Back to Journals » Cancer Management and Research » Volume 13
CT-Guided 125I Brachytherapy in the Treatment of Hepatocellular Carcinoma Refractory to Conventional Transarterial Chemoembolization: A Pilot Study
Authors Xu X, Ding Y, Pan T, Gao F, Huang X, Sun Q
Received 4 February 2021
Accepted for publication 25 March 2021
Published 15 April 2021 Volume 2021:13 Pages 3317—3326
DOI https://doi.org/10.2147/CMAR.S305422
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bilikere Dwarakanath
Xinjian Xu,1,* Yiwen Ding,1,* Tianfan Pan,1 Feng Gao,1 Xiangzhong Huang,1 Qiulian Sun2
1Department of Interventional Radiology, Jiangyin People’s Hospital, Jiang Yin City, Jiangsu Province, 214400, People’s Republic of China; 2Department of Radiology, Zhejiangtaizhou Hospital, Taizhou City, Zhejiang Province, 317000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiangzhong Huang; Qiulian Sun Email [email protected]; [email protected]
Purpose: To investigate the efficacy and safety of CT-guided 125I brachytherapy in the treatment of hepatocellular carcinoma (HCC) refractory to conventional transarterial chemoembolization (TACE).
Methods: Nineteen patients with TACE-refractory HCC treated with CT-guided 125I brachytherapy between June 2017 and June 2020 at Jiangyin People’s Hospital were enrolled in this study. In addition, we used the modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria to evaluate the treatment response after 125I brachytherapy.
Results: Twenty-one tumours were treated with CT-guided 125I brachytherapy in nineteen patients. Twelve tumours (57.1%) showed a complete response, and a partial response was observed in seven tumours (33.3%). The six-month objective response rate was 90.5% (19/21). The adverse effects of CT-guided 125I brachytherapy were tolerable.
Conclusion: Our preliminary clinical experience demonstrated that CT-guided 125I brachytherapy was effective and well tolerated for the treatment of TACE-refractory HCC, suggesting that CT-guided 125I brachytherapy has the potential to become an effective alternative treatment for TACE-refractory HCC.
Keywords: 125I brachytherapy, TACE, refractory, hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumours worldwide.1 Recently, the China National Cancer Center reported that HCC is the fourth most common malignancy and the second leading cause of cancer-related deaths in China.2 Moreover, China accounts for half of new HCC cases as well as HCC-related deaths worldwide.3,4
Transarterial chemoembolization (TACE) is the current standard therapy for unresectable intermediate-stage HCC.5,6 However, some HCC patients receiving repetitive TACE showed poor response to this treatment, such as poor tumour response, new lesions, extrahepatic metastasis, vascular invasion, or continued elevation of tumour markers. The Japan Society of Hepatology (JSH) and the Liver Cancer Study Group of Japan (LCSGJ) defined this phenomenon as “refractoriness or failure to TACE”7
For TACE-refractory HCC, continued TACE therapy would result in increased side effects, deteriorated liver function, or even reduced survival time.8,9 Therefore, switching the treatment modality for TACE-refractory HCC is necessary. Although sorafenib was recommended for the treatment of intermediate-stage HCC patients refractory to TACE,10 the main drawbacks of sorafenib, including a relatively low response rate,11,12 a common occurrence of drug resistance,13 intolerable adverse reactions14 (eg, severe hand-foot syndrome, severe diarrhoea, and intractable nausea or vomiting) and high drug expense,15 may limit its application in the treatment of TACE-refractory HCC. Consequently, more alternative treatments with high effectiveness and safety for TACE-refractory HCC continue to be required.
The data from several studies suggested that external beam radiotherapy could achieve good efficacy (> 85% local control) in treating HCC.16 However, external beam radiotherapy was associated with a relatively high occurrence rate (5–50%) of radiation-induced liver disease (RILD) related to the high dose of radiation.17,18 Iodine-125 (125I) is a radioisotope that emits low energy γ-rays and has a half-life of 60 days. The radiation diameter of radioactive 125I seeds for tissue is 1.7 cm. Theoretically, 125I brachytherapy could induce tumour cell apoptosis and spare the surrounding nontumour tissues, thus maximizing the antitumour effect and minimizing the side effects.19 Previous studies showed that 125I brachytherapy yielded good clinical efficacy and safety in patients with hepatic malignant tumours.20–24 However, evidence regarding 125I brachytherapy for TACE-refractory HCC is still lacking. Therefore, we conducted CT-guided 125I brachytherapy on seventeen patients with TACE-refractory HCC and evaluated the efficacy and safety of this treatment in this study.
Materials and Methods
Patients Refractory to Conventional TACE
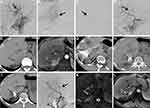
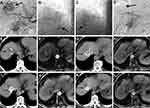
Between June 2017 and June 2020, 246 patients underwent TACE at Jiangyin People’s Hospital. Of those patients, nineteen consecutive patients with TACE-refractory HCC treated with CT-guided 125I brachytherapy were included in this study. A total of twenty-one tumours refractory to conventional TACE in the nineteen patients were analysed. TACE-refractory HCC (Figures 1A–D and 2A–D) was defined as those with more than two consecutive incomplete necrosis (lipiodol deposition <50%) (Figures 1E, F and 2E, F) on CT and/or MRI which were employed to evaluate tumour response to TACE.7 The tumour response evaluation with CT and MRI was performed at 1 to 2 months after conventional TACE.
The Procedure of the Preoperative Plan and CT-Guided 125I Brachytherapy
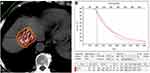
Abdominal CT images were obtained using spiral CT (PHILIPS Brilliance CT 64, Philips Healthcare, Netherlands) <1 week before the procedure. The DICOM format images were then imported into the Prowess treatment planning system (TPS) (Prowess Panther TPS, Chico, USA). The planning target volume of each tumour was delineated in every slice. A dose-volume histogram was generated by the TPS with a prescribed matched peripheral dose (MPD) of 130–160 Gy. The number of 125I seeds and isodose curves were calculated. The dose distribution of the target tumour and risk organs was demonstrated. The implantation position and the puncture approach were also planned based on the location of the target tumour.24 Following the planned positions and the puncture approach, an 18G puncture needle was inserted into the targeted tumour under CT guidance, and 125I seeds (activity,0.6mCi; manufacturer, Shanghai Xinke Pharmaceutical Co., Ltd, China) were implanted into tumour tissue in compliance with the plan (Figures 1G and 2G). Repeat CT was performed to check for possible complications. The image was imported into the TPS to verify the dose distribution (Figure 3A and B). Postoperative antibiotics and haemostatics were routinely administered for three days.
Assessment of the Therapeutic Effects
The antitumour effect was evaluated by comparing non-enhanced CT and dynamic enhanced MRI imaging before treatment to imaging 1, 2, 4, and 6 months after the CT-guided 125I brachytherapy procedure (Figures 1H, I, K, L and 2H–L), selective angiography was also performed if necessary (Figure 1J). The Modified Response Evaluation Criteria in Solid Tumors (mRECIST)25 criteria were employed to evaluate the treatment response of the target tumour (see the Appendix).
Statistical Analysis
In this study, normally distributed data are represented by the mean ± SD; otherwise, data are represented by the median. Categorical variables are represented by frequencies. Statistical analyses were conducted with SPSS version 18.0 software (SPSS Inc., Chicago, IL, USA).
Results
Patients and Tumour Characteristics
The baseline characteristics of the patients with TACE-refractory HCC are shown in Table 1. There were eighteen males and one female, aged 43 to 80 years, with a median age of 69 years. All nineteen patients had hepatitis B virus infection. Three patients were at stage A, and sixteen patients were at stage B based on the Barcelona Clinic for Liver Cancer (BCLC) staging classification. The mean tumour size was 4.3 cm before 125I brachytherapy. One tumour was of the infiltrating type, two were multinodular, and the others were of the nodular type. A median of 4 rounds (range, 2 to 8 times) of conventional TACE was performed in these patients before CT-guided 125I brachytherapy.
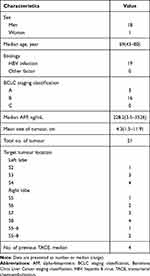 |
Table 1 Baseline Characteristics of HCC Patients Refractory to Conventional TACE |
Six-Month Tumour Response After CT-Guided 125I Brachytherapy
Six months after CT-guided 125I brachytherapy, CR was observed in twelve tumours (57.1%), PR was observed in seven tumours (33.3%), and SD was observed in two tumours (9.5%) (Table 2) according to the mRECIST criteria. The six-month objective response rate was 90.5% (19/21), and the six-month survival rate was 100% (21/21).
 |
Table 2 Response to Conventional TACE and CT-Guided 125I Brachytherapy |
CT-Guided 125I Brachytherapy-Related Complications
No serious intraoperative complications, such as massive bleeding, intestinal fistula, intestinal bleeding, bile fistula, or infection, occurred. CT-guided 125I brachytherapy-related complications included slight pneumothorax and slight perihepatic haemorrhage. Pneumothorax occurred in 3 cases, and perihepatic haemorrhage occurred in one case (Table 2). All these minor complications were tolerable, and they were managed conservatively with a favourable outcome.
Clinical Courses After CT-Guided 125I Brachytherapy
The clinical courses after CT-guided 125I brachytherapy are listed in Table 3. Four patients died, and fifteen patients survived to the end of the follow-up. Seven patients eventually suffered disease progression, and the median time to progression was 264 days. After 125I brachytherapy, additional treatments such as TACE, TIPSS, or sorafenib were given as necessary.
 |
Table 3 Clinical Courses After CT-Guided 125I Brachytherapy |
Discussion
TACE has been recommended as the standard therapy for unresectable intermediate and advanced liver cancer. However, for patients with TACE-refractory HCC, continuous TACE damages liver function and even shortens the life of the patients; therefore, effective treatment for TACE-refractory HCC is necessary.
Currently, the exact mechanism of TACE-refractory HCC is still not clear, and several possible explanations may be responsible for the occurrence of TACE-refractory HCC. First, HCC has a dual blood supply. The blood supply to the central area of the HCC is mainly from the hepatic artery, while the blood supply to the peripheral area is mainly from the portal vein. TACE could stop the blood supply to the central area of the tumour, thus leading to ischemic necrosis. However, the blood supply to the marginal area of the tumour is less affected by TACE, so residual cancer cells could lead to HCC recurrence.9 Second, lipiodol deposited in the tumour after TACE may be washed out by the blood flow or engulfed by Kupffer cells in the liver,26 which may affect the tumour response. Third, the acquisition of resistance to chemotherapeutic drugs after repeated TACE could also lead to TACE refractoriness.27 Fourth, the tumour tissue was in a state of ischemia and hypoxia after repeated TACE, which could upregulate VEGF expression and promote angiogenesis and revascularization, leading to tumour recurrence and metastasis.28,29 In addition, the HGF/c-Met signalling pathway plays an important role in tumour cell infiltration and metastasis, ischemia, and hypoxia after repeated TACE and can upregulate the expression of c-Met in HCC, resulting in tumour resistance and metastasis.30
HCC is sensitive to radiation, and previous studies have demonstrated that external beam radiotherapy with an effective dose (>60 Gy) could achieve a complete response in HCC.31,32 However, the maximum tolerated dose of normal liver tissue was 30 Gy;33 therefore, external beam radiotherapy for HCC was limited by radiation-induced liver injury. The 125I seeds (diameter 0.8 mm, length 4.5 mm) could emit continuous low-doseγ-rays (27.4–35.5 keV) with an effective tissue penetration of 17 mm. It was previously believed that γ-rays could cause unrepairable damage to cancer cells by damaging DNA (DNA double-strand breaks, single-strand breaks, and free radical damage).23 Recently, 125I brachytherapy was used to treat HCC with promising therapeutic effects.
Compared with external beam radiotherapy, 125I brachytherapy has several physical and biological advantages. The effective tissue penetration of 125I seeds was approximately 1.7 cm, and its effective radiation was mainly focused on the targeted tumour area, with minimal effects on normal liver tissue.21 In one session of external beam radiotherapy, only cancer cells in the sensitive phase (M phase and G2 phase) of the tumour cell cycle could be easily killed, whereas cancer cells in the non-sensitive phase (S phase) of the tumour cell cycle had resistance to radiation. However, the γ-rays released by 125I seeds could continue irradiating tumours for up to 200 days. During this period, the ratio of cells in the sensitive and non-sensitive phases of the cell cycle became redistributed, thereby increasing the total radiation dose of tumour cells in the sensitive phase, which would help to improve the killing effect of radiation on tumour cells.19 Moreover, although the dose rate of brachytherapy with 125I seeds was lower than that of external beam radiotherapy, continuous low-dose γ-rays could cause significant damage to cancer cells because of the cumulative effects of radiation. It was reported that the accumulated dose within the local tissue during the half-life of 125I could reach up to 120–160 Gy, which could cause devastating damage to the tumour in the target area.34 In the present study, the matched peripheral dose of the patients who received brachytherapy was 130–160 Gy, which may play an important role in the effective treatment of TACE-refractory HCC.
In the present study, for CT-guided 125I brachytherapy in treating TACE-refractory HCC, the six-month objective response rate was 90.5% (19/21), and the six-month survival rate was 100% (21/21), which suggested that 125I brachytherapy had satisfactory mid-term effects in controlling TACE-refractory HCC. These promising results may be explained by several possible reasons. The cancer cells in the marginal area of the HCC mainly supplied by the portal vein were still active after TACE, whereas the γ-rays released by 125I seeds could kill these cancer cells and achieve an effective tumour response. In addition, patients with TACE-refractory HCC underwent multiple TACE procedures; therefore, liver cancer cells were often in a hypoxic state. Recent evidence showed that 125I seeds had a low dose rate and low oxygen enhancement ratio, which could partially overcome the resistance of hypoxic tumour cells to radiation and increase the sensitivity of γ-rays to hypoxic tumour cells.35 This effect may also be a possible mechanism by which 125I seeds treat TACE-refractory HCC.
Moreover, hypoxia could induce increased expression of VEGF in HCC, and VEGF was a specific growth factor for vascular endothelial cells and could promote tumour angiogenesis. Vascular endothelial cells have active proliferation capacity and high sensitivity to radiation.36 Several studies reported that the radiation released by 125I seeds could damage vascular endothelial cells and inhibit the expression of VEGF in tumour tissue, thus inhibiting tumour neovascularization to prevent tumour growth.37 We speculated this may be another possible explanation for 125I seeds to treat TACE-refractory HCC.
During the procedure of CT-guided 125I brachytherapy, the vital blood vessels and organs around the tumour could be clearly displayed under CT guidance, which could help clinicians better control the direction and depth of the implanted needle and reduce the risk of damage to the intestines, important blood vessels or the bile duct. In the present study, no major intraoperative complications, such as intestinal fistula, intestinal bleeding, massive bleeding, bile fistula, or infection, occurred; however, some minor complications occurred. One patient had slight perihepatic haemorrhage during the procedure, which was associated with damage to intrahepatic small blood vessels. Slight pneumothorax occurred in 3 patients during the procedure, which was caused by the puncture path through the chest cavity. These minor complications were all relieved after conservative treatment. These findings in the present study were similar to those reported by previous researchers,19,38 suggesting that CT-guided 125I brachytherapy was a safe and tolerable treatment for TACE-refractory HCC.
The present study had several limitations. First, the relatively small sample size and the relatively short follow-up enabled us to draw only preliminary conclusions concerning the potential value of CT-guided 125I brachytherapy for TACE-refractory HCC. Further studies with larger sample sizes and longer follow-up periods are needed to confirm these preliminary findings. Second, although the results were encouraging, the results of this study were compromised by its retrospective nature and single-arm design. Third, the half-life of iodine-125 was 60 days, and as time extended, the radioactivity of iodine-125 gradually decreased, which may gradually diminish the inhibition of tumour cells. Therefore,to obtain better efficacy for TACE-refractory HCC, repeated CT-guided 125I brachytherapy or other combined therapies may be necessary. Fourth, because of the relatively short application time of 125I brachytherapy in the treatment of HCC and HCC having its own tumour characteristics, there is currently no standard treatment for 125I brachytherapy in treating HCC, and further research and exploration are still needed.
In conclusion, our preliminary practice demonstrated that CT-guided 125I brachytherapy was effective and well tolerated for the treatment of TACE-refractory HCC, suggesting that CT-guided 125I brachytherapy has the potential to become an effective alternative treatment for TACE-refractory HCC.
Research Involving Human Participants and/or Animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Jiangying Hospital Research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Medical Ethics Committee of Jiangyin People’s Hospital.
Informed Consent
This study was a retrospective study. During the follow-up, some patients have died, we were unable to obtain the informed consent of these patients. Therefore, the patient’s informed consent was not required by the ethics committee of our hospital. Patient confidentiality was maintained by anonymizing patient data to remove any identifying information.
Acknowledgments
No funding was received for this study. Xinjian Xu and Yiwen Ding are co-first authors for this study. Thanks are due to Yiyang Huang, Yiwen Ding, Tianfan Pan, Qiulian Sun and Feng Gao for assistance with the study and to Xiangzhong Huang for valuable discussion.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–1953. doi:10.1002/ijc.31937
2. Zheng RS, Sun KX, Zhang SW, et al. Report of cancer epidemiology in China, 2015. Chin J Oncol. 2019;41(1):19–28.
3. Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394(10204):1145–1158. doi:10.1016/S0140-6736(19)30427-1
4. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries: global cancer statistics 2018. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
5. Ikeda M, Kudo M, Aikata H, et al. Transarterial chemoembolization with miriplatin vs. epirubicin for unresectable hepatocellular carcinoma: a Phase III randomized trial. J Gastroenterol. 2018;53(2):281–290. doi:10.1007/s00535-017-1374-6
6. Yang JD, Heimbach JK. New advances in the diagnosis and management of hepatocellular carcinoma. BMJ. 2020;371:m3544. doi:10.1136/bmj.m3544
7. Kudo M, Matsui O, Izumi N, et al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology. 2014;87(Suppl 1):22–31. doi:10.1159/000368142
8. Ernst O, Sergent G, Mizrahi D, et al. Treatment of hepatocellular carcinoma by transcatheter arterial chemoembolization: comparison of planned periodic chemoembolization and chemoembolization based on tumour response. Am J Roentgenol. 1999;172(1):59–64. doi:10.2214/ajr.172.1.9888740
9. Yu CX, Teng GJ. Transarterial chemoembolization refractory: current research progress. J Interv Radiol. 2017;26(12):1063–1067.
10. Kokudo N, Hasegawa K, Akahane M, et al. Evidence-based clinical practice guidelines for hepatocellular carcinoma: the Japan society of hepatology 2013 update (3rd JSH-HCC guidelines). Hepatol Res. 2015;45(2):123–127. doi:10.1111/hepr.12464
11. Cheng AL, Kang YK, Chen ZD, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi:10.1016/S1470-2045(08)70285-7
12. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi:10.1056/NEJMoa0708857
13. Mendez-Blanco C, Fondevila F, García-Palomo A, et al. Sorafenib resistance in hepatocarcinoma: role of hypoxia-inducible factors. Exp Mol Med. 2018;50(10):1–9. doi:10.1038/s12276-018-0159-1
14. Rimassa L, Danesi R, Pressiani T, et al. Management of adverse events associated with tyrosine kinase inhibitors: improving outcomes for patients with hepatocellular carcinoma. Cancer Treat Rev. 2019;77:20–28. doi:10.1016/j.ctrv.2019.05.004
15. Hill A, Gotham D, Fortunak J, et al. Target prices for mass production of tyrosine kinase inhibitors for global cancer treatment. BMJ Open. 2016;6(1):e009586. doi:10.1136/bmjopen-2015-009586
16. Bae SH, Park HC, Lim DH, et al. Salvage treatment with hypofractionated radiotherapy in patients with recurrent small hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012;82(4):e603–607. doi:10.1016/j.ijrobp.2011.09.053
17. Dawson LA, Normolle D, Balter JM, et al. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53(4):810–821. doi:10.1016/S0360-3016(02)02846-8
18. Liang SX, Jiang GL, Zhu XD, et al. Radiation-induced liver disease: risk factors and liver irradiation tolerance. Oncol Prog. 2006;4(4):308–313.
19. Zheng JP, Shao GL, Luo J, et al. CT-guided 125I seeds interstitial implantation for the refractory liver cancers ineffective to commonly used therapies. J Interv Radiol. 2015;24(3):260–264.
20. Lv J, Cao XF, Zhu B. (125)I radioactive seeds implantation therapy for hepatocellular carcinoma. Gastroenterol Res. 2009;2(3):141–147. doi:10.4021/gr2009.05.1289
21. Luo KY, Zheng JH, Li B, et al. Clinical application of iodine-125 seeds for hepatic carcinoma. J Hepatobiliary Surg. 2004;16(1):29–31.
22. Ricke J, Wust P, Stohlmann A, et al. CT-guided interstitial brachytherapy of liver malignancies alone or in combination with thermal ablation: Phase I-II results of a novel technique. Int J Radiat Oncol Biol Phys. 2004;58(5):1496–1505. doi:10.1016/j.ijrobp.2003.09.024
23. Chen L, Zheng CS. Application of 125I particle implantation combined with other therapies in treating liver cancers of different stages. J Interv Radiol. 2019;28(9):910–913.
24. Song Z, Ye J, Wang Y, et al. Computed tomography-guided iodine-125 brachytherapy for unresectable hepatocellular carcinoma. J Cancer Res Ther. 2019;15(7):1553–1560. doi:10.4103/jcrt.JCRT_629_19
25. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi:10.1055/s-0030-1247132
26. Idée JM, Guiu B. Use of lipiodol as a drug-delivery system for transcatheter arterial chemoembolization of hepatocellular carcinoma: a review. Crit Rev Oncol Hematol. 2013;88(3):530–549. doi:10.1016/j.critrevonc.2013.07.003
27. Tak E, Lee S, Lee J, et al. Human carbonyl reductase 1 upregulated by hypoxia renders resistance to apoptosis in hepatocellular carcinoma cells. J Hepatol. 2011;54(2):328–339. doi:10.1016/j.jhep.2010.06.045
28. Li X, Feng GS, Zheng CS, et al. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol. 2004;10(19):2878–2882. doi:10.3748/wjg.v10.i19.2878
29. Wang B, Xu H, Gao ZQ, et al. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008;49(5):523–529. doi:10.1080/02841850801958890
30. Kajihara J, Tomimaru Y, Eguchi H, et al. The clinical impact of transcatheter arterial chemoembolization (TACE)-induced c-met upregulation on TACE refractoriness in hepatocellular carcinoma. Dig Dis Sci. 2016;61(6):1572–1581. doi:10.1007/s10620-015-4018-9
31. Zeng ZC, Tang ZY, Fan J, et al. A comparison of chemoembolization combination with and without radiotherapy for unresectable hepatocellular carcinoma. Cancer J. 2004;10(5):307–316. doi:10.1097/00130404-200409000-00008
32. Zeng ZC, Tang ZY, Fan J, et al. Consideration of role of radiotherapy for lymph node metastases in patients with HCC: retrospective analysis for prognostic factors from 125 patients. Int J Radiat Oncol Biol Phys. 2005;63(4):1067–1076. doi:10.1016/j.ijrobp.2005.03.058
33. Xu ZY, Liang SX, Zhu J, et al. Prediction of radiation-induced liver disease by Lyman normal-tissue complication probability model in three-dimensional conformal radiation therapy for primary liver carcinoma. Int J Radiat Oncol Biol Phys. 2006;65(1):189–195. doi:10.1016/j.ijrobp.2005.11.034
34. Cao GW, Du MM, Cui XJ, et al. Efficacy of TACE combined with 125I radioactive particle implantation in the treatment of paracardiac hepatic carcinoma. Shandong Med. 2017;57(10):55–57.
35. Lu X, Zeng GB, Lin LQ, et al. Transcatheter hepatic arterial oily chemoembolization combined with brightness mode guided125I seeds implantation for huge-type hepatoma. Chin Arch Gen Surg. 2014;8(2):125–129.
36. Li WS, Liu FK, Chen ZH, et al. Changes of angiogenesis in rectal cancer patients after preoperative radiotherapy. Chin J Gen Surg. 2004;19(6):331–333.
37. Tong WS, Pan JH, Qiu XL, et al. Influence of TACE combined with radioactive particle interstitial radiotherapy on malignant biological indexes in patients with primary hepatocellular carcinoma. Pract J Cancer. 2018;33(3):416–418.
38. Guo Q, Li Y, Yan YM, et al. Efficacy of TACE combined with 125I radioactive particle implantation in the treatment of hepatic carcinoma. J Med Imaging. 2012;22(7):1130–1132.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.