Back to Journals » OncoTargets and Therapy » Volume 13
CTHRC1 in Ovarian Cancer Promotes M2-Like Polarization of Tumor-Associated Macrophages via Regulation of the STAT6 Signaling Pathway
Authors Bai Y, Yin K, Su T , Ji F, Zhang S
Received 19 February 2020
Accepted for publication 18 May 2020
Published 17 June 2020 Volume 2020:13 Pages 5743—5753
DOI https://doi.org/10.2147/OTT.S250520
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjay Singh
Yihan Bai,* Kemin Yin,* Tong Su, Fang Ji, Shu Zhang
Department of Gynecology and Obstetrics, Shanghai Key Laboratory of Gynecology Oncology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shu Zhang; Fang Ji
Department of Gynecology and Obstetrics, Shanghai Key Laboratory of Gynecology Oncology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, 160 Pu Jian Road, Shanghai 200127, People’s Republic of China
Tel +86-2158752345
Fax +86-21-61557239
Email [email protected]; [email protected]
Purpose: The infiltration of tumor-associated macrophages (TAMs) facilitates the progression of epithelial ovarian cancer (EOC). TAMs are mainly M2-like due to exposure to various factors in the tumor microenvironment. In our previous study, we reported that collagen triple helix repeat containing 1(CTHRC1), a secreted protein, is associated with ovarian cancer progression and metastasis. However, the correlation between CTHRC1 and the immunological microenvironment in EOC remains unknown.
Methods: The association with the expression of CTHRC1 and CD68+CD163+ TAMs infiltration density and phosphorylation of STAT6 was analyzed in tumor tissues of ovarian cancer patients by immunohistochemistry. Western blot and flow cytometry analysis were used to analyze M2-like macrophage polarization induced by CTHRC1. Cell Counting Kit-8 and adhesion assays were used to detect cell proliferation and adhesion, respectively. Cell migration and invasion were detected using transwell assays.
Results: In the present study, we observed that the overexpression of CTHRC1 and increased TAMs infiltration density are closely correlated to an advanced stage of EOC. Meanwhile, CTHRC1 expression was positively associated with the infiltration density of M2-like CD68+CD163+TAMs and phosphorylation of STAT6 in EOC. In human PBMC-derived monocytes, recombinant CTHRC1 protein (rCTHRC1) induces an M2-like macrophage phenotype, in a dose-dependent manner, characterized by activating the STAT6 signaling pathway. The conditioned culture medium of Lenti-CTHRC1 EOC cells promoted M2 polarization of macrophages, and by contrast, CTHRC1 knockdown abolished STAT6-mediated M2 polarization of macrophages. Moreover, the culture supernatants of rCTHRC1-treated macrophages efficiently increased the migration and invasion abilities of ovarian cancer cells.
Conclusion: Our data indicate that CTHRC1 might play an important role in regulating M2 polarization of macrophages in the ovarian tumor microenvironment and suggest that it is a potential therapeutic target for antitumor immunity.
Keywords: CTHRC1, M2-like polarization macrophage, ovarian cancer, migration, invasion
Introduction
The main feature of ovarian cancer is metastasis, which is the leading cause of gynecologic cancer death. The tumor microenvironment (TME) has been regarded as a key contributor to the evolution of many cancers, including ovarian cancer.1–4 Various immune cells including myeloid and lymphoid cells are important constituents in TME. It is also worth noting that tumor-associated macrophages (TAMs) are the most abundant infiltrating immune cells the TME.5 TAMs have a strong environment-dependent plasticity and have been primarily classified into two major phenotypes—M1-like and M2-like macrophages—with opposite functions.6 Most studies indicated TAMs in malignant tumors as M2-like macrophages, which generally promote tumor growth and metastasis.7,8 Currently, it is found that macrophages can be recruited to the carcinoma tissue site through complex mechanisms and are further polarized into the M2-like subtype.9,10 It has been reported that increased levels of CCL2, CSF1 and VEGF-A are correlated with macrophage accumulation in breast, prostate, ovarian and lung tumor sites.11–13 Once macrophages are recruited to the tumor sites, they can be polarized into M2-like TAMs activated by tumor stroma-derived interleukin-4 (IL-4) or interleukin-13 (IL-13) via the STAT6 signaling pathway.14–17
Ovarian cancer is now the leading cause of death in women with gynecologic cancers. Recent studies have indicated that, in ovarian cancers where the protumor role of TAMs is dominant,18–21 ovarian cancer cells can recruit TAMs to the tumor site by upregulating TGF-β expression.22 Furthermore, ovarian cancer stem-like cells polarize TAMs into M2-like type via the COX-2/PGE2/JAK signaling pathway.23 Those suggest that M2-like macrophages are influenced by the surrounding TME and the dynamic interaction between M2-TAMs and TME, consequently favoring tumor progression and metastasis. Thus, advancing our understanding of the association between M2-TAMs and ovarian cancer cells might help us find a novel target for ovarian cancer therapies.
Collagen triple helix repeat containing 1 (CTHRC1) is an evolutionarily conserved extracellular matrix glycoprotein with a molecular weight of 28-kDa.24 It had been reported that CTHRC1 has important roles in promoting tumor progression in lung, gastrointestinal tract, ovary, breast, and pancreatic cancer.25–29 Our previous study indicated that CTHRC1 could promote ovarian cancer cells metastasis through the integrinβ3/FAK signaling pathway.29 Recently, CTHRC1 was found to be able to upregulate the number of macrophages via the TGF-β and Notch pathway during wound healing. We previously found that CTHRC1 could promote recruitment and infiltration of M2-like TAMs in endometrial cancer via the integrin-Akt signaling pathway.30 However, little is known about the association between CTHRC1 and TAMs in EOC.
In this study, we aimed to address the relationship between CTHRC1 expression and TAM level in EOC. Additionally, we examined the potential effect of CTHRC1 in M2-like macrophage polarization. Here, we showed that the level of TAM infiltration was correlated with CTHRC1 expression in EOC patients. We also detected that, in the tumor microenvironment, TAMs could be polarized into M2-like TAMs by CTHRC1 secreted from EOC cells through STAT6 signaling pathway activation and in turn, the polarized M2-like TAMs could promote the migration and invasion of ovarian cancer cells. Our findings revealed a novel role for CTHRC1 in activating the polarization of M2-like TAMs in TME and promoting tumor metastasis of EOC indirectly.
Materials and Methods
Cell Culture and Treatment
The human epithelial ovarian cancer (EOC) cell lines SKOV3 and HO8910 were purchased from the American Type Culture Collection (Rockville, MD, USA) and the Cell Bank of Chinese Academy of Science (Shanghai, China), respectively. SKOV3 cells were cultured in RPMI-1640 (Gibco, Foster City, CA) and HO8910 cells were maintained in DMEM/High Glucose (Gibco, Foster City, CA) supplemented with 10% fetal bovine serum (FBS, Gibco) and 1% penicillin-streptomycin (Gibco) at 37°C in 5% CO2. Peripheral venous blood was aseptically extracted and Ficoll-Hypaque was used for density gradient centrifugation. To acquire peripheral blood mononuclear cells (PBMCs), the buffy coat was aspirated, washed with PBS buffer, and evenly spread into a 6-well plate with 1×106 cells per well. Differentiation of PBMCs into monocyte-derived macrophages (MDMs) was performed according to our previous study.30
Human Tissue Collection
Primary human EOC tissues were collected from patients with primary ovarian cancer who underwent surgery in our hospital between 2016 and 2018. No patients received chemotherapy before surgery. Among the 50 primary ovarian cancer patients, 15 patients were affirmed to be FIGO stage I–II, and 35 patients were affirmed to be FIGO stage III–IV. The study was approved by the Institutional Ethics Committee of Ren Ji Hospital of Shanghai Jiao Tong University and was conducted following the ethical guidelines of the Declaration of Helsinki. All patients provided written informed consent prior to surgery.
Cells Transfection and Conditioned Media Concentration
The construction of the lentivirus vector (Lenti-CTHRC1 and Lenti-shCTHRC1) and transduction of SKOV3 cells were performed as described previously29 and verified by Western blot. Cells conditioned medium were centrifuged at 2000 g for 10 mins to clear cell debris and filtered with a 0.22 mm filter (Millipore). The cell conditioned media were referred to as SKOV3-NC medium, SKOV3-shCTHRC1 medium, SKOV3-CTHRC1 medium, and SKOV3-NC medium. Ultrafiltration tubes (Millipore 10KD 15 mL) were used to centrifuge conditioned media at 4000 g for 30 mins for the concentration.
Cell Migration and Invasion Assays
Cell migration and invasion were assessed using 24-well plates with 8 μm insert chambers (Corning, USA) transwell culture system. For the migration assay, cells (3×104 SKOV3 or HO8910) in serum-free medium were seeded onto the upper chambers. Six hundred and fifty microliters of conditioned medium of rCTHRC1-treated macrophages with 10% FBS was added into the bottom chambers. To perform the invasion assay, Matrigel (BD Biosciences, USA) was plated in the upper chambers. After incubation at 37°C for 12 hrs, cells (8×104 SKOV3 or HO8910) were seeded in the upper chamber. The conditioned medium was consistent with the migration assay. After 24 hrs, the cells on the bottom face of the insert chamber were stained with 0.1% crystal violet, photographed with LECAI DM 2500 (LECAI, Germany) and counted in five random fields for each sample.
Cell Adhesion Assays
In brief, exponentially growing cells were seeded in 96-well plates coated with vitronectin (Sigma, Germany) and incubated with the conditioned medium of rCTHRC1-treated macrophages for 4 hrs, 8 hrs and 12 hrs, at 37°C. After incubation, the non-adherent cells were removed and the adherent cells were treated with 10 μL/well CCK-8 reagent (Dojindo, Tokyo, Japan) and further incubated for 2 hrs at 37°C, under 5% CO2. Absorbance value was then read at 450 nm using Thermo Scientific Varioskan Flash (Thermo Fisher Scientific, USA).
Cell Proliferation Assays
3.5×103 cells per well were placed into 96-well plates. After incubation with conditioned medium of rCTHRC1-treated macrophages for 24 hrs, 48 hrs and 72 hrs, the medium was replaced with CCK-8 reagent (10 μL/well, Dojindo, Tokyo, Japan) and the cells were further incubated for 2 hrs at 37°C, under 5% CO2. Absorbance at 450 nm was measured with Thermo Scientific Varioskan Flash (Thermo Fisher Scientific, USA).
Western Blot
Lysis buffer composed with RIPA, 1% phenylmethanesulfonyl fluoride and 1% phosphatase inhibitor were used to obtain the total protein and the protein concentration was detected by the BCA protein assay kit. Ten percent SDS-PAGE was used for protein separation, followed by transfer onto PVDF membranes. Five percent BSA was used to block the membranes for 1 hr, and then incubated with the appropriate primary antibodies overnight, at 4ºC. Antibodies used in this study included CD163 (1:1000, Abcam, Cambridge, UK), CD206 (1:1000, Santa, USA), STAT6 (1:1000, Proteintech, Chicago USA), p-STAT6 (1:1000, CST, Boston, USA) and β-catenin (1:1000, Proteintech, Chicago, USA).
Immunohistochemistry (IHC)
A total of 50 paraffin-embedded human EOC tissues were assessed IHC staining. CTHRC1, CD68 and CD163 expression were measured with antibody-CTHRC1 (1:200, Proteintech Group, Chicago IL, USA), antibody-CD68 (1:200, Abcam, Cambridge, UK), antibody-CD163 (1:200, Abcam, Cambridge, UK) and antibody-pstat6 (1:500, Abcam, Cambridge, UK).The experimental steps followed standard procedures and score criteria were the same as in our previous study.30
Flow Cytometry Analysis
The cells were routinely resuspended in FACS staining buffer (BD Biosciences, NJ, USA) and stained with CD68 antibody (clone Y1/82A, BD Biosciences, NJ, USA), CD163 antibody (clone GHI/61, BD Biosciences, NJ, USA) or CD206 antibody (clone 19.2, BD Biosciences, NJ, USA) on ice for 30 min. The cells were further washed via centrifugation and resuspended in FACS buffer. Flow cytometry was conducted using an LSRII cytometer (BD Biosciences, NJ, USA). At least 20,000 events were acquired per sample and data were analyzed using BD FACSDIVA v6.1.3 software. Debris and dead cells were gated out using forward and side light scatter.
Statistical Analysis
All experiments were performed in triplicate. The data were presented as mean ± standard deviation (SD). Unpaired Student’s t-test was performed for comparison between the two groups. The Chi-square test was performed to account for clinicopathological characteristics. All analyses were performed using the SPSS17.0 software (SPSS Inc., Chicago, IL, USA). Value of P<0.05 was considered statistically significant.
Results
CTHRC1 High Expression and High Density of CD68+CD163+TAMs are Associated with Advanced-Stage EOC
Our former study has demonstrated that the CTHRC1 overexpression in EOC tissues was strongly related to advanced FIGO clinical stage, which predicts a poor prognosis of ovarian cancer.29 Several studies have shown that the EOC microenvironment plays an important role during tumor development and that TAMs are the main immunosuppressive cells that accelerate tumor growth.31–33
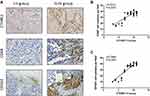
In this study, we not only found that protein levels of CTHRC1 had a positive correlation with clinical stage (FIGO III–IV) of EOC (P=0.002, Table 1) but also demonstrated that there was a significant relationship between CD68+CD163+ TAM density and FIGO stage of EOC (P=0.0033, Table 2). In 50 human EOC samples, we found that 91.4% (32 out of 35) of EOC in stage III–IV showed moderate to strong CTHRC1 staining in cell membrane and cytoplasm and that 60% (9 out of 15) of EOC in stage I–II showed weak staining (Table 1, Figure 1A). Consistent with our previous report, there is no association between CTHRC1 expression and patient age and tumor histologic grade. At the same time, we assessed the infiltration of CD68+ and CD163+ (M2-specific markers) TAMs in EOC. Of the 35 advanced stage (FIGO III–IV) cases, 29 (83%) showed high CD68+ TAM infiltration density and 25 (71.4%) showed high CD163+ TAM infiltration density, whereas in early stage cases (FIGO I–II), 6 (40%) showed high CD68+ TAM infiltration density and for the CD163+ TAMs and 4 (26.7%) showed high concentration (Table 2). Our data showed that high CD68+ and CD163+ TAM density tend to be associated with advanced FIGO stage (III–IV) of patients with EOC. Moreover, our results revealed that high CTHRC1 expression indicated high CD68+ (26/35, 74.3%) and CD163+ (29/35, 82.9%) TAM infiltration, while the infiltration density of CD68+/CD163+ TAMs was significantly lower in tissue samples with low CTHRC1 expression (Table 3). High CTHRC1 expression had a positive correlation with the high density of CD68+ TAMs and CD163+ TAMs (CD68 r=0.5723, CD163 r=0.7886, Figure 1B). Our findings indicated that CTHRC1 affected the differentiation of macrophages in EOC tissue.
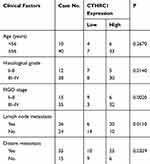 |
Table 1 Correlations Between CTHRC1 Expression Levels in Ovarian Cancer and Clinical Features |
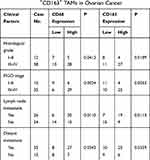 |
Table 2 Correlation Between Clinical Features and Infiltration Density of CD68+CD163+ TAMs in Ovarian Cancer |
 |
Table 3 Correlations Between CTHRC1, CD68+ and CD163+ TAMs Expression Levels |
CTHRC1 Polarized M2-Like Macrophages Through the STAT6 Signaling Pathway
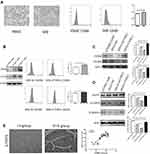
Multiple studies have suggested that M2-like polarized TAMs play an important role in solid tumor progression and metastasis.10,18,30,34,35 We investigated if CTHRC1 expression may promote macrophage polarization into an M2-like phenotype. We evaluated the expression levels of CD206 and CD163, M2 phenotype markers, on human macrophages derived from peripheral blood after treatment of rCTHRC1. Human peripheral blood mononuclear cells (PBMCs) were harvested and cultured with 50 ng/mL M-CSF for 7 days until differentiated into macrophages, as previously described.30 As shown in Figure 2A, after the treatment of M-CSF, the morphological characteristics of macrophages were observed under a microscope. Besides, the flow cytometry assay showed that the expression of CD68, a cell surface marker for macrophages, was higher in PBMC-derived macrophages (*P<0.05). Because the CTHRC1 is a secreted protein, we first analyzed the functional role of recombinant CTHRC1 (rCTHRC1) on M2-like macrophage polarization in vitro. We found that the expression of classical M2 phenotype markers (CD206 and CD163) had been significantly increased by rCTHRC1 in a dose-dependent manner (Figure 2B), suggesting that CTHRC1 could be involved in M2-like polarization of macrophages. Furthermore, flow cytometry analysis showed that 5 nM rCTHRC1 treatment increased CD206 and CD163 expression in macrophages (Figure 2B, *P<0.05). Next, we investigated the crosstalk between EOC cells and macrophages in a co-culture system. Stable CTHRC1 knockdown (SKOV3-siCTHRC1) and CTHRC1 over-expression (SKOV3-CTHRC1) cells were established as previously described.29 Similar to the treatment of rCTHRC1 (5 nM), the addition of culture supernatants of SKOV3-CTHRC1 cells increased CD206 and CD163 expression in PBMC-derived macrophages (Figure 2C, *P<0.05, **P<0.001, ***P<0.0001). However, the expression of CD206 and CD163 in macrophages was reduced after co-culture with supernatants of SKOV3-siCTHRC1 cells. These results supported our hypothesis that CTHRC1 expression in ovarian cancer promotes M2-like macrophage polarization.
Next, we explored the underlying mechanism involved in macrophage polarization by CTHRC1. It has been reported that Wnt/β-catenin/STAT6 signaling can activate the differentiation of human monocytes into macrophages36–38 and CTHRC1 promotes tumor invasion and metastasis in ovarian cancer39 and gastrointestinal stromal tumors40 by activating the Wnt/β-catenin pathway. We wondered whether CTHRC1 could activate Wnt/β-catenin signaling in macrophage polarization at the same time in EOC. We found that after co-culture with 5 nM rCTHRC1, both β-catenin expression and phosphorylation of STAT6 were significantly upregulated in macrophages compared with control, determined by Western blot (Figure 2D, *P<0.05, **P<0.001, ***P<0.0001). Following treatment of culture supernatants of SKOV3-CTHRC1 cells, both β-catenin expression and STAT6 phosphorylation levels increased dramatically in macrophages and decreased when treated with SKOV3-siCTHRC1 cell supernatant (Figure 2D, *P<0.05, **P<0.001, ***P<0.0001). In order to relate our in vitro findings to actual human patient tumor specimens, we investigated the levels of phosphorylated STAT6 by immunohistochemistry in tissue samples of ovarian cancer patients. We found that among the 35 advanced stage (FIGO III–IV) cases, 26 (74%) showed high phosphorylation of STAT6 while in 15 early-stage(FIGO I–II) cases, 5 (33%) showed high phosphorylation of STAT6. Our results suggested that the levels of phosphorylated STAT6 were higher in the advanced FIGO stage (FIGO III–IV) and the over-expression of CTHRC1 in EOC was strongly correlated with the phosphorylation of STAT6 (r=0.7037, Figure 2E). Taken together, our results preliminarily suggested that overexpression of CTHRC1 promotes M2-like macrophage polarization via the β-catenin/STAT6 signaling pathway.
Polarized Macrophages Promote Ovarian Cancer Cell Migration and Invasion
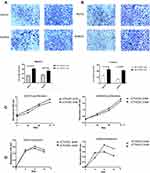
There is growing evidence30–34 suggesting that M2-like macrophages are involved in tumor progression. To explore the effects of rCTHRC1-initiated M2-like macrophages on ovarian cancer cells, we assessed the proliferation, migration, invasion and adhesion in SKOV3 and HO8910 ovarian cancer cells after incubation with the conditioned medium of polarized M2-like macrophages. We found that, after treatment of rCTHRC1, the conditioned medium of macrophages increased migration and invasion abilities of SKOV3 and HO8910 cells significantly when compared to the control (Figure 3A and B, *P<0.05, ***P<0.0001). However, rCTHRC1-treated macrophages have no effect on their proliferation and adhesion abilities (P>0.05, P>0.05, Figure 3C and D). Our results indicated that CTHRC1-initiated M2-like macrophages promoted EOC cell migration and metastasis.
Discussion
Recently, CTHRC1 has been recognized as a positive regulator of cancer growth, invasion, metastasis and angiogenesis in many kinds of cancer, including hepatocellular carcinoma, colorectal cancer, lung cancer and gynecologic cancers.9,29,30,35,41 We also found that CTHRC1 could promoted endometrial cancer myometrial invasion by recruiting M2-like TAMs.30 In the present study, we showed for the first time that number of M2-type TAMs in clinical ovarian cancer tumor tissues was positively correlated with CTHRC1 expression. This prompted us to investigate whether CTHRC1 can mediate macrophage polarization. In cancers, TAM recruitment and polarization play an important role in regulating tumor growth and invasion.8–10,17,36 In ovarian cancer, it was reported that M2-polarized TAMs induced the stemness characteristics of SKOV3 cell via the IL8/STAT3 signaling pathway.42 M2-polarized TAMs also induced metastasis of ovarian cancer through activated toll-like receptors pathway.20 Furthermore, macrophages-derived exosomes elicited a chemoresistant phenotype in ovarian cancer cells.43 TAMs need to be polarized into M2-like subtype to play a role in promoting tumor progression. In tumor tissues, macrophage polarization is regulated by various tumor microenvironmental signals derived from tumor cells.30,42–44 The polarization of TAMs is mainly mediated by the JAK/STAT, PI3K/Akt and Wnt/β-catenin signaling pathways. In pancreatic ductal adenocarcinoma, macrophages could be polarized into M2 phenotype through activated JAK/STAT3 signaling pathway.45 Lu et al reported that in animal models of emphysema, polarization of M2 macrophages could be regulated via the PI3K/AKT pathway.46 It is well documented that in hepatocellular carcinoma cells, the canonical Wnt/β-catenin signaling pathway was activated in M2-polarized macrophages.36 Deng et al reported that ovarian cancer stem cells (OCSCs) induced the M2-like macrophages through the PPARγ and NF-κB pathways;47 however, another researcher discovered that ovarian cancer stem cells elicited macrophages polarization via the COX/PGE2/JAK signaling pathway in a co-culture system of OCSCs and macrophages.23 In this study we found that both rCTHRC1 and overexpression of CTHRC1 in ovarian cancer cells induced the M2 polarization in vivo. Furthermore, we demonstrated that M2-type macrophages polarized by CTHRC1 promoted the migration and invasion of ovarian cancer cells in vivo.
In tumor microenvironment, tumor cell-derived molecules could promote M2 phenotype TAM polarization. The signal transducer and activator of transcription (STAT) protein family has been recognized as a potential activator in promotion of M2 macrophages.45,48-50 Studies have shown that STAT6 (a critical member of the STAT family) was potentially involved in regulating the M2 phenotypic macrophage activation.51 As studies have shown that after treatment with a glycoprotein, the M2-like surface markers of macrophages, including CD206, CD163 and IL-10 were upregulated while M1-like surface markers were downregulated in a STAT3 and STAT6-dependent.50 Of interest, Cosin-Roger et al have reported that a STAT6-dependent macrophage phenotype mediated mucosal repair by activating the Wnt signaling pathway.51 Previous studies have reported that β-catenin as a transcription was highly expressed in the EOC and STAT6 was identified as the downstream molecule of the Wnt/β-catenin signaling pathway.52,53 In this study, we found that STAT6 phosphorylation was upregulated after treatment with rCTHRC1 in M2-like macrophages, suggesting there appears to be a correlation between CTHRC1 over-expression and STAT6 phosphorylation. To determine whether CTHRC1 regulates STAT6 activation in EOC, we analyze the levels of phosphorylated STAT6 in EOC tissue samples. Our results showed that phosphorylated STAT6 was highly expressed in the advanced stage (FIGO III–IV) and statistical analysis revealed a strong correlation between CTHRC1 and phosphorylated STAT6 expression in EOC. Therefore, we concluded that CTHRC1 promoted macrophages towards M2-like TAMs via the STAT6 signaling pathway.
Conclusions
In summary, our study reports a novel role for CTHRC1 in promoting M2-TAM polarization and inducing ovarian cancer metastasis. We demonstrated that CTHRC1 activated M2-TAM polarization by activating STAT6 phosphorylation. These findings suggest that further understanding of the molecular mechanisms in the regulation of macrophage polarization might provide a new target for the therapy of ovarian cancer.
Abbreviations
TAM, tumor-associated macrophages; EOC, epithelial ovarian cancer; CTHRC1, collagen triple helix repeat containing 1; IHC, immunohistochemistry; TME, tumor microenvironment; PBMC, peripheral blood mononuclear cells; STAT, signal transducer and activator of transcription.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (ID: 81672564 to S. Zhang). Yihan Bai and Kemin Yin are co-first authors for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siveen KS, Raza A, Ahmed EI, et al. The role of extracellular vesicles as modulators of the tumor microenvironment, metastasis and drug resistance in colorectal cancer. Cancers. 2019;11(6):746. doi:10.3390/cancers11060746
2. Racioppi L, Nelson ER, Huang W, et al. CaMKK2 in myeloid cells is a key regulator of the immune-suppressive microenvironment in breast cancer. Nat Commun. 2019;10(1):2450. doi:10.1038/s41467-019-10424-5
3. Shi JJ, Xing H, Wang YX, et al. PI3Kα inhibitors sensitize esophageal squamous cell carcinoma to radiation by abrogating survival signals in tumor cells and tumor microenvironment. Cancer Lett. 2019;459:145–155. doi:10.1016/j.canlet.2019.05.040
4. Wen Z, Liang Y, Deng S, et al. Talin2 regulates invasion of human breast cancer MDA-MB-231 cells via alteration of the tumor microenvironment. Oncol Lett. 2019;17(6):4835–4842. doi:10.3892/ol.2019.10175
5. Stakheyeva M, Riabov V, Mitrofanova I, et al. Role of the immune component of tumor microenvironment in the efficiency of cancer treatment: perspectives for the personalized therapy. Curr Pharm Des. 2017;23(32):4807–4826. doi:10.2174/1381612823666170714161703
6. Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229(2):176–185. doi:10.1002/path.4133
7. Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi:10.1016/j.cell.2010.03.014
8. Biswas SK, Gangi L, Paul S, et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation). Blood. 2006;107(5):2112–2122. doi:10.1182/blood-2005-01-0428
9. Yin Z, Ma T, Lin Y, et al. IL-6/STAT3 pathway intermediates M1/M2 macrophage polarization during the development of hepatocellular carcinoma. J Cell Biochem. 2018;119(11):9419–9432. doi:10.1002/jcb.27259
10. Martinez VG, Rubio C, Martinez-Fernandez M, et al. BMP4 induces M2 macrophage polarization and favors tumor progression in bladder cancer. Clin Cancer Res. 2017;23(23):7388–7399. doi:10.1158/1078-0432.CCR-17-1004
11. Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–225. doi:10.1038/nature10138
12. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–1437. doi:10.1038/nm.3394
13. Linde N, Lederle W, Depner S, van Rooijen N, Gutschalk CM, Mueller MM. Vascular endothelial growth factor-induced skin carcinogenesis depends on recruitment and alternative activation of macrophages. J Pathol. 2012;227(1):17–28. doi:10.1002/path.3989
14. Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61. doi:10.1016/j.immuni.2014.06.010
15. Waqas SFH, Ampem G, Roszer T. Analysis of IL-4/STAT6 signaling in macrophages. Methods Mol Biol. 2019;1966:211–224.
16. Gupta S, Jain A, Syed SN, et al. IL-6 augments IL-4-induced polarization of primary human macrophages through synergy of STAT3, STAT6 and BATF transcription factors. Oncoimmunology. 2018;7(10):e1494110. doi:10.1080/2162402X.2018.1494110
17. Rahal OM, Wolfe AR, Mandal PK, et al. Blocking interleukin (IL)4- and IL13-mediated phosphorylation of STAT6 (Tyr641) decreases M2 polarization of macrophages and protects against macrophage-mediated radioresistance of inflammatory breast cancer. Int J Radiat Oncol Biol Phys. 2018;100(4):1034–1043. doi:10.1016/j.ijrobp.2017.11.043
18. Zeng XY, Xie H, Yuan J, et al. M2-like tumor-associated macrophages-secreted EGF promotes epithelial ovarian cancer metastasis via activating EGFR-ERK signaling and suppressing lncRNA LIMT expression. Cancer Biol Ther. 2019;20(7):956–966. doi:10.1080/15384047.2018.1564567
19. Colvin EK. Tumor-associated macrophages contribute to tumor progression in ovarian cancer. Front Oncol. 2014;4:137. doi:10.3389/fonc.2014.00137
20. Ke X, Zhang S, Wu M, et al. Tumor-associated macrophages promote invasion via Toll-like receptors signaling in patients with ovarian cancer. Int Immunopharmacol. 2016;40:184–195. doi:10.1016/j.intimp.2016.08.029
21. Yin M, Li X, Tan S, et al. Tumor-associated macrophages drive spheroid formation during early transcoelomic metastasis of ovarian cancer. J Clin Invest. 2016;126(11):4157–4173. doi:10.1172/JCI87252
22. Tang M, Liu B, Bu X, Zhao P. Cross-talk between ovarian cancer cells and macrophages through periostin promotes macrophage recruitment. Cancer Sci. 2018;109(5):1309–1318. doi:10.1111/cas.13567
23. Zhang Q, Cai DJ, Li B. Ovarian cancer stem-like cells elicit the polarization of M2 macrophages. Mol Med Rep. 2015;11(6):4685–4693. doi:10.3892/mmr.2015.3323
24. Pyagay P, Heroult M, Wang Q, et al. Collagen triple helix repeat containing 1, a novel secreted protein in injured and diseased arteries, inhibits collagen expression and promotes cell migration. Circ Res. 2005;96(2):261–268. doi:10.1161/01.RES.0000154262.07264.12
25. Ma MZ, Zhuang C, Yang XM, et al. CTHRC1 acts as a prognostic factor and promotes invasiveness of gastrointestinal stromal tumors by activating Wnt/PCP-Rho signaling. Neoplasia (New York, NY). 2014;16(3):
26. Kim JH, Baek TH, Yim HS, et al. Collagen triple helix repeat containing-1 (CTHRC1) expression in invasive ductal carcinoma of the breast: the impact on prognosis and correlation to clinicopathologic features. Pathol Oncol Res. 2013;19(4):731–737. doi:10.1007/s12253-013-9636-y
27. Park EH, Kim S, Jo JY, et al. Collagen triple helix repeat containing-1 promotes pancreatic cancer progression by regulating migration and adhesion of tumor cells. Carcinogenesis. 2013;34(3):694–702. doi:10.1093/carcin/bgs378
28. He W, Zhang H, Wang Y, et al. CTHRC1 induces non-small cell lung cancer (NSCLC) invasion through upregulating MMP-7/MMP-9. BMC Cancer. 2018;18(1):400. doi:10.1186/s12885-018-4317-6
29. Guo B, Yan H, Li L, Yin K, Ji F, Zhang S. Collagen triple helix repeat containing 1 (CTHRC1) activates Integrin beta3/FAK signaling and promotes metastasis in ovarian cancer. J Ovarian Res. 2017;10(1):69. doi:10.1186/s13048-017-0358-8
30. Li LY, Yin KM, Bai YH, Zhang ZG, Di W, Zhang S. CTHRC1 promotes M2-like macrophage recruitment and myometrial invasion in endometrial carcinoma by integrin-Akt signaling pathway. Clin Exp Metastasis. 2019;36(4):351–363. doi:10.1007/s10585-019-09971-4
31. Zhang LL, Zhang LF, Shi YB. Down-regulated paxillin suppresses cell proliferation and invasion by inhibiting M2 macrophage polarization in colon cancer. Biol Chem. 2018;399(11):1285–1295. doi:10.1515/hsz-2018-0002
32. Liu L, Wang X, Li X, Wu X, Tang M, Wang X. Upregulation of IGF1 by tumor-associated macrophages promotes the proliferation and migration of epithelial ovarian cancer cells. Oncol Rep. 2018;39(2):818–826. doi:10.3892/or.2017.6148
33. Winslow S, Scholz A, Rappl P, et al. Macrophages attenuate the transcription of CYP1A1 in breast tumor cells and enhance their proliferation. PLoS One. 2019;14(1):e0209694. doi:10.1371/journal.pone.0209694
34. Guo Z, Song J, Hao J, et al. M2 macrophages promote NSCLC metastasis by upregulating CRYAB. Cell Death Dis. 2019;10(6):377. doi:10.1038/s41419-019-1618-x
35. Chen Y, Zhang S, Wang Q, Zhang X. Tumor-recruited M2 macrophages promote gastric and breast cancer metastasis via M2 macrophage-secreted CHI3L1 protein. J Hematol Oncol. 2017;10(1):36. doi:10.1186/s13045-017-0408-0
36. Yang Y, Ye YC, Chen Y, et al. Crosstalk between hepatic tumor cells and macrophages via Wnt/beta-catenin signaling promotes M2-like macrophage polarization and reinforces tumor malignant behaviors. Cell Death Dis. 2018;9(8):793. doi:10.1038/s41419-018-0818-0
37. L’Episcopo F, Tirolo C, Serapide MF, et al. Microglia polarization, gene-environment interactions and wnt/beta-catenin signaling: emerging roles of glia-neuron and glia-stem/neuroprogenitor crosstalk for dopaminergic neurorestoration in aged parkinsonian brain. Front Aging Neurosci. 2018;10:12. doi:10.3389/fnagi.2018.00012
38. Feng Y, Ren J, Gui Y, et al. Wnt/beta-catenin-promoted macrophage alternative activation contributes to kidney fibrosis. J Am Soc Nephrol. 2018;29(1):182–193. doi:10.1681/ASN.2017040391
39. Hou M, Cheng Z, Shen H, et al. High expression of CTHRC1 promotes EMT of epithelial ovarian cancer (EOC) and is associated with poor prognosis. Oncotarget. 2015;6(34):35813–35829. doi:10.18632/oncotarget.5358
40. Liu J, Li W, Liu S, et al. Knockdown of collagen triple helix repeat containing 1 (CTHRC1) inhibits epithelial-mesenchymal transition and cellular migration in glioblastoma cells. Oncol Res. 2017;25(2):225–232. doi:10.3727/096504016X14732772150587
41. Chen W, Xu Y, Zhong J, et al. MFHAS1 promotes colorectal cancer progress by regulating polarization of tumor-associated macrophages via STAT6 signaling pathway. Oncotarget. 2016;7(48):78726–78735. doi:10.18632/oncotarget.12807
42. Ning Y, Cui Y, Li X, et al. Co-culture of ovarian cancer stem-like cells with macrophages induced SKOV3 cells stemness via IL-8/STAT3 signaling. Biomed Pharmacother. 2018;103:262–271. doi:10.1016/j.biopha.2018.04.022
43. Zhu X, Shen H, Yin X, et al. Macrophages derived exosomes deliver miR-223 to epithelial ovarian cancer cells to elicit a chemoresistant phenotype. J Exp Clin Cancer Res. 2019;38(1):81. doi:10.1186/s13046-019-1095-1
44. Chen XJ, Deng YR, Wang ZC, et al. Hypoxia-induced ZEB1 promotes cervical cancer progression via CCL8-dependent tumour-associated macrophage recruitment. Cell Death Dis. 2019;10(7):508. doi:10.1038/s41419-019-1748-1
45. Salmiheimo A, Mustonen H, Vainionpaa S, et al. Tumour-associated macrophages activate migration and STAT3 in pancreatic ductal adenocarcinoma cells in co-cultures. Pancreatology. 2017;17(4):635–641. doi:10.1016/j.pan.2017.04.013
46. Lu J, Xie L, Liu C, Zhang Q, Sun S. PTEN/PI3k/AKT regulates macrophage polarization in emphysematous mice. Scand J Immunol. 2017;85(6):395–405. doi:10.1111/sji.12545
47. Deng X, Zhang P, Liang T, Deng S, Chen X, Zhu L. Ovarian cancer stem cells induce the M2 polarization of macrophages through the PPARgamma and NF-kappaB pathways. Int J Mol Med. 2015;36(2):449–454. doi:10.3892/ijmm.2015.2230
48. Szelag M, Piaszyk-Borychowska A, Plens-Galaska M, Wesoly J, Bluyssen HA. Targeted inhibition of STATs and IRFs as a potential treatment strategy in cardiovascular disease. Oncotarget. 2016;7(30):48788–48812. doi:10.18632/oncotarget.9195
49. Yokota N, Burne-Taney M, Racusen L, Rabb H. Contrasting roles for STAT4 and STAT6 signal transduction pathways in murine renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2003;285(2):F319–F325. doi:10.1152/ajprenal.00432.2002
50. Choi JW, Kwon MJ, Kim IH, Kim YM, Lee MK, Nam TJ. Pyropia yezoensis glycoprotein promotes the M1 to M2 macrophage phenotypic switch via the STAT3 and STAT6 transcription factors. Int J Mol Med. 2016;38(2):666–674. doi:10.3892/ijmm.2016.2656
51. Cosin-Roger J, Ortiz-Masia D, Calatayud S, Hernandez C, Esplugues JV, Barrachina MD. The activation of Wnt signaling by a STAT6-dependent macrophage phenotype promotes mucosal repair in murine IBD. Mucosal Immunol. 2016;9(4):986–998. doi:10.1038/mi.2015.123
52. Hai Zhu HG. Increased Expression of PITX2, LEF-1,beta-Catenin in epithelial ovarian cancer. J Cytol Histol. 2015;06(04). doi:10.4172/2157-7099.1000333
53. Gong M, Zhuo X, Ma A. STAT6 upregulation promotes M2 macrophage polarization to suppress atherosclerosis. Med Sci Monit Basic Res. 2017;23:240–249. doi:10.12659/MSMBR.904014
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.