affect the ability of these cells to effect self-renewal and differentiation.40
It is also known that treatment with etoposide is associated with neutropenia. In retrospective studies, Buckley et al32 demonstrated that severe neutropenia is the major risk factor for adverse outcomes after induction chemotherapy, increasing the probability of morbidity and mortality in patients with AML. The cause of etoposide-induced neutropenia may be not only myelosuppression, but also induction of apoptosis/necrosis of mature neutrophils. Topoisomerase II, an etoposide handle, has weak activity in nonproliferating cells. However, etoposide can induce the cell death of neutrophils by increasing oxidative stress. In previous studies, it has been demonstrated that etoposide increases the level of ROS in resting neutrophils.41 Therefore, it seems important to investigate the effect of curcumin, which is a factor modulating ROS-sensitive signaling pathways, in cells treated with etoposide.
We have shown in vitro that curcumin does not potentiate the cytogenotoxic etoposide effect in resting granulocytes, and that it can even be seen to reduce the tendency toward apoptosis exerted by the cytostatic drug, as indicated by an insignificant but clear decrease in the percentage of the sub-G1 fraction and the level of phosphorylated H2AX after treatment with curcumin (20 μM) and etoposide in comparison to the results with use of the cytotoxic drug alone.
The in vitro influence of curcumin on the antileukemic action of etoposide has been confirmed in further in vivo studies performed on a BNML model. This model of transplantable leukemia turned out to be useful in the design and translation into clinical practice of new therapeutic strategies.24,29 Therefore, we chose it to analyze the antileukemic activity of curcumin. Curcumin administration throughout the period of leukemia development resembles the situation when the disease recurs after remission, and this polyphenol reduces the growth of leukemia.
Our study shows that curcumin, at a higher dose (200 mg/kg) and given every second day after injection of BNML cells, reduces the progression of leukemia. This observation, which confirms the antileukemic potential of curcumin, is consistent with earlier studies performed in murine WEHI-3 myeloid leukemia models.42 Moreover, we demonstrated significant improvement in therapeutic efficacy when pretreatment with curcumin was combined with administration of etoposide. Hence, the BNML cells were sensitive to etoposide alone, and administration of the drug during the final 3 days of the experiment was sufficient to effect a reduction of more than twofold in the number of leukemic cells. Importantly, pretreatment with curcumin resulted in a further enhancement of the antileukemic activity of etoposide.
We also propose a possible mechanism that may underlie the observed effect. Our results show that curcumin itself triggered apoptosis of leukemic BNML cells in the rat spleen. As could be expected, etoposide administration also induced apoptosis in leukemic cells. Importantly, the combination of etoposide and curcumin treatments increased apoptosis rates more than curcumin or etoposide administered separately. Therefore, it can be concluded that curcumin enhances the proapoptotic activity of etoposide in BNML cells in vivo.
We have found in vivo that administration of etoposide induced expression of survivin in BNML cells growing in the rat spleen, an effect clearly attenuated by pretreatment with curcumin. However, we could not confirm these observations in vitro in HL-60 cells. Therefore, it can be assumed that curcumin does not significantly affect the expression of survivin, a downstream target of NF-kB, in BNML cells growing in the rat spleen and in HL-60 cells treated with curcumin/etoposide.
Importantly, we did not detect any increase in apoptosis rates in nonleukemic splenic B-cells in response to either etoposide or the higher dose of curcumin. Unexpectedly, a low dose of curcumin (100 mg/kg) significantly increased the percentage of apoptotic B-cells. The difference in dose–response profiles could depend on the quantity of curcumin metabolites that may block its action. Furthermore, there was a statistically significant decrease in apoptosis in B-cells isolated from animals treated with a combination of etoposide and curcumin, although the biological significance of such a reduction is uncertain due to the very low apoptosis rate found in the control B-cells. It should be added that we have shown in earlier studies that curcumin enhances the antileukemic activity of etoposide in the bone marrow of BNML rats, while leading to an increase in the percentage of normal granulocyte and erythrocyte precursors compared to the group treated with etoposide alone. Thus, curcumin may protect normal myeloid precursors against the cytotoxic effects of etoposide. Alternatively, due to a significant decrease in the percentage of leukemic cells after treatment with etoposide and curcumin, the normal cells of the myeloid lineage began to dominate.43
Generally, our results indicate that curcumin enhances the proapoptotic activity of etoposide, acting selectively in leukemic cells. A new element of our research is the demonstration that curcumin in combination with etoposide acts synergistically in myeloid leukemia cells. In this activity of curcumin, oxidative stress undoubtedly plays an important role. Its effect is much stronger in malignant myeloid cells than in their normal counterparts. This suggests that curcumin could be useful in the design of new anticancer strategies to intensify the proapoptotic potential of etoposide in myeloid leukemia cells.
Acknowledgments
The study was partially supported by the Jagiellonian University Programs number K/ZDS/005620 and by grants from the National Science Centre (2012/06/M/NZ1/00008 and 2013/11/N/NZ3/00956). Research was conducted under the scope of the MiR-TANGO International Associated Laboratory. Faculty of Biochemistry, Biophysics and Biotechnology is a beneficiary of National Research Leading Center (KNOW) grant from the Polish Ministry for Science and Higher Education. BNML cells and mouse primary monoclonal anti-RM124 antibody were kindly provided by Professor ACM Martens, Utrecht University, the Netherlands.
Disclosure
The authors report no conflicts of interest in this work.
References
Ravindran J, Prasad S, Aggarwal BB. Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? AAPS J. 2009;11(3):495–510. | ||
Zhou QM, Wang XF, Liu XJ, et al. Curcumin improves MMC-based chemotherapy by simultaneously sensitising cancer cells to MMC and reducing MMC-associated side-effects. Eur J Cancer. 2011;47:2240–2247. | ||
Goel A, Aggarwal BB. Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr Cancer. 2010;62:919–930. | ||
Sallmyr A, Fan J, Rassool FV. Genomic instability in myeloid malignancies: increased reactive oxygen species (ROS), DNA double strand breaks (DSBs), and error-prone repair. Cancer Lett. 2008;270:1–9. | ||
Syng-Ai C, Kumari AL, Khar A. Effect of curcumin on normal and tumor cells: role of glutathione and bcl-2. Mol Cancer Ther. 2004;3:1101–1108. | ||
Ahsan H, Parveen N, Khan NU, et al. Pro-oxidant, anti-oxidant and cleavage activities on DNA of curcumin and its derivatives demethoxycurcumin and bisdemethoxycurcumin. Chem Biol Interact. 1999;121:161–175. | ||
Notarbartolo M, Poma P, Perri D, et al. Antitumor effects of curcumin, alone or in combination with cisplatin or doxorubicin, on human hepatic cancer cells: analysis of their possible relationship to changes in NFκB activation levels and in IAP gene expression. Cancer Lett. 2005;224:53–65. | ||
Zaffaroni N, Daidone MG. Survivin expression and resistance to anticancer treatments: perspectives for new therapeutic interventions. Drug Resist Updat. 2002;5:65–72. | ||
Kanwar JR, Kamalapuram SK, Kanwar RK. Targeting survivin in cancer: the cell-signalling perspective. Drug Discov Today. 2011;16:485–494. | ||
Slevin ML. The clinical pharmacology of etoposide. Cancer. 1991;67:319–329. | ||
Hussain AR, Ahmed M, Al-Jomah NA, et al. Curcumin suppresses constitutive activation of nuclear factor-KB and requires functional Bax to induce apoptosis in Burkitt’s lymphoma cell lines. Mol Cancer Ther. 2008;7(10):3318–3329. | ||
Yu LL, Wu JG, Dai N, et al. Curcumin reverses chemoresistance of human gastric cancer cells by downregulating the NF-κB transcription factor. Oncol Rep. 2011;26:1197–1203. | ||
Corbett AH, Osheroff N. When good enzymes go bad: conversion of topoisomerase II to a cellular toxin by antineoplastic drugs. Chem Res Toxicol. 1993;6:585–597. | ||
Saleh EM. Inhibition of topoisomerase IIα sensitizes FaDu cells to ionizing radiation by diminishing DNA repair. Tumour Biol. Epub 2015 Jun 17. | ||
Haim N, Nemec J, Roman J, et al. Peroxidase-catalyzed metabolism of etoposide (VP-16–213) and covalent binding of reactive intermediates to cellular macromolecules. Cancer Res. 1987;47:5835–5840. | ||
Kagan VE, Yalowich JC, Borisenko GG, et al. Mechanism-based chemopreventive strategies against etoposide-induced acute myeloid leukemia: free radical/antioxidant approach. Mol Pharmacol. 1999;56:494–506. | ||
Soucek P, Anzenbacher P, Skoumalová I, et al. Expression of cytochrome P450 genes in CD34+ hematopoietic stem and progenitor cells. Stem Cells. 2005;23:1417–1422. | ||
Whitlock JA, Greer JP, Lukens JN. Epipodophyllotoxin related leukemia. Identification of a new subset of secondary leukemia. Cancer. 1991;68:600–604. | ||
Vlasova II, Feng W, Goff JP, et al. Myeloperoxidase-dependent oxidation of etoposide in human myeloid progenitor CD34+ cells. Mol Pharmacol. 2011;79:448–479. | ||
Chou TC, Talalay P. Analysis of combined drug effects: a new look at a very old problem. Trends Pharmacol Sci. 1983;4:450–454. | ||
Rogakou EP, Pilch DR, Orr AH, et al. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. | ||
Sedelnikova OA, Rogakou EP, Panyutin IG, et al. Quantitative detection of (125) IdU-induced DNA double-strand breaks with gamma-H2AX antibody. Radiat Res. 2002;158:486–492. | ||
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. | ||
Martens AC, Van Bekkum DW, Hagenbeek A. The BN acute myelocytic leukemia (BNML) (a rat model for studying human acute myelocytic leukemia (AML)). Leukemia. 1990;4:241–257. | ||
Papież MA, Kaja M, Gębarowska A. Age-dependent different action of curcumin in thyroid of rat. Folia Histochem Cytobiol. 2008;46(2):205–211. | ||
Mosieniak G, Sliwinska M, Piwocka K, et al. Curcumin abolishes apoptosis resistance of calcitriol-differentiated HL-60 cells. FEBS Lett. 2006;580:4653–4660. | ||
Sen GS, Mohanty S, Hossain DM, et al. Curcumin enhances the efficacy of chemotherapy by tailoring p65NFκB-p300 cross-talk in favor of p53-p300 in breast cancer. J Biol Chem. 2011;286(49):42232–42247. | ||
Misra R, Sahoo SK. Coformulation of doxorubicin and curcumin in poly(D,L-lactide-co-glycolide) nanoparticles suppresses the development of multidrug resistance in K562 cells. Mol Pharm. 2011;8(3):852–866. | ||
McCormack E, Bruserud O, Gjertsen BT. Animal models of acute myelogenous leukaemia – development, application and future perspectives. Leukemia. 2005;19:687–706. | ||
Shman TV, Fedasenka UU, Savitski VP, et al. CD34+ leukemic subpopulation predominantly displays lower spontaneous apoptosis and has higher expression levels of Bcl-2 and MDR1 genes than CD34- cells in childhood AML. Ann Hematol. 2008;87:353–360. | ||
Eisele L, Klein-Hitpass L, Chatzimanolis N, et al. Differential expression of drug resistance-related genes between sensitive and resistant blasts in acute myeloid leukemia. Acta Haematol. 2007;117:8–15. | ||
Buckley SA, Othus M, Vainstein V, et al. Prediction of adverse events during intensive induction chemotherapy for acute myeloid leukemia or high-grade myelodysplastic syndromes. Am J Hematol. 2014;89:423–428. | ||
Papież MA. The influence of curcumin and (–)-epicatechin on the genotoxicity and myelosuppression induced by etoposide in bone marrow cells of male rats. Drug Chem Toxicol. 2013;36(1):93–101. | ||
Rao J, Xu DR, Zheng FM, et al. Curcumin reduces expression of Bcl-2, leading to apoptosis in daunorubicin-insensitive CD34+ acute myeloid leukemia cell lines and primary sorted CD34+ acute myeloid leukemia cells. J Transl Med. 2011;9:71. | ||
Ramachandran C, Nair SM, Escalon E, et al. Potentiation of etoposide and temozolomide cytotoxicity by curcumin and turmeric force™ in brain tumor cell lines. J Complement Integr Med. 2012;9:Article 20. | ||
Sreenivasan S, Krishnakumar S. Synergistic effect of curcumin in combination with anticancer agents in human retinoblastoma cancer cells lines. Curr Eye Res. 2014;11:1–13. | ||
Sánchez Y, Simón GP, Calviño E, et al. Curcumin stimulates reactive oxygen species production and potentiates apoptosis induction by the antitumor drugs arsenic trioxide and lonidamine in human myeloid leukemia cell lines. J Pharmacol Exp Ther. 2010;335:114–123. | ||
Yoshino M, Haneda M, Naruse M, et al. Prooxidant activity of curcumin: copper-dependent formation of 8-hydroxy-2′-deoxyguanosine in DNA and induction of apoptotic cell death. Toxicol In Vitro. 2004;18:783–789. | ||
Aggarwal BB, Deb L, Prasad S. Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses. Molecules. 2014;20(1):185–205. | ||
Wang K, Zhang T, Dong Q, et al. Redox homeostasis: the linchpin in stem cell self-renewal and differentiation. Cell Death Dis. 2013;4:e537. | ||
Kapiszewska M, Cierniak A, Elas M, et al. Lifespan of etoposide-treated human neutrophils is affected by antioxidant ability of quercetin. Toxicol In Vitro. 2007;21:1020–1030. | ||
Su CC, Yang JS, Lin SY, et al. Curcumin inhibits WEHI-3 leukemia cells in BALB/c mice in vivo. In Vivo. 2008;22:63–68. | ||
Papież MA, Krzyściak W. The dual effect of curcumin on etoposide action in leukemic and healthy bone marrow cells of rats with acute myeloid leukemia. Folia Med Cracov. 2014;54(2):71–79. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.