Back to Journals » Breast Cancer: Targets and Therapy » Volume 11
Cryoablation In The Management Of Breast Cancer: Evidence To Date
Authors Pusceddu C, Paliogiannis P, Nigri G , Fancellu A
Received 23 July 2019
Accepted for publication 27 September 2019
Published 10 October 2019 Volume 2019:11 Pages 283—292
DOI https://doi.org/10.2147/BCTT.S197406
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Pranela Rameshwar
Claudio Pusceddu,1 Panagiotis Paliogiannis,2 Giuseppe Nigri,3 Alessandro Fancellu4
1Division of Interventional Radiology, Department of Oncologic Radiology, Businco Hospital, Cagliari, Italy; 2Unit of Experimental Pathology and Oncology, Department of Medical Surgical and Experimental Sciences, University of Sassari, Sassari, Italy; 3Department of Medical and Surgical Sciences and Translational Medicine, Sapienza University of Rome, St. Andrea University Hospital, Rome, Italy; 4Unit of General Surgery 2 - Clinica Chirurgica, Department of Medical, Surgical and Experimental Sciences, University of Sassari, Sassari, Italy
Correspondence: Alessandro Fancellu
University of Sassari, Department of Medical, Surgical and Experimental Sciences, Unit of General Surgery 2 - Clinica Chirurgica, V.le San Pietro 43, Sassari 07100, Italy
Tel +39 079 228432
Fax +39 079 228394
Email [email protected]
Abstract: Cryoablation has been successfully used to treat various type of solid tumors, including breast carcinomas. This ablation method has the advantage of being a minimally invasive procedure useful in various clinical situations, including early breast cancer and metastatic breast cancer, when co-morbidities preclude the use of surgical treatment. However, due to the small sample size of the available studies, reliable and definitive conclusions on the usefulness of cryoablation in patients with breast cancer could not be drawn. In fact, many aspects necessitate to be elucidated, regarding technical issues, indications, efficacy, imaging follow-up, and possible advantages over other percutaneous ablative methods. This review article has the aim to clarify the current evidence supporting cryoablation of breast cancer, and discuss the future perspectives, including those arising from the new studies on immunological effects related to cryoablation.
Keywords: cryoablation, breast cancer, ablation, treatment, interventional radiology
Introduction
In recent years, there has been a surge of ablative methods for the treatment of different solid neoplasms. Those techniques were initially used as alternative to surgery in either inoperable patients or in those with metastatic disease in order to achieve cytoreduction of the primary tumor.1–5 Ablation techniques offer the advantage of being done percutaneously under image guidance, thus obtaining in general low rates of complications and patients’ discomfort. As regards breast cancer, a large amount of articles in the recent literature reports on the use of radiofrequency, microwave ablation, cryoablation, interstitial laser therapy, and focused ultrasound in different clinical scenarios.3,6–9 Among them, cryoablation represents an emerging treatment, which has been used in the treatment of both benign and malignant breast diseases. A systematic literature search using the PubMed, WOS, and Scopus databases was performed in June 2019 to identify studies in English language reporting on outcomes of cryoablation in patients with breast cancer. The following terms were used for the search: “cryoablation”, or “cryotherapy, or “cryosurgery”, and “breast cancer”, or “breast diseases”. The “related articles” function was used to broaden the search and all abstracts, citations, and studies scanned as well as the references of relevant articles were reviewed (see search strategy in Figure 1). The aim of this review is to provide a synthesis on the use of cryoablation as ablative treatment in breast cancer at different stages. In fact, some authors have assessed cryoablation in patients with metastatic disease, whereas others have used it in those who were unsuitable for surgery, or refused surgical treatment. More recently, cryoablation has been proposed as an alternative to surgery in selected patients with early breast cancer. In addition, studies investigating the correlations between cryoablation, immunotherapy, and systemic anti-tumoral agents have been reviewed.
 |
Figure 1 Search strategy. |
Cryoablation Technique And Application In Breast Cancer
The efficacy of cryoablation is based on the cytotoxic effects of cold that produce both instant and delayed destruction of cellular ultrastructure. Tissue destruction occurs when tissues are frozen to lethal temperatures lower than −40°C.3,10–12 Cold temperatures result in increasing of intracellular osmolarity and freezing of extracellular water; this causes, in turn, drawing water out of the cells and cellular dehydration. During the passive thaw phase, cell swelling and subsequent rupture occurs. Additionally, ice crystals in the intracellular milieu damage organelles and plasma membranes. Cryoablation also damages tumor cells by causing endothelial cell dysfunction, microthrombus formation, ischemia, and platelet aggregation.1–5,13,14
Cryoablation consists of cycles of first freeze, a passive thaw phase, and a second freeze.12,15 It usually takes less than 45 mins to be completed. A second freeze is necessary because tissues that have been injured during the first freeze conduct cold temperatures more efficiently, thus enhancing the damaging effects of cold and expanding the area of tumor necrosis. The duration of the thaw phase varies according to the size and position of the tumor, the device in use, and the size of margins around the target lesion. It is known that breast cancer nodules require longer freeze time than fibroadenomas. In general, cryoablation of invasive breast cancer is supposed to create an ice ball extended at least 1 cm beyond the tumor margins. Breast cryoablation may be done with US, CT, or MRI guidance. Although the first cases were performed under MRI guidance,16,17 cryoablation is usually performed under US- or CT-guidance for probe(s) placement and monitoring the ice ball formation in the target lesion.8
Cryoablation can be carried out with little anesthesia, because the cooling produced by the probes provides analgesia. In fact, it can be also performed as an outpatient basis.8 Cryoablation is a minimally invasive method capable of obtaining satisfying aesthetical results.18 More importantly, it is a repeatable procedure in case of local relapse or incomplete ablation. Furthermore, it does not interrupt other systemic therapies, ie, systemic treatment for metastatic disease, and can act in synergy with them.1,19,20 Cryoablation is not without possible adverse effects. One of the disadvantages is the low capability of limiting the ablation area, thus large “ice balls” created by the probes during the treatment may cause fat necrosis and infection. Skin necrosis or pectoralis muscle necrosis hasbeen rarely reported, and instilling warm saline between the ice ball and skin surface during freezing helps in reducing the risk.21 Other minor adverse effects include breast pain, swelling, ecchymosis, and skin burns.1,8
Cryoablation For Early Breast Cancer
Advances in systemic treatments and early diagnosis due to mammographic screening have led to a progressive de-escalation of breast cancer treatment towards less invasive forms of loco-regional interventions.22–24 Thus, breast-conserving surgery has become the treatment of choice for the majority of patients with early breast cancer. Recently, in line with ongoing researches in different fields of surgical oncology, nonoperative approaches such as percutaneous ablative techniques have been proposed such as possible alternative to surgery in selected patients with early breast cancer. Conventional surgical treatment of early breast cancer may have different complications or sequels which can prolong hospital stay, such as chronic pain, infection, hematoma, and seroma formation.24,25 Cryoablation treatment has some potential advantages over surgical operation. Because of the natural pain relieving effect of cold, cryoablation is usually painless. Therefore, it can be applied using either local anesthesia or peripheral nerve block, without general anesthesia. Since it is done percutaneously through a small needle hole, there is no incision, suture, and scar, and in general no deformation occurs in the breast with consequent aesthetics advantages. The patient can be discharged the same day of treatment and return to normal life almost immediately following the operation.18 Cryoablation for breast cancer represents an extension of its use in benign breast diseases. In fact, it was initially used in the treatment of fibroadenomas, with encouraging results in terms of ablation of the target lesions.26–29
In 2004, Sabel et al reported on 27 patients with invasive breast cancer who underwent US-guided cryoablation followed by surgical resection. Cryoablation destroyed successfully 100% of cancers ≤1.0 cm, whereas in patients with invasive ductal carcinomas between 1.0 and 1.5 cm, 100% success rate was obtained only for tumors without a significant ductal carcinoma in situ (DCIS) component. They concluded that cryoablation should be limited to invasive ductal carcinoma up to 1.5 cm in maximum diameter having <25% DCIS component in the core biopsy.30
Poplack et al published in 2015 a retrospective study on 20 patients with invasive ductal carcinoma up to 15 mm, with limited or no DCIS who underwent US-guided cryoablation followed by surgical resection. They concluded that cryoablation was technically feasible and well tolerated by patients, although a clinical failure rate of 15% was observed. Interestingly, the authors highlighted that technical failures occurred when DCIS component was outside the cryoablation field.31
To date, the most important trial exploring the usefulness of cryoablation in the treatment of early breast cancer with curative intent was the level II trial ACOSOG Z1072. The primary endpoint was the rate of complete tumor ablation, defined as no remaining foci of either invasive or DCIS on pathological examination of the targeted lesion. Eighty-six patients with 87 unifocal invasive ductal carcinoma ≤2 cm underwent cryoablation and subsequent surgical resection of the target tumor within 28 days. Central pathologic review showed successful cryoablation in 75.9% of cancer lesions and residual invasive carcinoma and/or DCIS in 24.1%. There was 100% ablation in all tumors smaller than 1 cm. The authors concluded that technical adjustments and modifications in patient selection were needed to ameliorate cryoablation results in the non-surgical treatment of early breast cancer.32
Literature search reveals a high heterogeneity on this topic. Some studies enrolled a small number of patients at various stages of disease,18,33 in others surgical tumor removal was performed after cryoablation,31,34 and in others again percutaneous ablation was the only modality of treatment,6,35 thus reliable conclusions on the usefulness of cryoablation in the subgroup with early breast cancer could not be drawn.
The role of cryoablation in early breast cancer is under investigation in two ongoing trials in the US, the FROST (Freezing Instead of Removal Of Small Tumors) activated in 2016, and Ice3 (Cryoablation of Low Risk Small Breast Cancer) activated in 2014.36,37 Both studies seek to evaluate the role of cryoablation instead of surgery in women aged 50 years or older, having as primary endpoint assessment of 5-year local recurrence rate. Inclusion criteria in the FROST trial are: unifocal primary invasive breast carcinoma ≤1.5 cm in its greatest diameter, clinically node-negative, hormone receptor-positive, Her2 negative, with <25% intraductal component in the aggregate. Recruited patients will undergo US-guided cryoablation. After 6 months, an US-guided core biopsy of the cryoablated lesion will be performed, in order to confirm the absence of residual viable disease. Following cryoablation treatment, subjects will begin a minimum 5-year course of adjuvant hormonal therapy and serial mammography, US, and MRI. All subjects found to have residual or recurrent disease at follow-up image exams will undergo standard surgical resection.36 The ICE-3 trial has similar inclusion criteria: unifocal primary ducal invasive breast cancer ≤1.5 cm in greatest diameter, Nottingham grade 1–2, estrogen receptor-positive, progesterone receptor-positive, Her2 negative tumors. Final data collection for primary outcome measure are expected in December 2023.37
It is important to emphasize that at least 1 cm visible ice coverage beyond all tumor margins should be achieved when dealing with treatment of early breast cancer with curative attempt.18 In fact, the concept of obtaining “free margins” after breast conservation may be translated into “iced coverage beyond the tumor” after cryoablation and other ablative techniques. A 1-cm ice margin is generally considered as a conservative estimate of cytotoxic temperatures beyond the tumor.
To note, at present cryoablation has been reserved for treatment of patients with invasive ductal carcinoma. In fact, patients having pure DCIS or invasive ductal carcinoma with an extensive intraductal component cannot be considerate good candidates because those forms of breast cancer have indefinite margins at breast imaging (especially US and MRI) and may extend outside of the cryoablation-targeted ablation zone.15,30,38 Also, patients with invasive lobular carcinoma have generally been excluded because of frequent multicentricity and scarce correlation between tumor extension at preoperative imaging and final pathology examination.1
Cryoablation In Patients With Metastatic Disease
The effectiveness of removing the primary tumor in patients presenting with metastatic breast cancer (Stage IV at diagnosis) remains under debate. The only recognized indication for salvage surgery exists for locally advanced tumors with poor or nil response after systemic pharmacologic treatment. In these cases, some forms of treatment of the primary tumor are required to avoiding bleeding and/or ulceration of the tumor mass.20 However, things are different for patients presenting at Stage IV and having nonpalpable or clinically indolent tumors who have stable metastatic disease. In those patients, lumpectomy or mastectomy may represent an unnecessary treatment, which also may have aesthetical and psychological consequences related to the surgical procedure. In addition, changes in breast tumor size can be used as a measure of chemosensitivity of the primary tumor.
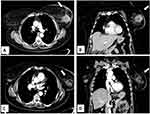
However, there is a growing interest in percutaneous ablation in patients presenting at Stage IV, because it permits to obtain tumor necrosis without the inconveniences of surgical procedures (Figure 2). Pusceddu et al reported on 35 patients at stage IV with mean tumor size of 3 cm submitted to CT-guided cryoablation. Complete tumor necrosis was achieved in 85.7% and 100% of cases at 2-month and 6-month follow-up, respectively. No patient developed major complications, while minor side effects occurred in 30 patients (82%). After a mean follow-up of 46 months, 7 patients (20%) experienced local recurrences that were treated with redo cryoablation. The authors concluded that cryoablation of the primary tumor is safe and effective in the treatment of patients presenting with metastatic disease.20 In a recent report, Beji et al evaluated results of cryoablation in 17 patients with stable metastatic breast cancer. In 15 patients, a complete regression of the primary breast lesion without recurrence was observed, whereas local recurrence developed in 2 patients with tumors ≥40 mm. Both of them underwent a second cryotherapy.39 The possibility to redo cryoablation after local failure is one of the points of strength of that ablative method (Figure 3).
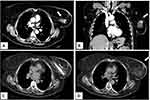 |
Figure 3 Same patient as shown in Figure 2. She developed a local recurrence after 12 months from the cryoablation procedure that was treated with redo cryoablation. Axial (A) and coronal (B) contrast-enhanced CT scan images showing a 2 cm nodule with enhancement in the previous ablation zone. (C) Second cryoablation with insertion of two cryo-probes. (D) Complete tumor ablation with no contrast-enhancement at the end of the procedure. |
Cryoablation In Patients Unsuitable Of Surgery
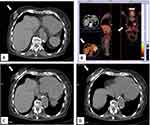
Cryoablation is nowadays widely used to treat breast cancer and other solid tumors, as this technique bears the major advantage of allowing accurate monitoring of iceball formation with different imaging modalities avoiding the use of contrast medium, i.e. unenhanced computed tomography. Although breast surgery can be usually safely performed in patients with relevant co-morbidities, there are some who are unsuitable for this approach due to general contra-indications (primarily cardiac or pulmonary insufficiency) or lack of consent for surgery (Figure 4). Cazzato et al carried out cryoablation in 23 elderly patients unsuitable for surgery, with median age of 86 years. After a median follow-up of 14.6 months, 5 patients developed local recurrence and 2 were successfully re-treated with cryoablation. At MRI follow-up, rates of primary complete local tumor control were 95.6%, 76.9%, 13.4%, and 9.4% at 3-, 12-, 18-, and 24-month, respectively.35
Cryoablation Of Breast Cancer In Comparison With Other Ablative Techniques
Few studies have compared cryoablation with other percutaneous ablative methods for breast cancer.1 Manenti et al compared radiofrequency ablation (40 patients) and cryoablation (40 patients) in the treatment of early breast cancer. All patients received sentinel lymph node biopsy. After 30–45 days from the ablation treatment, surgical resection of the tumor was scheduled. After 18-month follow-up, no local recurrences occurred. The authors observed complete necrosis in 75 patients (93.8%) and residual disease in 5 (6.2%). There was a good correlation between MRI volume and histologic samples. The conclusion of the study was that both percutaneous ablative methods achieved good clinical and cosmetic outcomes, but cryotherapy was the preferred method due to the analgesic effect of freezing with better patients compliance.34
Mauri et al assessed in a meta-analysis the technical success, technique efficacy, and complications of minimally invasive imaging-guided percutaneous ablation procedures for breast cancer. The study included 1156 patients with 1168 lesions from 45 studies. Cryoablation was used in 13% of patients, whereas radiofrequency the most used ablation method. Technique efficacy of cryoablation was 75%. General conclusions of the authors were that imaging-guided ablation techniques for breast cancer considered as a whole were 96% technically successful, but technique efficacy remains suboptimal. However, technique efficacy was significantly better in patients submitted to radiofrequency and cryoablation compared to laser, microwaves, and high intensity focused ultrasound.7
Imaging Follow-Up After Cryoablation Treatment
The extent of the ice ball caused by cryoablation can be recognized on US, CT scan, and MRI.8 However, timing and imaging methods of surveillance after breast cancer cryoablation remain to be better defined. In fact, early diagnosis of local recurrence after either surgical removal or ablation of the primary tumor is of paramount importance for disease-free survival, and perhaps for overall survival of patients with breast cancer. Contrast-enhanced MRI is a relatively new method in breast cancer detection and diagnosis, based on vascularization of tumor lesions. It has widely been used for planning surgical treatment in selected patients with invasive breast cancer and DCIS, and for assessing response to neoadjuvant systemic therapy.40,41 After cryoablation treatment, damaged cancer cells stay located in the treated area, thus MRI may be the most accurate image tool for evaluating responses to cryoablation.6,34,42 Manenti et al reported a good correlation between MRI volume and histological samples size in their series of 80 patients submitted to either radiofrequency ablation or cryoablation and surgical removal of the tumor.34 However, the role of MRI has been questioned by some authors. In the Z1072 trial, the negative predictive value of MRI was 81.2%.32 Poplack et al, in their retrospective study, observed that MRI was not useful to predict cryoablation results accurately, such as the detection of residual cancer and the recognition of benign cryoablation-related change.31
Immunologic Effects Of Cryoablation
In the era of introduction of immunotherapy in the treatment of various malignancies, increasing interest has been directed toward the cryoablation-induced anti-tumor immune response, which may aid in tumor control and cure.3,21,43 The key point is that antigenic tissue remains in the breast after local ablation. Cryoablation of tumor lesions causes the coagulative necrosis of neoplastic cells. During the thawing phase, tumor cells within the iceball release in blood circulation intact tumor antigens, as well as other “danger signals”, such as nuclear proteins, proinflammatory cytokines, and HMGB1, a molecule that stimulates antitumor immunity response through interactions with Toll-like receptors. These signals act as a stimulus for the natural immune response by attracting macrophages, NK cells, and granulocytes. These cells, in addition, cause cytokines release and dendritic cells, the professional antigen-presenting cells, to reach the cryoablated tissue.44,45 There are many studies showing the clinical benefit of cancer antigens. In fact those antigens can stimulate the production of antitumor antibodies, cytotoxic T-cells and induce a vigorous cytokine response targeted toward malignant cells. Thus, tumor-derived self-antigens can be released into circulation. The most advantageous method for the immune system to identify these new circulating cryoablated self-antigens may be the enhanced immune response activation caused by blocking tumor checkpoints. Experimental and clinical studies demonstrated the complementary roles for cytotoxic T-lymphocyte-associated protein (CTLA-4) and PD-1 antagonists in influencing adaptive immunity. Combination immunotherapy followed by cryosurgery seems to provide a more targeted immune response to distant lesions.43,46
Various immunostimulation and immunomodulation pathways are activated by necrotic tissue damage-associated molecular products.20,43 Furthermore, increased interest has been raised regarding the possible synergic effects of cryoablation and systemic treatments.
In an experimental model of triple-negative breast cancer, Chandra et al tested cryoablation combined with Meriva (a lecithin delivery system of curcumin with improved bioavailability) founding that cryoablation delayed the development of pulmonary metastases on the short term, and that post-cryoablation Meriva administration was significantly better in delaying the development of pulmonary metastases, and affected survival on the long-term period.19
In a recent study, breast cryoablation was combined with immune therapy in 19 women scheduled for mastectomy in whom pre-operative tumor cryoablation, single-dose Ipilimumab, or both were administrated. Interestingly, in the patients who received the combination of cryoablation and Ipilimumab, synergistic antitumor immunity effects were observed.47
The clinical benefits of a combination of tumor cryoablation with natural killer cells therapy and Herceptin were studied from Liang et al. In 48 patients with HER2- overexpressing recurrent breast cancer, the three-therapy combination treatment resulted in reduced levels of circulating tumor cells, reduced tumor markers such as CEA and CA15-3, and significant prolongation of progression free survival.48 The synergetic effect of cryoablation and doxorubicin nanoparticles was assessed as effective in MCF-7 model, a widely studied epithelial cancer cell line derived from breast adenocarcinoma.49
State Of The Art And Future Perspectives
Many studies have demonstrated that cryoablation represents a useful ablation method for patients with breast cancer, being a well-tolerated procedure that permits to obtain complete tumor necrosis in a high percentage of cases. However, robust evidence is lacking on long-term benefits of its use in the different clinical scenarios. Well designed randomized trials are needed to better elucidate the role of cryoablation in multimodality treatment of breast cancer. Overall, the literature data are heterogeneous, as highlighted in a recent systematic review from Lanza et al who reported a variable local tumor control ranging from 19% to 95%.8 Cryoablation may represent a valid technique for local treatment of patients who are unsuitable for, or refuse surgery. Moreover, it should be taken into account as a method of primary tumor ablation in patients presenting with distant metastases, who may benefit of a percutaneous procedure instead of surgical removal of the tumor, given the systemic diffusion of the neoplastic disease and the doubted utility associated to lumpectomy in those situations.
Cryoablation as an alternative to surgery in patients with early breast cancer is the most interesting aspect, because it may represent a conceptual shift toward a minimally invasive treatment. In spite of encouraging results, to date no studies have demonstrated that cryoablation is equal to breast-conserving surgery in terms of local control, disease-free survival, or overall survival. Interestingly, the ongoing trials are evaluating cryoablation as curative treatment in a highly selected patients, namely those older than 50 years having low risk breast cancer no larger than 15 mm. While waiting for the results of those trials, breast-conserving surgery must be considered as the treatment of choice for patients with early breast cancer.
Studies are also needed regarding timing and modalities of imaging follow-up after cryoablation, because the importance to early detect local recurrences after tumor ablation cannot be overemphasized.
Effects of cryoablation on immune system and possible synergic effects with systemic therapies are open and fascinating fields that deserve further attention. In fact, immune therapy has recently emerged as a useful treatment for various solid tumors. Currently, immune strategies include the use of drugs that modulate key T cell inhibitory checkpoints and vaccines. In particular, checkpoint blockade along with other treatments such as systemic therapies and localized therapy including tumor cryoablation seems to be a promising strategy in the treatment of breast cancer.
Author contributions
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cazzato RL, Garnon J, Ramamurthy N, et al. Percutaneous image-guided cryoablation: current applications and results in the oncologic field. Med Oncol. 2016;33:140. doi:10.1007/s12032-016-0848-3
2. Pusceddu C, Melis L, Ballicu N, et al. Percutaneous microwave ablation under CT guidance for hepatocellular carcinoma: a single institutional experience. J Gastrointest Cancer. 2018;49(3):295–301. doi:10.1007/s12029-017-9951-8
3. Mahnken AH, König AM, Figiel JH. Current Technique and Application of Percutaneous Cryotherapy. Rofo. 2018;190:836–846. doi:10.1055/a-0598-5134
4. Pusceddu C, Melis L, Sotgia B, Guerzoni D, Porcu A, Fancellu A. Usefulness of percutaneous microwave ablation for large non-small cell lung cancer: apreliminary report. Oncol Lett. 2019;18:659–666. doi:10.3892/ol.2019.10375
5. Zhao Z, Wu F. Minimally-invasive thermal ablation of early-stage breast cancer: a systematic review. Eur J Surg Oncol. 2010;36:1149–1155. doi:10.1016/j.ejso.2010.09.012
6. Machida Y, Shimauchi A, Igarashi T, Fukuma E. MRI findings after cryoablation of primary breast cancer without surgical resection. Acad Radiol. 2019;26:744–751. doi:10.1016/j.acra.2018.07.012
7. Mauri G, Sconfienza LM, Pescatori LC, et al. Technical success, technique efficacy and complications of minimally-invasive imaging-guided percutaneous ablation procedures of breast cancer: A systematic review and meta-analysis. Eur Radiol. 2017;27(8):3199–3210. doi:10.1007/s00330-016-4668-9
8. Lanza E, Palussiere J, Buy X, et al. Percutaneous image-guided cryoablation of breast cancer: a systematic review. J Vasc Interv Radiol. 2015;26:1652–1657. doi:10.1016/j.jvir.2015.07.020
9. Nguyen T, Hattery E, Khatri VP. Radiofrequency ablation and breast cancer: a review. Gland Surg. 2014;3:128–135. doi:10.3978/j.issn.2227-684X.2014.03.05
10. Rui J, Tatsutani KN, Dahiya R, Rubinsky B. Effect of thermal variables on human breast cancer in cryosurgery. Breast Cancer Res Treat. 1999;53:185–192. doi:10.1023/A:1006182618414
11. Pusceddu C, Melis L, Sotgia B, Fancellu A, Meloni GB. Computed tomography-guided cryoablation of local recurrence after primary resection of pancreatic adenocarcinoma. Clin Pract. 2015;5:741. doi:10.4081/cp.2015.741
12. Pusceddu C, Sotgia B, Amucano G, et al. Breast cryoablation in patients with bone metastatic breast cancer. J Vasc Interv Radiol. 2014;25:1225–1232. doi:10.1016/j.jvir.2014.05.001
13. Chu KF, Dupuy DE. Thermal ablation of tumors: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14:199–208. doi:10.1038/nrc3672
14. Tafra L, Smith SJ, Woodward JE, Fernandez KL, Sawyer KT, Grenko RT. Pilot trial of cryoprobe-assisted breast-conserving surgery for small ultrasound-visible cancers. Ann Surg Oncol. 2003;10:1018–1024. doi:10.1245/aso.2003.04.002
15. Tarkowski R, Rzaca M. Cryosurgery in the treatment of women with breast cancer—a review. Gland Surg. 2014;3:88–89. doi:10.3978/j.issn.2227-684X.2014.03.04
16. Morin J, Traore A, Dionne G, et al. Magnetic resonance guided percutaneous cryosurgery of breast carcinoma: technique and early clinical results. Can J Surg. 2004;47:347–351.
17. Pusztaszeri M, Vlastos G, Kinkel K, et al. Histopathological study of breast cancer and normal breast tissue after magnetic resonance-guided cryotherapy ablation. Cryobiology. 2007;55:44–51. doi:10.1016/j.cryobiol.2007.05.002
18. Littrup PJ, Jallad B, Chandiwala-Mody P, D’Agostini M, Adam BA, Bouwman D. Cryotherapy for breast cancer: a feasibility study without excision. J Vasc Interv Radiol. 2009;20:1329–1341. doi:10.1016/j.jvir.2009.06.029
19. Chandra D, Jahangir A, Cornelis F, et al. Cryoablation and Meriva have strong therapeutic effect on triple-negative breast cancer. Oncoimmunology. 2015;5:e1049802. doi:10.1080/2162402X.2015.1049802
20. Pusceddu C, Melis L, Ballicu N, et al. Cryoablation of primary breast cancer in patients with metastatic disease: considerations arising from a single-centre data analysis. Biomed Res Int. 2017;2017:3839012. doi:10.1155/2017/3839012
21. Fleming MM, Holbrook AI, Newell MS. Update on image-guided percutaneous ablation of breast cancer. AJR. 2017;208:267–274. doi:10.2214/AJR.16.16984
22. Fancellu A, Sanna V, Sedda ML, et al. Benefits of organized mammographic screening programs in women aged 50 to 69 years: a surgical perspective. Clin Breast Cancer. 2019. doi:10.1016/j.clbc.2019.04.013
23. Fancellu A, Sanna V, Cottu P, et al. Mastectomy patterns, but not rates, are changing in the treatment of early breast cancer. Experience of a single European institution on 2315 consecutive patients. Breast. 2018;39:1–7. doi:10.1016/j.breast.2018.02.003
24. Fancellu A, Cottu P, Feo CF, et al. Sentinel node biopsy in early breast cancer: lessons learned from more than 1000 cases at a single institution. Tumori. 2012;98:413–420. doi:10.1700/1146.12633
25. Gambardella C, Clarizia G, Patrone R, et al. Advanced hemostasis in axillary lymph node dissection for locally advanced breast cancer: new technology devices compared in the prevention of seroma formation. BMC Surg. 2019;18(Suppl 1):125. doi:10.1186/s12893-018-0454-8
26. Niu L, Wu B, Xu K. Cryosurgery for breast fibroadenomas. Gland Surg. 2012;1:128–131. doi:10.3978/j.issn.2227-684X.2012.08.02
27. Kaufman CS, Littrup PJ, Freeman-Gibb LA, et al. Office-based cryoablation of breast fibroadenomas with long-term follow-up. Breast J. 2005;11:344–350. doi:10.1111/j.1075-122X.2005.21700.x
28. Littrup PJ, Freeman-Gibb L, Andea A, et al. Cryotherapy for breast fibroadenomas. Radiology. 2005;234:63–72. doi:10.1148/radiol.2341030931
29. Paepke S, Metz S, Brea Salvago A, Ohlinger R. Benign breast tumours - diagnosis and management. Breast Care (Basel). 2018;13:403–412. doi:10.1159/000495919
30. Sabel MS, Kaufman CS, Whitworth P, et al. Cryoablation of early-stage breast cancer: work-in-progress report of a multi-institutional trial. Ann Surg Oncol. 2004;11:542–549. doi:10.1245/ASO.2004.08.003
31. Poplack SP, Levine GM, Henry L, et al. A pilot study of ultrasound-guided cryoablation of invasive ductal carcinomas up to 15 mm with MRI follow-up and subsequent surgical resection. AJR Am J Roentgenol. 2015;204:1100–1108. doi:10.2214/AJR.13.12325
32. Simmons RM, Ballman KV, Cox C, et al.; ACOSOG investigators. A Phase II Trial Exploring the Success of Cryoablation Therapy in the Treatment of Invasive Breast Carcinoma: results from ACOSOG (Alliance) Z1072. Ann Surg Oncol. 2016;23:2438–2445. doi:10.1245/s10434-016-5275-3
33. Gajda MR, Mireskandari M, Baltzer PA, et al. Breast pathology after cryotherapy. Histological regression of breast cancer after cryotherapy. Pol J Pathol. 2014;65:20–28. doi:10.5114/pjp.2014.42665
34. Manenti G, Scarano AL, Pistolese CA, et al. Subclinical breast cancer: minimally invasive approaches. Our experience with percutaneous radiofrequency ablation vs. cryotherapy. Breast Care (Basel). 2013;8:356–360. doi:10.1159/000355707
35. Cazzato RL, de Lara CT, Buy X, et al. Single-centre experience with percutaneous cryoablation of breast cancer in 23 consecutive non-surgical patients. Cardiovasc Intervent Radiol. 2015;38:1237–1243. doi:10.1007/s00270-015-1181-5
36. Cryoablation of Small Breast Tumors in Early Stage Breast Cancer (FROST). Available from: https://clinicaltrials.gov/ct2/show/NCT01992250.
37. Cryoablation of Low Risk Small Breast Cancer- Ice3 Trial. Available from: https://clinicaltrials.gov/ct2/show/NCT02200705.
38. Pfleiderer SO, Marx C, Camara O, Gajda M, Kaiser WA. Ultrasound-guided, percutaneous cryotherapy of small (< or = 15 mm) breast cancers. Invest Radiol. 2005;40:472–477. doi:10.1097/01.rli.0000166935.56971.ff
39. Beji H, Pilleul F, Picard R, et al. Percutaneous cryoablation of breast tumors in patients with stable metastatic breast cancer: safety, feasibility and efficacy. Br J Radiol. 2018;91(1083):20170500. doi:10.1259/bjr.20170500
40. Fancellu A, Soro D, Castiglia P, et al. Usefulness of magnetic resonance in patients with invasive cancer eligible for breast conservation: a comparative study. Clin Breast Cancer. 2014;14:114–121. doi:10.1016/j.clbc.2013.10.002
41. Fancellu A, Turner RM, Dixon JM, Pinna A, Cottu P, Houssami N. Meta-analysis of the effect of preoperative breast MRI on the surgical management of ducal carcinoma in situ. Br J Surg. 2015;102:883–893. doi:10.1002/bjs.9797
42. Pediconi F, Marzocca F, Cavallo Marincola B, Napoli A. MRI-guided treatment in the breast. J Magn Reson Imaging. 2018;48:1479–1488. doi:10.1002/jmri.26282
43. Sabel MS, Nehs MA, Su GK, et al. Immunologic response to cryoablation of breast cancer. Breast Cancer Res Treat. 2005;90:97–104. doi:10.1007/s10549-004-3289-1
44. Abdo J, Cornell DL, Mittal SK, Agrawal DK. Immunotherapy plus cryotherapy: potential augmented abscopal effect for advanced cancers. Front Oncol. 2018;8:85. doi:10.3389/fonc.2018.00085
45. Keisari Y. Tumor abolition and antitumor immunostimulation by physico-chemical tumor ablation. Front Biosci. 2017;22:310–347. doi:10.2741/4487
46. Sidana A. Cancer immunotherapy using tumor cryoablation. Immunotherapy. 2014;6:85–93. doi:10.2217/imt.13.151
47. McArthur HL, Diab A, Page DB, et al. A pilot study of preoperative single-dose ipilimumab and/or cryoablation in women with early stage breast cancer with comprehensive immune profiling. Clin Cancer Res. 2016;22:5729–5737. doi:10.1158/1078-0432.CCR-16-0190
48. Liang S, Niu L, Xu K, et al. Tumor cryoablation in combination with natural killer cells therapy and Herceptin in patients with HER2-overexpressing recurrent breast cancer. Mol Immunol. 2017;92:45–53. doi:10.1016/j.molimm.2017.10.003
49. Ye P, Yin H, Gu X, et al. Improved synergetic therapy efficiency of cryoablation and nanoparticles for MCF-7 breast cancer. Nanomedicine (Lond). 2018;13:1889–1903. doi:10.2217/nnm-2018-0168
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.