Back to Journals » International Journal of General Medicine » Volume 15
CT Characteristics of Acute Pancreatitis with Preexisting Fatty Liver and Its Impact on Pancreatitis Severity and Persistent Systemic Inflammatory Response Syndrome
Authors Liu W , Li Z , Zhang X , Du J , Liang R, Ji Y, Tang W, Zhang X
Received 15 July 2022
Accepted for publication 29 August 2022
Published 5 September 2022 Volume 2022:15 Pages 7017—7028
DOI https://doi.org/10.2147/IJGM.S382287
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Wei Liu, Zenghui Li, Xinyu Zhang, Juanjuan Du, Rui Liang, Yifan Ji, Wei Tang, Xiaoming Zhang
Medical Imaging Key Laboratory of Sichuan Province and Department of Radiology, Affiliated Hospital of North Sichuan Medical College, Nanchong, People’s Republic of China
Correspondence: Xiaoming Zhang; Wei Tang, Medical Imaging Key Laboratory of Sichuan Province and Department of Radiology, Affiliated Hospital of North Sichuan Medical College, No. 1 South Maoyuan Road, Nanchong, Sichuan, 637000, People’s Republic of China, Tel +86 13808271001 ; +86 1369600 2904, Email [email protected]; [email protected]
Purpose: To study the CT characteristics of acute pancreatitis (AP) associated with preexisting fatty liver (FL) and the impact of preexisting FL on the severity of AP and persistent systemic inflammatory response syndrome (SIRS).
Patients and Methods: A total of 189 patients with AP were divided into AP with and without preexisting FL. The CT features, clinical characteristics, severity of AP, and presence of persistent SIRS between the two groups were compared. Univariate and multivariate analyses were performed to determine the risk factors for predicting SIRS. The diagnostic performances of the risk factors were evaluated by receiver operating characteristic (ROC) curve analysis.
Results: Among the 189 patients, 49.7% (94/189) had preexisting FL. On CT, AP patients with preexisting FL were more likely to develop necrosis (23.4% vs 10.5%, p=0.021), local complications (45.7% vs 29.5%, p=0.025) and persistent SIRS (59.6% vs 27.4%, p< 0.001). Multivariate analysis showed that preexisting FL (OR=2.863, 95% CI: 1.264– 6.486, p=0.012), APACHE II≥ 6 (OR=1.334, 95% CI: 1.117– 1.594, p=0.002), and MCTSI ≥ 4 (OR=1.489, 95% CI: 1.046– 2.119, p=0.027) could be independent risk factors for persistent SIRS. The areas under the ROC curve of preexisting FL, APACHE II, and MCISI in diagnosing AP patients with persistent SIRS were 0.664, 0.703, and 0.783, respectively.
Conclusion: Patients with preexisting FL were more likely to develop necrosis and local complications on CT and present more severe AP and persistent SIRS. Preexisting FL can be an independent risk factor in predicting the presence of persistent SIRS in patients with AP.
Keywords: acute pancreatitis, fatty liver, severity, systemic inflammatory response syndrome, Atlanta classification
Introduction
Acute pancreatitis (AP) is a type of acute inflammatory disorder that exists in the pancreas and may be fatal due to a severe inflammatory response. The global pooled incidence of acute pancreatitis is 34 cases (95% confidence interval (CI) 23–49) per 100,000 general population per year.1 The global Incidence of AP is increasing over time.2 Although the majority of AP cases are mild and resolve spontaneously, severe AP has a high mortality rate.3 During the process of severe AP, the massive release of inflammatory cytokines may result in multiple organ dysfunction syndrome (MODS) together with systemic inflammatory response syndrome (SIRS).4
It was reported that multiple risk factors take part in the disease process of AP and can lead to secondary complications, which is bad for pathology or changes the degree of response.5 Notably, the number of AP patients is increasing, parallel to the increase in fatty liver (FL) patients.1,6 In recent years, it has become increasingly aware that metabolic syndrome (MS) exerts a principal effect in AP,7 and it is widely assumed that there is a strong relationship between MS and inflammation. Previous study found that MS together with its components are associated with the occurrence of AP in China.8 MS is a clinical syndrome with insulin resistance (IR) as the central link, accompanied by multiple metabolic abnormalities, such as hyperglycemia, hyperlipidemia and obesity.9 Some studies have found that hyperglycemia, hyperlipidemia and obesity are involved in the occurrence and development of severe AP,10 promoting the transition from SIRS to MODS in severe AP.11
FL and MS have common risk factors, and FL is often accompanied by MS.12 FL can be regarded as a type of chronic low-level inflammatory state characterized by high proinflammatory cytokine levels in serum. In the condition of FL, in AP pathogenesis, Kupffer cells are important due to their release of several inflammatory factors, which may lead to excessive activation of the inflammatory process and increase the body’s proneness to the SIRS response.13 FL is thought to be a metabolically active fatty state that may increase proinflammatory cytokine secretion and amplify the inflammatory response.14 A previous study reported that the incidence of FL was not uncommon in AP, and the condition of some AP patients with FL was more serious.15,16 Previous studies have found that preexisting diabetes has an effect on the severity of AP,17 So we speculate that preexisting FL also has an effect on the severity of AP, but the relationship of preexisting FL with AP remains unclear, and we have not found any literature on the correlation between preexisting AP and FL.
We conducted this research to explore the CT characteristics of AP with preexisting FL and the correlation of preexisting FL with the severity and presence of persistent SIRS after the onset of AP.
Materials and Methods
Ethical Approval
The institutional review committee of the Affiliated Hospital of North Sichuan Medical College(2021ER160-1) approved this research study and waived the informed consent requirement and all procedures involving humans maintained adherence to the Declaration of Helsinki.
Study Population
A retrospective study was performed for patients with AP who underwent treatment in the two hospitals from March 2016 to April 2021. At least two of the following factors can be defined as AP: (1) abdominal pain in accordance with AP (acute, severe, persistent upper abdominal pain attack, generally radiating to the back); (2) an increase in amylase or serum lipase that exceeds the normal level by at least three times or the upper limit; and (3) the characteristic findings of AP on computed tomography (CT), magnetic resonance imaging (MRI) or abdominal ultrasound.18
The inclusion criteria included the following: (1) AP patients over 18 years old; (2) patients with the first AP attack; (3) available clinical and laboratory data at three days before and after CT, MRI or ultrasound; and (4) patients with AP who underwent liver imaging examination for routine physical examination or other reasons within one year before admission or during hospitalization.
The exclusion criteria were as follows: (1) AP patients under 18 years old; (2) patients with chronic pancreatitis or AP recurrence; (3) AP induced by tumor; (4) patients with AP complicated with viral hepatitis or liver cirrhosis; (5) patients with unclear images and incomplete information; and (6) FL diagnosed within one year before admission that was found to have recovered on imaging during hospitalization or clinical intervention was performed within one year in AP patients. The flowchart of this research is exhibited in Figure 1.
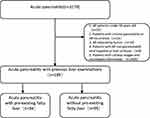 |
Figure 1 Flow chart of patient recruitment in this study. |
Eventually, a total of 189 patients satisfied the strict inclusion criteria, including 67 females and 122 males, and the average age was 53.06 ± 15.33 years (ranging from 18 to 87 years).
Laboratory Data Collection
All laboratory data were collected during the three days after admission in patients with AP. Two researchers who were given detailed information about the study reviewed the medical records and recorded the required data, including clinical baseline characteristics, physiological indicators, and laboratory indicators. The information gathered came from medical reports, and it was double-checked by another researcher to ensure that there were no inconsistencies or errors.
CT Images Observation
All imaging data were collected by two additional radiologists with extensive experience (≥3 years) in abdominal imaging who were not aware of the patients’ laboratory values or clinical course. Two radiologists reviewed and analyzed the CT features of AP, including the presence or absence of pancreatic parenchyma/peripancreatic necrosis and local complications such as acute peripancreatic fluid collection (APFC), acute necrotic collection (ANC), pancreatic pseudocyst and walled-off necrosis (WON). Finally, the MCTSI score was also evaluated. These imaging data were collected during the three days after admission in patients with AP. The liver findings on CT, MRI, or ultrasound were reviewed and analyzed by the two radiologists to determine whether patients had FL, and when there were differences, a consensus was reached through negotiation.
Definition of Persistent SIRS and Severity of Patients with AP
Based on the collected data, the presence or absence of persistent SIRS in patients with AP was determined. The diagnostic criteria for persistent SIRS (SIRS beyond 48 hours) referred to the existence of at least two of the following factors: (1) heart rate greater than 90 beats/minute; (2) breathing rate greater than 20 beats/minute or carbon dioxide partial pressure <32 mmHg; (3) body temperature <36 or >38 °C; and (4) white blood cell count <4000/mm3 or >12,000/mm3.19 The severity of patients with AP was analyzed in accordance with the 2012 RAC,20 APACHE II score system,21 and MCTSI.22
Definition of FL in Patients with AP
By reviewing imaging data within one year before admission and during hospitalization, the patients with AP can be defined as having preexisting FL if any of the following conditions is met: (1) The liver density is attenuated by 10 HU compared with the spleen, or less than 40 HU on nonenhanced CT;23 (2) The near field diffuse punctate hyperecho (stronger than spleen and kidney) and the far-field echo attenuation (sparse light spots) in the liver area on ultrasound;24 (3) The signal intensity of liver is dropped off on out-of-phase MRI.25 Patients without preexisting FL who experienced FL after AP were defined as new-onset FL.
Statistical Analysis
The patients were divided into AP with and without preexisting FL. The basic clinical characteristics, the severity of AP, and the presence of persistent SIRS between the two groups were compared. Univariate analysis was utilized to analyze the basic clinical characteristics and severity of AP associated with persistent SIRS, and in the univariate analysis, the variables with P < 0.2 were chosen to perform multivariate analysis. In the multivariate analysis, the variables with P < 0.05 were chosen to perform receiver operating characteristic (ROC) curve analysis. The AUC comparisons were carried out in accordance with the approach put forward by DeLong.26 The outcomes of the multivariate analysis were described as odds ratios (ORs) containing 95% confidence intervals (CIs) and associated P values. The categorical variables could be reported as percentages or frequencies, which were compared with Fisher’s exact test or the χ2 test as proper. Continuous variables were reported as the median or means ± standard deviations (SD), which were compared with the Mann‒Whitney U-test or independent samples t test. The reliability of the MCTSI between the two reviewers was evaluated using the interclass correlation coefficient (ICC). An ICC higher than 0.75 indicated excellent agreement. R (v. 3.4.1, https://www. r-project.org/) and SPSS 26.0 software package (SPSS Inc., Chicago, Illinois, USA) was employed for implementing the statistical analysis; for all of the detections, it can be regarded as statistically significant when the value of p is less than 0.05.
Results
The Clinical Characteristics of Recruited Patients
Among 189 AP patients, 49.7% (94/189) had a history of FL, and 29.4% (28/95) had new-onset FL after AP. The fundamental clinical traits of the 189 individuals are summarized in Table 1. There was a significant difference between AP with preexisting FL and without preexisting FL for sex (p=0.026), etiology (p≤0.001), and hospital stay (p=0.028), Patients in the preexisting FL group had a longer hospital stay (Figure 2). Age, body mass index (BMI), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and maximum serum C-reactive protein (CRP) were not different between AP patients with and without preexisting FL (p>0.05). The level of triglycerides (TGs) was higher in AP patients with preexisting FL than in those without preexisting FL, but the difference was not statistically significant (5.91 versus 4.78, p=0.791).
 |
Table 1 Clinical Characteristics of Patients with AP Stratified by Presence of Preexisting FL (n=189) |
 |
Figure 2 Distribution of hospitalization days in different groups. |
The CT Features of Recruited Patients
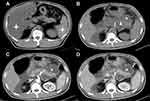
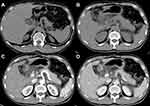
Among 189 AP patients, 16.9% (32/189) had acute necrotizing pancreatitis (ANP), and 37.6% (71/189) had local complications. Compared with patients without preexisting fatty liver, AP with preexisting FL were more likely to present with ANP (p = 0.021) and develop local complications (p = 0.025) (Table 1).
Comparison of Severity and Persistent SIRS Between AP Patients with and without Preexisting FL
The severity of AP and the presence and absence of persistent SIRS in the 94 patients with preexisting FL and 95 patients without preexisting FL are described in Table 2. According to the 2012 RAC, the percentages of severe (21.3% versus 8.4%) and moderate (34% versus 27.4%) AP in AP patients with preexisting FL were higher than those in AP patients without preexisting FL, and the percentage of mild AP (44.7% versus 64.2%) was lower than that in AP patients without preexisting FL (Figures 3 and 4). The mean APACHE II (6.28±3.77 points versus 5.07±3.05 points) and MCTSI (4.94 points versus 4.04 points) scores of the AP patients with preexisting FL were higher than those of the AP patients without preexisting FL. The agreement between the observers for MCTSI was satisfactory (ICC = 0.784, 95% CI: 0.721–0.833). Preexisting FL was evidently related to the severity of AP assessed by 2012 RAC (p=0.01), APACHE II (p=0.017), and MCTSI (p=0.004) (Table 2). In addition, persistent SIRS was found in 56 (59.6%) of 94 AP patients with per-existing FL and 26 (27.4%) of 95 AP patients without per-existing FL, and there was a significant difference for preexisting FL association with the presence of persistent SIRS (p<0.001) (Table 2).
 |
Table 2 Comparison of Severity and Persistent SIRS Between AP with and without Preexisting FL (n=189) |
Patients’ Basic Clinical Characteristics and Severity of AP Associated with the Presence of Persistent SIRS
In the 189 patients with AP, 43.4% (82/189) of patients presented persistent SIRS during hospitalization. Univariate analysis (Table 3) showed that the etiology (p=0.002), maximum serum CRP (p<0.01), TG (p<0.002), preexisting FL (p<0.001), and severity of AP assessed by 2012 RAC (p<0.001), APACHE II (p<0.001), and MCTSI (p<0.001) were remarkably related to the presence of persistent SIRS. Multivariate analysis (Table 4) showed that preexisting FL (OR: 2.868, 95% CI: 1.266–6.495, p=0.012), APACHE II≥ 6 (OR: 1.333, 95% CI: 1.117–1.592, p= 0.001), and MCTSI≥ 4 (OR: 1.500, 95% CI: 1.147–1.961, p=0.003) could be independent risk factors for predicting the presence of persistent SIRS in patients with AP.
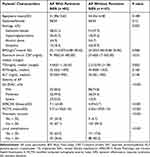 |
Table 3 Univariate Analysis of Patients’ Clinical Characteristics and Severity of AP Associated with Presence of Persistent SIRS (n=189) |
 |
Table 4 Multivariate Analysis of Risk Factors Associated with Persistent SIRS |
ROC Curve Analysis of Independent Risk Factors Associated with Persistent SIRS
The areas under the ROC curve of preexisting FL, APACHE II, and MCTSI in diagnosing AP patients with the presence of persistent SIRS were 0.664, 0.703, and0.783, respectively (Table 5, Figure 5). The AUC for predicting severe AP was highest for the MCTSI. When compared to the APACHE II score (P = 0.1167) and preexisting FL (P = 0.0234), the MCTSI score produced a higher AUC.
 |
Table 5 ROC Curve Analysis of Independent Risk Factors Associated with Persistent SIRS |
Discussion
In the current research, we found that AP patients with preexisting FL were more likely to have necrosis and local complications on CT and present with more severe AP (based on the 2012 RAC) and were more likely to be diagnosed with persistent SIRS within 48 h of admission. Even after modulating for numerous confounding factors, such as age, male sex, CRP, TG, AST, ALT, 2012 RAC, APACHE II, MCTSI score, pancreatic necrosis and local complications, preexisting FL was still a principal risk factor for persistent SIRS. Our results are the first to explore the relationship between preexisting FL and CT findings of AP. This is also the first research study to exhibit the correlation between preexisting FL and SIRS, which is critical for timely treatment and clinical management.
CT is clinically a first choice for AP, and can diagnose pancreatic necrosis and local complications. The MCTSI score and the 2012 RAC well reflect the severity and prognosis of patients with AP. The presence or absence of preexisting FL also affects the CT findings of AP. In our study, we found that compared with patients without preexisting FL, patients with preexisting FL were more prone to pancreatic necrosis. The specific mechanism is still unclear, but one of the possible reasons may be that the fat content of organs increases in FL patients, and the content of unsaturated fatty acids released by hydrolyzing lipase increases in AP. According to previous studies, unsaturated fatty acids can aggravate pancreatic necrosis.27 Second, we also found that patients with preexisting FL are more prone to local complications, which have not been reported in previous studies. The possible reason is that patients with preexisting FL have been in a chronic proinflammatory state for a long time, which is also verified by the higher incidence of SIRS in patients with preexisting FL in our results, and it may be that this long-term chronic proinflammatory state leads to an increased incidence of local complications.28
CRP is an acute-phase reactant produced by hepatocytes in response to IL-1 and IL-6 stimulation. It is the most widely used and widely available indicator of the severity of the acute inflammatory response.29 In this study, we found higher serum maximum CRP levels in AP patients with preexisting FL and persistent SIRS, and the results also confirmed that a chronic proinflammatory state in patients with FL may worsen the course of AP. However, in our study, serum maximum CRP was not an independent risk factor for persistent SIRS. The reason may be that the delayed elevation of serum maximum CRP makes it less useful in predicting the SIRS of AP.30
In this study, we also found higher serum TG levels in AP patients with persistent SIRS. The elevation of serum TG is one of the four components of MS.11 MS may trigger inflammatory responses and lead to pancreatic acinar cell injury.8 Previous studies have shown that TG levels are elevated in FL patients.31 However, in our study, the level of triglycerides (TGs) was higher in AP patients with preexisting FL than in those without preexisting FL, however, there was no statistically significant change. The tiny patient sample size may help to explain this. Previous studies found higher BMI in FL patients.32 However, in our study there were no significant difference in BMI between two groups, The tiny patient sample size may help to explain this.
Various scoring systems were used to stratify the degree of inflammation of AP. However, the sensitivity and the specificity of the used methods for determining severity of AP is different,33 and they are frequently limited by their low predictive values at best, the availability of resources, and the need to observe trends.34 As a result, it cannot be used to stratify risk in the early stages of AP and direct earlier intervention. Compared with these scoring systems, SIRS may be an earlier and simpler indicator for predicting severe AP or ongoing organ failure.35 Our results showed that 2012 RAC, MCTSI, and APACHE II scores were significantly associated with persistent SIRS, indicating that SIRS is feasible as an early index to predict the severity of AP. However, only the MCTSI and APACHE II scores can be independent risk factors for persistent SIRS. The presence and persistence of organ failure is emphasized in the 2012 RAC.20 SIRS does resolve and will not develop organ failure after appropriate resuscitation in some patients,36 which can explain why 2012 RAC cannot be an independent risk factor for persistent SIRS. In addition, we found that AP is more severe (based on 2012 RAC) in preexisting FL patients. A possible explanation is that FL is frequently associated with hyperlipidemia, which can result in free radical accumulation, microcirculatory disruption, oxidative stress, and acinar necrosis in AP.37
Persistent SIRS is a very important feature in the clinical course of AP, and early recognition of persistent SIRS can encourage clinicians to take intervention measures, improving the prognosis of AP patients.35 In our study, the frequency of persistent SIRS was higher than that of AP patients without preexisting FL, and AP patients with preexisting FL were 2.863 times more likely to develop persistent SIRS than AP patients without preexisting FL. Furthermore, pre-existing FL was a separate risk factor for the presence of persistent SIRS in AP patients. The possible reason may be that FL participates in and amplifies the inflammatory process of AP;38 furthermore, since the process of inflammation will amplify and evolve over time, there is probably a window of opportunity to determine whether AP patients will inevitably develop persistent SIRS and intervene. Within the pre-SIRS window, both FL and AP should be considered as the overall disease trajectory, and AP patients with preexisting FL should consider early treatment and obtain better results.
It is worth mentioning that the prevalence of FL was higher in the patients with AP in our study. According to previous study, approximately one-third of AP patients had variations in fatty liver on nonenhanced CT images.39 Previous study reported that approximately half of patients with AP had a history of nonalcoholic FL.31 These findings confirmed that FL is generally reflected in patients with AP, since the two diseases share risk factors such as alcoholism, obesity, and hyperlipidemia.
There were several limitations on this work. The study was retrospective in nature, and some patients were excluded due to inadequate data, which might lead to selection bias. Second, only persistent SIRS was analyzed, and transient SIRS was not analyzed in our study. It is uncertain whether some patients have transient SIRS because some patients were transferred from other hospitals, and the data of referring hospital patients were incomplete. Third, there was no pathological examination to confirm the severity of FL. Previous studies have reported that the imaging and pathological examinations of FL are in good agreement. Therefore, we believe that it is feasible to use imaging examinations to diagnose FL.
Conclusion
We found that AP was more likely to have necrosis and local complications on CT and was more severe in preexisting FL patients. AP patients with preexisting FL were prone to developing persistent SIRS, and preexisting FL can be a separate risk factor in predicting the presence of persistent SIRS in AP patients.
Abbreviations
AP, acute pancreatitis; FL, fatty liver; CT, computed tomography; SIRS, systemic inflammatory response syndrome; RAC, revised Atlanta classification; APACHE, Acute Physiology and Chronic Health Evaluation; MCTSI, Modified CT severity index; MRI, magnetic resonance imaging; ROC, receiver operating characteristic; US, ultrasound sonography; SD, standard deviation; OR, odds ratio; CI, confidence interval; TG, triglyceride.
Ethics Statement
This study was approved by the ethical review board of North Sichuan Medical College (2021ER160-1) and conducted as per the Declaration of Helsinki. The ethic committees approved the request to waive the informed consent. The research presented no risk of harm to subjects and involved no procedures for which written consent is normally required for this retrospective study. All data were anonymized or maintained with confidentiality.
Acknowledgments
This work was supported by the City-School Science and Technology Strategic Cooperation Project in Nanchong city (grant No. 20SXPTJS0001).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16(3):175–184.
2. Iannuzzi JP, King JA, Leong JH, et al. Global incidence of acute pancreatitis is increasing over time: a systematic review and meta-analysis. Gastroenterology. 2021.
3. Forsmark CE, Vege SS, Wilcox CM. Acute Pancreatitis. New Engl J Med. 2016;375(20):1972–1981.
4. Beger HG, Rau B, Mayer J, Pralle U. Natural course of acute pancreatitis. World J Surg. 1997;21(2):130–135.
5. Barreto SG, Habtezion A, Gukovskaya A, et al. Critical thresholds: key to unlocking the door to the prevention and specific treatments for acute pancreatitis. Gut. 2020;2:45.
6. Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18(4):223–238.
7. Szentesi A, Parniczky A, Vincze A, et al. Multiple hits in acute pancreatitis: components of metabolic syndrome synergize each other’s deteriorating effects. Front Physiol. 2019;10:1202.
8. Shen Z, Wang X, Zhen Z, Wang Y, Sun P. Metabolic syndrome components and acute pancreatitis: a case-control study in China. BMC Gastroenterol. 2021;21(1):17.
9. da Silva AA. Role of hyperinsulinemia and insulin resistance in hypertension: metabolic syndrome revisited. Can J Cardiol. 2020;36(5):671–682.
10. Mosztbacher D, Hanak L, Farkas N, et al. Hypertriglyceridemia-induced acute pancreatitis: a prospective, multicenter, international cohort analysis of 716 acute pancreatitis cases. Pancreatology. 2020;20(4):608–616.
11. Niknam R, Moradi J, Jahanshahi KA, Mahmoudi L, Ejtehadi F. Association Between Metabolic Syndrome and Its Components with Severity of Acute Pancreatitis. Diabetes Metab Syndr Obes. 2020;13:1289–1296.
12. Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(5):1264–1281 e1264.
13. Folch-Puy E. Importance of the liver in systemic complications associated with acute pancreatitis: the role of Kupffer cells. J Pathol. 2007;211(4):383–388.
14. Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2(11):901–910.
15. Xiao B, Zhang XM, Jiang ZQ, et al. Fatty liver in acute pancreatitis: characteristics in magnetic resonance imaging. J Comput Assisted Tomogr. 2012;36(4):400–405.
16. Vancsa S, Nemeth D, Hegyi P, et al. Fatty Liver Disease and non-alcoholic fatty liver disease worsen the outcome in acute pancreatitis: a systematic review and meta-analysis. J Clin Med. 2020;9:9.
17. Miko A, Farkas N, Garami A, et al. Preexisting diabetes elevates risk of local and systemic complications in acute pancreatitis: systematic review and meta-analysis. Pancreas. 2018;47(8):917–923.
18. Boxhoorn L, Voermans RP, Bouwense SA, et al. Acute pancreatitis. Lancet. 2020;396(10252):726–734.
19. Nystrom PO. The systemic inflammatory response syndrome: definitions and aetiology. J Antimicrob Chemother. 1998;41(Suppl):
20. Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2012;62(1):102–111.
21. Larvin M, McMahon M. Apache-Ii score for assessment and monitoring of acute pancreatitis. Lancet. 1989;334(8656):201–205.
22. Mortele KJ, Wiesner W, Intriere L, et al. A modified CT severity index for evaluating acute pancreatitis: improved correlation with patient outcome. AJR Am J Roentgenol. 2004;183(5):1261–1265.
23. Duman DG, Celikel C, Tuney D, Imeryuz N, Avsar E, Tozun N. Computed tomography in nonalcoholic fatty liver disease: a useful tool for hepatosteatosis assessment? Dig Dis Sci. 2006;51(2):346–351.
24. Esterson YB, Grimaldi GM. Radiologic imaging in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Clin Liver Dis. 2018;22(1):93–108.
25. Cowin GJ, Jonsson JR, Bauer JD, et al. Magnetic resonance imaging and spectroscopy for monitoring liver steatosis. J Magn Reson Imaging. 2008;28(4):937–945.
26. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845.
27. Khatua B, El-Kurdi B, Singh VP. Obesity and pancreatitis. Curr Opin Gastroenterol. 2017;33(5):374–382.
28. Frossard JL, Lescuyer P, Pastor CM. Experimental evidence of obesity as a risk factor for severe acute pancreatitis. World J Gastroenterol. 2009;15(42):5260–5265.
29. van den Berg FF, de Bruijn AC, van Santvoort HC, Issa Y, Boermeester MA. Early laboratory biomarkers for severity in acute pancreatitis; A systematic review and meta-analysis. Pancreatology. 2020;20(7):1302–1311.
30. Staubli SM, Oertli D, Nebiker CA. Laboratory markers predicting severity of acute pancreatitis. Crit Rev Clin Lab Sci. 2015;52(6):273–283.
31. Yki-Jarvinen H, Luukkonen PK, Hodson L, Moore JB. Dietary carbohydrates and fats in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2021;18(11):770–786.
32. Fan R, Wang J, Du J. Association between body mass index and fatty liver risk: a dose-response analysis. Sci Rep. 2018;8(1):15273.
33. Miko A, Vigh E, Matrai P, et al. Computed tomography severity index vs. other indices in the prediction of severity and mortality in acute pancreatitis: a predictive accuracy meta-analysis. Front Physiol. 2019;10:1002.
34. Buxbaum J, Quezada M, Chong B, et al. The Pancreatitis Activity Scoring System predicts clinical outcomes in acute pancreatitis: findings from a prospective cohort study. Am J Gastroenterol. 2018;113(5):755–764.
35. Singh VK, Wu BU, Bollen TL, et al. Early systemic inflammatory response syndrome is associated with severe acute pancreatitis. Clin Gastroenterol Hepatol. 2009;7(11):1247–1251.
36. Davies MG, Hagen PO. Systemic inflammatory response syndrome. Br J Surg. 1997;84(7):920–935.
37. Ditzel J, Thaysen EH. Increased hemoglobin-oxygen affinity in patients with pancreatitis associated with type I and V hyperlipoproteinemia. Adv Exp Med Biol. 1977;94:423–428.
38. Wu D, Zhang M, Xu S, et al. Nonalcoholic fatty liver disease aggravated the severity of acute pancreatitis in patients. Biomed Res Int. 2019;2019:9583790.
39. Yoon SB, Lee IS, Choi MH, et al. Impact of fatty liver on acute pancreatitis severity. Gastroenterol Res Pract. 2017;2017:4532320.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.