Back to Journals » Neuropsychiatric Disease and Treatment » Volume 16
CSF VEGF Was Positively Associated with Neurogranin Independent of β-Amyloid Pathology
Authors Huang Y, Wang J, Zhu B, Fu P
Received 1 March 2020
Accepted for publication 21 April 2020
Published 22 July 2020 Volume 2020:16 Pages 1737—1744
DOI https://doi.org/10.2147/NDT.S252008
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Yangping Huang, Jun Wang, Bihong Zhu, Pan Fu
Department of Neurology, Taizhou First People’s Hospital, Taizhou, Zhejiang, People’s Republic of China
Correspondence: Pan Fu Email [email protected]
Purpose: Increasing evidence suggests that both vascular endothelial growth factor (VEGF) and synaptic failure have been involved in the pathogenesis of Alzheimer’s disease (AD). However, it is not clear whether CSF VEGF levels are associated with synaptic function in living human.
Patients and Methods: In the present study, we included a total of 291 older individuals, including 83 individuals with normal cognition (NC), 143 individuals with mild cognitive impairment (MCI) and 65 patients with AD. Several linear regression models were conducted to examine the associations of CSF VEGF with CSF neurogranin levels (NG, reflecting synaptic degeneration) when controlling for other potential confounding factors, including age, gender, years of education, clinical diagnosis, APOE4 genotype and CSF β-amyloid 42 (Aβ 42) levels.
Results: There was no significant difference in VEGF levels between the three diagnostic groups. In the pooled sample, females had significantly lower levels of VEGF than males. Aβ-positive (CSF Aβ 42 < 192 pg/mL) individuals had lower levels of VEGF than Aβ-negative individuals. However, the relationships between VEGF and NG levels were not modified by disease stage. Finally, we found that CSF VEGF levels were associated with NG levels with adjustment of age, gender, years of education, clinical diagnosis, APOE4 genotype and CSF Aβ 42 levels.
Conclusion: CSF VEGF levels were associated with NG independent of AD pathology and disease stage.
Keywords: vascular endothelial growth factor, neurogranin, synaptic dysfunction, Alzheimer’s disease
Introduction
Synaptic failure has been implicated as an important feature of Alzheimer’s disease (AD) pathophysiology.1 Neurogranin (NG), a postsynaptic protein, was reported to be increased in the CSF of patients with mild cognitive impairment (MCI) and AD compared to cognitively normal older people.2–4 In addition, other studies indicated that elevated CSF NG levels may be specific for AD.5–7 Recent studies found that higher levels of NG in CSF can predict faster rates of cognitive decline in MCI and progression from MCI to AD.8 Thus, NG appears to be a promising biomarker reflecting AD-related synaptic degeneration.
Vascular endothelial growth factor (VEGF) plays crucial roles in modulation of vascular remodeling, permeability, angiogenesis, repair and inflammation.9 Recent studies revealed that VEGF is also involved in the pathogenesis of AD. For example, preclinical studies revealed that the transplantation of VEGF-overexpressing stem cells or VEGF-releasing nanospheres to AD mice improves cognitive deficits and reduces amyloid deposition.10,11 Additionally, several longitudinal prospective studies suggested that increased VEGF levels in CSF are associated with reduced hippocampal atrophy and less cognitive decline,12,13 suggesting that VEGF may be neuroprotective. However, it is not clear whether levels of VEGF in CSF are associated with NG (reflecting synaptic degeneration) in the living human brain.
In the present study, we aimed to examine the associations of CSF VEGF levels with NG in individuals with normal cognition (NC), individuals with MCI and AD from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database. These findings might provide critical insight into the neuropathological mechanisms by which VEGF affects AD.
Patients and Methods
Alzheimer’s Disease Neuroimaging Initiative Study
Data used for this analysis were extracted from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (http://adni.loni.usc.edu). The ADNI study was launched in 2003 with the primary goal of investigating whether neuropsychological assessments, serial MRI, PET and other biomarkers can be integrated to predict the decline of cognition. The ADNI centers obtained local institutional review board approval, and all subjects provided written informed consent. Further information can be found at the ADNI website (http://adni.loni.usc.edu).
Participants
We included individuals who had baseline CSF VEGF and NG samples. In this study, there were 83 individuals with NC, 143 individuals with MCI and 65 patients with mild AD dementia. Individuals with NC had a mini-mental state examination (MMSE) score of 24 or higher and a clinical dementia rating (CDR) score of 0. Individuals with MCI had a subjective memory complain, a CDR score of 0, a MMSE score ranging between 24 and 30, an objective verbal memory impairment as examined by Wechsler Memory Scale Logical memory II, largely preserved activities of daily living, and absence of dementia. Patients with mild AD met the NINCDS/ADRDA criteria for probable AD, and had a CDR score of 1 or lower and a MMSE score ranging from 20 to 26.
Measurement of VEGF in CSF
VEGF levels were measured as part of a CSF multiplex proteomic processing stream using an xMAP multiplex panel (MyriadRBM), details of which can be found at the ADNI website (http://adni.loni.usc.edu/wp-content/uploads/2012/01/2011Dec28-Biomarkers-Consortium-DataPrimer-FINAL1.pdf). In brief, analytes were removed if they can not pass the ANDI quality-control procedures: (1) the test-retest sample was ≥ 7; (2) the mean percentage difference was ≤ 35%; (3) the mean absolute percentage difference was ≤ 60%. The VEGF analyte successfully passed all these procedures. Values are given in pg/mL. The data were log transformed before statistical analysis.
Measurement of NG in CSF
Levels of NG in CSF were measured using electrochemiluminescence technology (Meso Scale Discovery, Gaithersburg, Maryland, USA) using Ng 7 (a monoclonal antibody specific for Ng) as a coating antibody and polyclonal NG anti-rabbit (ab 23,570, Upstate) as a detector antibody.14 The information about the measurement of NG in CSF has been detailed elsewhere.15
Measurement of CSF Aβ42 Levels
Levels of Aβ42 in CSF were measured using xMAP Luminex platform and Innogenetics/Fujirebio AlzBio3 immunoassay, which has been detailed elsewhere.16 Values are given in pg/mL. Aβ status was defined based on a previously established cut-off point (Aβ+: CSF Aβ42 ≤ 192 pg/mL; Aβ-: CSF Aβ42 > 192 pg/mL).17
Statistical Analysis
The one-way ANOVA was conducted to investigate the differences in continuous variables, and the Pearson x2 was applied to compare the distributions of categorical parameters among the three groups. The Pearson correlation test was performed to examine the relationship between CSF VEGF and NG levels in the pooled sample and within each diagnostic group. Finally, several linear regression models were used to investigate the associations between CSF VEGF and NG levels in the pooled sample: model 1 was unadjusted; model 2 was adjusted for age, gender, educational attainment, APOE4 genotype and clinical diagnosis; model 3 was additionally adjusted for Aβ status (Aβ-negative vs Aβ-positive). All data analyses were conducted using R software. The level of statistical significance was set at p < 0.05.
Results
Demographic and Clinical Characteristics
In this analysis, a total of 291 participants were included: 83 individuals with NC, 143 individuals with MCI and 65 patients with mild AD dementia (Table 1). There were no significant differences in age and educational attainment among the three groups. Expectedly, a significant difference in MMSE score across the three groups was observed (NC > MCI > AD, p < 0.001). Consistent with previous studies, there were significant differences in CSF Aβ 42 levels across the three groups (Aβ42: NC > MCI >AD, p < 0.001).
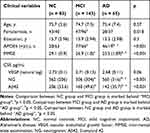 |
Table 1 Demographic and Clinical Variables |
CSF VEGF Levels in the Three Groups
As shown in Table 1 and Figure 1, no significant differences in CSF VEGF levels were found across the three groups (p = 0.06).
Relationships Between CSF VEGF and Other Variables in the Pooled Sample
As shown in Figure 2, CSF VEGF was associated with age (r = 0.24, p < 0.001) and MMSE scores (r = 0.16, p = 0.006) in the pooled sample. However, no relationship between VEGF and years of education was observed (r = 0.012, p = 0.84). Compared with males, females had significant lower levels of VEGF (Females: 2.68 ± 0.11; Males: 2.73 ± 0.13; t = 3.4, p < 0.001). Aβ+ individuals had lower levels of VEGF than Aβ- individuals (Aβ+: 2.7 ± 0.12; Aβ-: 2.75 ± 0.12; t = 3.4, p < 0.001). However, no significant difference in VEGF between APOE4 carriers and noncarriers was observed (p > 0.05).
Relationships Between CSF NG and Other Variables in the Pooled Sample
As shown in Figure 3, CSF NG was associated with age (r = −0.14, p = 0.02) and MMSE scores (r = −0.19, p = 0.001) in the pooled sample. Compared with males, females had significant higher levels of NG (t = −3, p = 0.003). Aβ+ individuals had higher levels of NG than Aβ- individuals (t = −7, p < 0.001).
 |
Figure 3 Relationships between NG and age, MMSE scores, gender and Aβ status in the pooled sample. Abbreviations: NG, neurogranin; MMSE, mini-mental state examination; Aβ, β-amyloid. |
Correlations Between CSF VEGF and NG in Each Diagnostic Group
In the pooled sample, levels of VEGF in CSF were positively associated with CSF NG (r = 0.23, p < 0.001). In the diagnosis-stratified analyses, we found that VEGF was associated with NG (NC: r = 0.335, p = 0.002; MCI: r = 0.257, p = 0.037; AD: r = 0.259, p = 0.037; Figure 4).
Association with CSF VEGF and NG
To examine the association between CSF VEGF and NG, several linear regression models were conducted (Table 2). In the first model, CSF VEGF levels were significantly associated with NG levels (unstandardized β = 538.9 (135); p < 0.001) without adjusting for other variables. In the second model, a significant association of CSF VEGF with NG was observed after controlling for age, gender, educational attainment, APOE4 genotype and clinical diagnosis (unstandardized β = 862 (126); p < 0.001). Finally, in the third model, the association of CSF VEGF with NG was still present after adjusting for Aβ status (unstandardized β = 948 (124); p < 0.001).
 |
Table 2 Modeling of Potential Association of VEGF with NG |
Discussion
To the best of our knowledge, this is the first study to report that CSF VEGF levels were positively associated with CSF NG (reflecting synaptic degeneration) independent of AD pathology among older individuals with different severities of cognitive impairment.
Regarding the biology of VEGF, it is abundantly expressed in the brain and plays important roles in angiogenesis, blood production and neural development.18 It has also been implicated as a beneficial factor in AD pathogeneses.19 For instance, patients with AD had lower serum VEGF levels20 and lower cerebral capillary VEGF expression in hippocampus and other AD-related brain regions21 compared with control subjects. In addition, previous longitudinal studies investigating the association of CSF VEGF levels with brain aging outcomes found that higher VEGF levels were associated with reduced hippocampal atrophy and reduced decline of cognition over time.12,13 Furthermore, preclinical studies found that the transplantation of nanospheres releasing VEGF or stem cells overexpressing VEGF to AD mice decreases Aβ accumulation and ameliorates cognitive deficits,10,11 suggesting that VEGF treatment may be neuroprotective in AD.12
Regarding the biology of neurogranin, it is a postsynaptic protein implicated in long-term potentiation (LTP) and cognition.2,22 Neurogranin is primarily expressed in the hippocampus and cortex,23,24 which are the same cerebral regions that are affected in patients with AD. It has been reported that neurogranin levels are substantially lower in the cortex and hippocampus of AD patients compared to control individuals.24,25 Additionally, Thorsell and his colleagues reported a significant increase of CSF neurogranin levels in patients with AD compared to controls4. One possible explanation is that dying neurons may lead to the increased neurogranin levels in CSF in patients of AD. Furthermore, greater levels of neurogranin in CSF predicted faster rates of cognitive decline and hippocampal atrophy and progression from MCI to AD.2,26 More importantly, Casaletto and colleagues found that neurogranin, but not amyloid or tau pathology, was associated with memory performance in cognitively normal older people, suggesting that neurogranin is highly sensitive to subtle memory abilities and that synaptic dysfunction may precede early AD pathogenesis.27
Regarding VEGF and synaptic function, a previous animal study using mice model of chronic cerebral hypoperfusion (CCH) has shown that VEGF could ameliorate impaired hippocampal synaptic function, including LTP, basal synaptic transmission, depotentiation and paired-pulse facilitation.28 In addition, De Rossi et al found that both VEGF and VEGF receptor 2 (VEGFR2) play important roles in the hippocampal synaptic function and consolidation of fear-related memory.29 It has been reported that in cultured hippocampal neurons, VEGF induces the elevation of protein synthesis, partially by regulating the levels of mammalian target of rapamycin (mTOR) and calcium/calmodulin protein kinase II (CaMKII), indicating that it may contribute to protracted alterations of synaptic efficacy.30 Taken together, these findings highlighted a potentially beneficial role of VEGF in the synaptic structure and function.
In the present study, we found that CSF VEGF levels were positively associated with NG in subjects with different severities of cognitive impairment. One possible explanation for this finding is that VEGF upregulation acts as a compensatory mechanism to reduce synaptic loss caused by AD. It has been reported that cerebral hypoperfusion has been considered as a key feature in AD brain.31 The long-lasting hypoperfusion could contribute to both upregulation of VEGF and compromise of neuronal and synaptic survival. Thus, VEGF elevations may play an important role in counteracting the detrimental effects of the AD pathological cascade on synaptic structure and function. However, further preclinical and clinical studies are needed to clarify the mechanisms by which VEGF affects synaptic function in AD.
In the present study, we did not observe a significant difference in CSF VEGF between APOE4 carriers and noncarriers in the whole sample (NC, MCI and mild AD). However, a previously published study32 showed that in AD patients, the severity of dementia was positively associated serum VEGF levels in APOE4 carriers, but not in APOE4 non-carriers. This inconsistency may be due to several differences between studies, such as the sample (CSF vs serum) and participants with different severities of cognitive impairment.
Several limitations should be noted. First, the cross-sectional design does not allow us to explore the temporal relationship between VEGF and NG. The prospective longitudinal studies are needed to examine the association of baseline CSF VEGF with change in CSF NG over time. Second, the ADNI cohort represents a convenience sample of volunteers, which may lead to selection bias. Therefore, our findings need to be replicated in other samples, particularly in a population-based sample.
In summary, CSF VEGF levels were associated with NG independent of AD pathology and disease stage.
Acknowledgments
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC; Johnson & Johnson Pharmaceutical Research & Development LLC; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Hardy J. The amyloid hypothesis for Alzheimer’s disease: a critical reappraisal. J Neurochem. 2009;110(4):1129–1134. doi:10.1111/j.1471-4159.2009.06181.x
2. Kvartsberg H, Duits FH, Ingelsson M, et al. Cerebrospinal fluid levels of the synaptic protein neurogranin correlates with cognitive decline in prodromal Alzheimer’s disease. Alzheimers Dement. 2015;11(10):1180–1190. doi:10.1016/j.jalz.2014.10.009
3. Sun X, Dong C, Levin B, et al. APOE epsilon4 carriers may undergo synaptic damage conferring risk of Alzheimer’s disease. Nat Commun. 2016;12(11):1159–1166.
4. Thorsell A, Bjerke M, Gobom J, et al. Neurogranin in cerebrospinal fluid as a marker of synaptic degeneration in Alzheimer’s disease. Brain Res. 2010;1362:13–22.
5. Janelidze S, Hertze J, Zetterberg H, et al. Cerebrospinal fluid neurogranin and YKL-40 as biomarkers of Alzheimer’s disease. Ann Clin Transl Neurol. 2016;3(1):12–20.
6. Remnestal J, Just D, Mitsios N, et al. CSF profiling of the human brain enriched proteome reveals associations of neuromodulin and neurogranin to Alzheimer’s disease. Proteomics Clin Appl. 2016;10(12):1242–1253.
7. Wellington H, Paterson RW, Portelius E, et al. Increased CSF neurogranin concentration is specific to Alzheimer disease. Neurology. 2016;86(9):829–835.
8. Portelius E, Zetterberg H, Skillback T, et al. Cerebrospinal fluid neurogranin: relation to cognition and neurodegeneration in Alzheimer’s disease. Brain. 2015;138(Pt 11):3373–3385.
9. Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9(6):653–660.
10. Herran E, Perez-Gonzalez R, Igartua M, Pedraz JL, Carro E, Hernandez RM. VEGF-releasing biodegradable nanospheres administered by craniotomy: a novel therapeutic approach in the APP/Ps1 mouse model of Alzheimer’s disease. J Control Release. 2013;170(1):111–119. doi:10.1016/j.jconrel.2013.04.028
11. Garcia KO, Ornellas FL, Martin PK, et al. Therapeutic effects of the transplantation of VEGF overexpressing bone marrow mesenchymal stem cells in the hippocampus of murine model of Alzheimer’s disease. Front Aging Neurosci. 2014;6:30. doi:10.3389/fnagi.2014.00030
12. Hohman TJ, Bell SP, Jefferson AL. The role of vascular endothelial growth factor in neurodegeneration and cognitive decline: exploring interactions with biomarkers of Alzheimer disease. JAMA Neurol. 2015;72(5):520–529. doi:10.1001/jamaneurol.2014.4761
13. Paterson RW, Bartlett JW, Blennow K, et al. Cerebrospinal fluid markers including trefoil factor 3 are associated with neurodegeneration in amyloid-positive individuals. Transl Psychiatry. 2014;4(7):e419. doi:10.1038/tp.2014.58
14. Headley A, De Leon-benedetti A, Dong C, et al. Neurogranin as a predictor of memory and executive function decline in MCI patients. Neurology. 2018;90(10):e887–e895. doi:10.1212/WNL.0000000000005057
15. Portelius EA-O, Olsson B, Höglund K, et al. Cerebrospinal fluid neurogranin concentration in neurodegeneration: relation to clinical phenotypes and neuropathology. Acta Neuropathologica. 2018;136(3):363–376. doi:10.1007/s00401-018-1851-x
16. Jagust WJ, Landau SM, Shaw LM, et al. Relationships between biomarkers in aging and dementia. Neurology. 2009;73(15):1193–1199. doi:10.1212/WNL.0b013e3181bc010c
17. Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65(4):403–413. doi:10.1002/ana.21610
18. Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci. 2001;114(Pt 5):853–865.
19. Storkebaum E, Carmeliet P. VEGF: a critical player in neurodegeneration. J Clin Invest. 2004;113(1):14–18. doi:10.1172/JCI20682
20. Mateo I, Llorca J, Infante J, et al. Low serum VEGF levels are associated with Alzheimer’s disease. Acta Neurol Scand. 2007;116(1):56–58. doi:10.1111/j.1600-0404.2006.00775.x
21. Provias J, Jeynes B. Reduction in vascular endothelial growth factor expression in the superior temporal, hippocampal, and brainstem regions in Alzheimer’s disease. Curr Neurovasc Res. 2014;11(3):202–209. doi:10.2174/1567202611666140520122316
22. Zhong L, Gerges NZ. Neurogranin and synaptic plasticity balance. Commun Integr Biol. 2010;3(4):340–342. doi:10.4161/cib.3.4.11763
23. Represa A, Deloulme JC, Sensenbrenner M, Ben-Ari Y, Baudier J. Neurogranin: immunocytochemical localization of a brain-specific protein kinase C substrate. J Neurosci. 1990;10(12):3782–3792. doi:10.1523/JNEUROSCI.10-12-03782.1990
24. Guadano-Ferraz A, Vinuela A, Oeding G, Bernal J, Rausell E. RC3/neurogranin is expressed in pyramidal neurons of motor and somatosensory cortex in normal and denervated monkeys. J Comp Neurol. 2005;493(4):554–570. doi:10.1002/cne.20774
25. Davidsson P, Blennow K. Neurochemical dissection of synaptic pathology in Alzheimer’s disease. Int PsychogeriatrInt Psychogeriatr. 1998;10(1):11–23. doi:10.1017/S1041610298005110
26. Kester MI, Teunissen CE, Crimmins DL, et al. Neurogranin as a cerebrospinal fluid biomarker for synaptic loss in symptomatic Alzheimer disease. JAMA Neurol. 2015;72(11):1275–1280. doi:10.1001/jamaneurol.2015.1867
27. Casaletto KB, Elahi FM, Bettcher BM, et al. Neurogranin, a synaptic protein, is associated with memory independent of Alzheimer biomarkers. Neurology. 2017;89(17):1782–1788. doi:10.1212/WNL.0000000000004569
28. Wang L, Wang J, Wang F, et al. VEGF-mediated cognitive and synaptic improvement in chronic cerebral hypoperfusion rats involves autophagy process. Neuromolecular Med. 2017;19(2–3):423–435. doi:10.1007/s12017-017-8458-6
29. De Rossi P, Harde E, Dupuis JP, et al. A critical role for VEGF and VEGFR2 in NMDA receptor synaptic function and fear-related behavior. Mol Psychiatry. 2016;21(12):1768–1780. doi:10.1038/mp.2015.195
30. Kim BW, Choi M, Kim YS, et al. Vascular endothelial growth factor (VEGF) signaling regulates hippocampal neurons by elevation of intracellular calcium and activation of calcium/calmodulin protein kinase II and mammalian target of rapamycin. Cell Signal. 2008;20(4):714–725. doi:10.1016/j.cellsig.2007.12.009
31. Schuff N, Matsumoto S, Kmiecik J, et al. Cerebral blood flow in ischemic vascular dementia and Alzheimer’s disease, measured by arterial spin-labeling magnetic resonance imaging. Alzheimers Dement. 2009;5(6):454–462.
32. Alvarez XA, Alvarez I, Aleixandre M, et al. Severity-related increase and cognitive correlates of serum VEGF levels in Alzheimer’s disease ApoE4 carriers. J Alzheimers Dis. 2018;63(3):1003–13.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



