Back to Journals » Patient Preference and Adherence » Volume 13
Cross-Cultural Adaptation Of The Persian Version Of Test Of The Adherence To Inhalers (TAI)
Authors Khosravi S , Rafiei F, Norozy M, Khanmohamadi Hezave A , Ebrahimabadi M
Received 6 July 2019
Accepted for publication 18 September 2019
Published 3 October 2019 Volume 2019:13 Pages 1693—1699
DOI https://doi.org/10.2147/PPA.S222096
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Sharareh Khosravi,1 Fatemeh Rafiei,2 Maryam Norozy,3 Ali Khanmohamadi Hezave,4 Maryam Ebrahimabadi5
1Department of Pediatric, School of Nursing, Arak University of Medical Sciences, Arak, Iran; 2Department of Biostatistics and Epidemiology, School of Health, Scientific Research Center, Tehran University of Medical Sciences, Tehran, Iran; 3Infectious Ward, Vali-Asr Hospital, Arak University of Medical Sciences, Arak, Iran; 4Student Research Committee, School of Nursing, Arak University of Medical Sciences, Arak, Iran; 5Department of Medical-Surgical, School of Nursing, Arak University of Medical Sciences, Arak, Iran
Correspondence: Maryam Ebrahimabadi
Department of Medical-Surgical, School of Nursing, Basij Square, Academic Complex Prophet (PBUH), Blue Wings, Arak 3848176941, Iran
Tel +98 861 9187640617
Fax +98 861 8634173524
Email [email protected]
Purpose: Despite the importance of using inhalers concerning chronic obstructive pulmonary disease (COPD), patients generally have problems regarding adherence to medication regimen. The first step in understanding medication adherence is its assessment which requires a reliable tool. The aim of this study was to translate and perform the psychometric assessment of Test of Adherence to Inhalers (TAI).
Patients and methods: In this cross-sectional study, the 10-item TAI was utilized. Patients with COPD referring to Amir-al-Momenin and Vali-Asr hospitals in Arak, Iran, were the study population. The tool was translated using forward–backward translation, and its validity was evaluated via face validity, content validity, and concurrent validity. The reliability of the tool was assessed using test–retest and Cronbach’s alpha.
Results: A total of 100 patients with COPD participated in the study, where the results showed that the tool has a good face validity. The CVR was 0.83, the CVI was 0.95, and the concurrent validity with General Medication Adherence Scale (GMAS) was moderate which was not significant (r = 0.613, p = 0.06). The results of the reliability test further showed that in the test–retest, Pearson correlation coefficient was 0.986, ICC was 0.972, and Cronbach’s alpha was 0.986.
Conclusion: The tool was translated to Farsi language, with the results indicating that Farsi TAI is a valid and reliable tool for measuring inhaler adherence in patients with chronic pulmonary disease.
Keywords: chronic illness, drug, compliance, nursing care, validity, reliability
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive and life-threatening illness. According to the WHO, the burden of the disease will increase in the coming decades due to exposure to risk factors (such as smoking), population growth, and aging, and it will become the third cause of mortality by 2030.1,2 In 2016, according to the Global Burden of Disease Study, the incidence of COPD worldwide was 251 million. However, 3.71 million COPD-associated deaths occurred throughout the world in 2015.3
Based on the available statistics, on average, 10% of Iranians are suffering from COPD, which varies from 1% to 40% in different communities with different climatic conditions.4 The prevalence of COPD is augmenting day by day, while there is no definitive treatment;5 however, the signs and symptoms can be relieved, and life quality can be ameliorated by treatment, thereby reducing hospitalization and death rates.6 The inhalers such as bronchodilators and anti-inflammatory drugs are the main treatments in patients with COPD, while oral medications such as theophylline are only occasionally used, hence the importance of sprays in managing the disease.7
In spite of the importance of taking medication, research has shown that people have currently problems adhering to their drug regimens.8,9 Various studies have indicated that adherence to the prescribed regimen regarding disorders such as COPD and asthma is at most 50%.10 Factors such as age, disease-related variables (duration, severity), and disease understanding affect adherence to inhalers.11–15
Non-adherence to the treatment regimen is a major obstacle in the health system, with ramifications such as disease recurrence, frequent admissions, and more medications, accompanied by increased health care costs.3,16 Non-adherence to medication regimen has various reasons such as the multiplicity of drugs used by patients, concerns about the side effects of medication, misunderstanding the benefits of drugs, and forgetfulness.17,18 In a qualitative study performed in Pakistan, barriers to medication adherence were identified as patient behavior, comorbidity and pill burden, cost-related non-adherence, and low levels of knowledge.19
Adherence is a behavioral process, as decision-making towards taking or non-taking medicine can be considered as a behaviour.20 The first step in understanding adherence is its evaluation, hence the necessity of valid, reliable, and cost-effective tools accepted by both health care providers and patients. The widespread use of such tools in different societies can provide a better understanding of adherence-related variables, non-adherence, and basis for interventions to ultimately increase the adherence to therapies.21
Self-reporting questionnaires are currently the most common and cost-effective tools.20 However, they are rarely used in clinics due to their inadequate validity, particularly for inhalers.22
Commonly employed, inhalers are a major component of drug therapy in patients with COPD, whose correct usage is of great importance. Therefore, as concerns measuring the adherence of patients to inhalers, the general adherence to medication assessment tools is not appropriate given the manner of their use, hence the need for a specific instrument for inhalers. TAI is a tool specifically designed in this area.
Therefore, the researchers decided to assess the validity and reliability of the Farsi version of TAI in COPD patients.
Materials And Methods
Study Design And Patients
This is a cross-sectional study conducted for a cultural adaptation of the Persian version of TAI. Participating in the research were 100 patients with COPD referred to Amir-al-Momenin and Vali-Asr educational hospitals in Arak, Iran. All patients were under treatment by two pulmonologists.
This work was supported by the Office of the Vice Chancellor for Research, Arak University of Medical Sciences (IR) under Grant 2825. The Ethics Committee of Arak University of Medical Sciences further approved this project by IR.ARAKMU.REC.1396. 135.
Test Of Adherence To Inhalers (TAI)
TAI was designed and validated by researchers in the Spanish Society of Pulmonology and Thoracic Surgery during an accurate research process. This self-report tool is suitable for daily use in clinical practice owing to its ease of use and the low amount of time required for completion.22 In a study, it became clear that TAI can be used to identify the types of drug non-adherence in COPD patients to plan comprehensive interventions and correct the identified deficiencies.6
This questionnaire has two forms, the first type being a 10-item questionnaire completed by the patient, identifying the patient’s adherence status ranging from poor adherence to optimal adherence. There is also a 12-item questionnaire containing 2 other questions answered by health care providers. If the questionnaire is aimed to check the degree of compliance, the 10-item form is used, and in the case of determining the pattern and type of non-compliance, the 12-item form is employed. In the present study, as in the research of Tegegn and Ayele (2017), the 10-item form was examined.23
The 10-item form is scored from 1 (the poorest) to 5 (the best) for each item, and the total score of the questionnaire ranges from 10 to 50 (the score of 45 or less indicates poor adherence, 46 to 49 is intermediate adherence, and 50 indicates good adherence).
Translation
The tool was translated separately by two translators fluent in English. During a session, the translated tools were compared to conclude a general result, where a single translation was prepared. Two experts checked the accuracy of the translated tool and made the necessary changes. The translated tool was then re-translated to English by a translator fluent in English and compared with the original text. The translated tool was reviewed and approved by the researchers.
Psychometric Properties Of The Instrument
Face validity, content validity, and concurrent validity were assessed to validate the tool. To test the reliability and internal consistency, the test–retest and Cronbach’s alpha were employed, respectively.
Face validity was assessed by 10 patients and 5 specialists.24 The translated questionnaire was primarily provided to 10 hospitalized patients with COPD to complete based on the description provided by one of the researchers; the patients shared their opinions with the researchers regarding the comprehensibility and possible misunderstandings associated with the questions. After that, the tool was given to five experts to confirm the concept and the correctness of the items; their opinions were then applied. The experts were nursing educators, pharmacologists, and pulmonologist who were accessed in their work place.
Content validity was determined by 10 experts. First, the content validity ratio (CVR) was specified based on the Lawshe criteria. Considering the number of contributor experts, the ratio of 64% was considered as appropriate.25 Next, the content validity index (CVI) was scored by 10 experts based on Waltz and Basel indicators (relevance, clarity, and fluency). In this method, the items with a score of over 0.79 are appropriate, those between 0.79 and 0.70 are to be corrected, and the scores less than 0.70 are unacceptable.26–28
Concurrent validity was specified by TAI questionnaire and General Medication Adherence Scale (GMAS). GMAS is developed by Naqvi et al (2018). Its content validity index (CVI) was reported 0.8 and Cronbach’s alpha 0.84. The test–retest Pearson’s correlation coefficient was reported 0.996 (p-value <0.01). Also, in another study to translate and validate GMAS, its internal consistency was 0.82 and the tool established convergent, discriminant, and concurrent validities. The two scales were completed simultaneously by the patients and the correlation between the results of the two questionnaires was further examined.29,30
For Cronbach’s alpha, the tool was completed by 30 patients, and the Cronbach’s alpha was calculated for each item as well as for the whole instrument. The Cronbach’s alpha over 0.70 was considered as appropriate.26,31
In test–retest, the questionnaire was completed by the 10 patients in two stages within a week. The first stage was done face to face and the second stage by telephone. Pearson correlation coefficient and intra-class correlation coefficient (ICC) were determined, with the index higher than 0.8% considered as desirable.32–34
Following the translation and psychometric evaluation of the scale and the preparation of the correct version of the Farsi scale, 100 patients were studied by the scale to evaluate their inhaler medication adherence.
The research was conducted after obtaining permission from the original designer of the questionnaire (Plaza et al) and written informed consent of all the participants in the study; this study was conducted in accordance with the Declaration of Helsinki.35
Results
Validity
Face Validity
The translated questionnaire had a good face validity after applying the opinions of the patients and specialists.
Content Validity
The content validity ratio and the content validity index of the tool are shown in Table 1.
 |
Table 1 Content Validity Of The Questionnaire |
The CVR and CVI of the whole questionnaire were 0.83 and 0.95, respectively. All the items had good CVR and CVI. Table 2 shows the content validity index of the questionnaire in terms of relevance, clarity, and fluency.
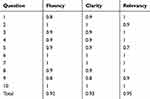 |
Table 2 Content Validity Index (Relevancy, Clarity, And Fluency) |
Concurrent Validity
The results of the concurrent validity of the instrument together with GMAS are presented in Table 3, where there is a moderate correlation between the two questionnaires which is not significant (r= 0.613, p= 0.06).
 |
Table 3 Concurrent Validity Of TAI & GMAS |
Reliability
Table 4 presents the results of the Pearson correlation coefficient and the ICC and Cronbach’s alpha of the questions and the whole instrument. The results show that in test–retest, the Pearson correlation coefficient of the questions ranged between 0.822 and 0.939, averaging 0.986 for the whole questionnaire. The ICC of the questions ranged from 0.819 to 0.939, averaging 0.972 for the whole questionnaire. The Cronbach’s alpha of the questions varied from 0.901 to 0.968, with an average of 0.986 for the whole questionnaire.
 |
Table 4 Reliability Measures Of TAI |
Pilot Study
100 patients were evaluated by the scale. The mean (SD) age was 53 (6.17) years. 57% of study participants were male and 31% had high school education. The demographic characteristics of the patients are presented in Table 5.
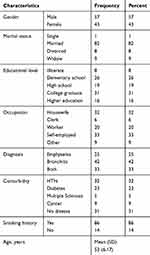 |
Table 5 Demographic Characteristics Of Study Participants |
The adherence to medication rate of patients is provided in Table 6, where most had a poor medication adherence, probably due to the high cut off presented for TAI. Based on the statistical analysis, there was no relationship between the demographic characteristics of the patients and their medication adherence.
 |
Table 6 Patient Adherence To Medication |
Table 7 indicates some information about the item scores.
 |
Table 7 The Information About The Item Scores |
Discussion
It is believed that lack of adherence by patients is a serious global health problem affecting all areas, cultures, ages, and diseases. This problem has remained unchanged over the past 30 years. The poor treatment adherence of the patients ensues higher mortality, frequent attacks, poorer control, and higher social and economic costs, particularly concerning diseases such as asthma and COPD. The clinical significance of this issue has led to guidelines for identifying and then modifying poor treatment adherence. Given that the main treatment of these patients is through inhalers, a tool is required to check adherence to inhalers.36
The present cross-sectional study focused on the translation and psychometry of TAI. The results showed that the Farsi version of TAI has good validity and reliability. In determining the validity of the tool, it was observed that all items had a CVR ranging from 0.8 to 1, and a CVI varying from 0.7 to 1, which is acceptable. The designers of this questionnaire further concluded that TAI has good psychometric properties, and is suitable for assessing medication adherence regarding asthma and COPD patients.22 To evaluate the concurrent validity of the TAI tool, GMAS was considered, where a moderate but not significant correlation was observed between the two questionnaires (r= 0.613, p= 0.06). In their study on compliance with inhalers in the COPD patients from seven Latin American countries, Montes de Oca et al (2017) made use of TAI and Morisky questionnaires, observing that the coefficient of agreement between the two questionnaires was moderate (Kappa index: 0.42; agreement: 64.7%).37 In Spain, a multiple study was conducted to combine the results of the TAI tool and electronic registry recording of drugs used to improve the understanding of medication adherence status. In this research, however, the results of the preliminary study, done on a limited number of samples, showed that the information obtained by the TAI was valid in contrast to the information associated with the electronic registry.36
In determining the reliability of the tool, it was observed that in the test–retest, the correlation coefficient of the whole questionnaire was 0.973, the ICC was 0.972, and Cronbach’s alpha was 0.986, all of which are acceptable. The designers of the questionnaire further conclude that the tool has excellent internal validity (Cronbach’s alpha = 0.873) and appropriate test–retest reliability (ICC = 0.883).36
The pilot study showed poor medication adherence, probably, due to the high cut off the original study (Plaza et al). If the cut off was considered lower, medication adherence scores might be better.
Limitations
In the test–retest, the first stage of instrument completion was performed in the presence of the patients; the second stage, on the other hand, was conducted via telephone calls due to lack of access to the patients, which might limit the accuracy of responses by the patients.
Conclusion
It is highly important to have a valid and reliable tool to assess the adherence to inhalers, especially in COPD patients. In this regard, TAI is a tool designed by Plaza et al for this purpose. The present study aimed to translate and determine the psychometric properties of the tool to provide an effective step towards identifying the patient adherence to medication status and planning for their medication adherence. The results showed that the Farsi version of the tool has good validity and reliability; however, the result of concurrent validity needs more studies. TAI seems a suitable, accessible, and inexpensive tool for daily use in clinical practice.
Acknowledgments
The authors thank and appreciate all the participants in this study. They are also grateful to Plaza et al and Naqvi et al for the permission to use their questionnaires.
Disclosure
The authors of this paper declare no competing interests in this work. The authors alone are responsible for the content and writing of the paper.
References
1. Lin W-C, Huang T-Y, Liu C-Y, Yeh M-L, Yu C-H, Hwang S-L. Validation of the clinical COPD questionnaire in Taiwan. Copd. 2016;13(3):360–366. doi:10.3109/15412555.2015.1094456
2. Blanco I, Diego I, Bueno P, et al. Geographical distribution of COPD prevalence in Europe, estimated by an inverse distance weighting interpolation technique. Int J Chron Obstruct Pulmon Dis. 2018;13:57. doi:10.2147/COPD.S150853
3. Amininasab S, Lolaty H, Moosazadeh M, Shafipour V. Medication adherence and its predictors among patients with heart failure. Nursing Midwifery Stud. 2018;7(2):81–86. doi:10.4103/nms.nms_9_17
4. Sharifi H, Masjedi MR, Emami H, et al. Interim report from burden of obstructive lung disease (BOLD Study) in Tehran: prevalence and risk factors of chronic obstructive pulmonary disease. Tanaffos. 2014;13(3):6–13.
5. Pitta F, Troosters T, Probst VS, Langer D, Decramer M, Gosselink R. Are patients with COPD more active after pulmonary rehabilitation? Chest J. 2008;134(2):273–280. doi:10.1378/chest.07-2655
6. Henrich R, Logue M. Feasibility of using the test for adherence to inhalers tool and tailoring education to the individual with chronic obstructive pulmonary disease. J Nurse Pract. 2017;13(10):e481–e483. doi:10.1016/j.nurpra.2017.07.025
7. Bonini M, Usmani OS. The importance of inhaler devices in the treatment of COPD. COPD Res Pract. 2015;1(1):9. doi:10.1186/s40749-015-0011-0
8. Farahani DF, Shamsi M, Khorsandi M, Rezanfar MR, Ranjbaran M. The relationship between perceived barriers and non-medication adherence in type 2 diabetic patients in Arak in 2014. Daneshvar Med. 2015;22:49–58.
9. Krska J, Katusiime B, Corlett SA. Validation of an instrument to measure patients’ experiences of medicine use: the living with medicines questionnaire. Patient Prefer Adherence. 2017;11:671. doi:10.2147/PPA.S134792
10. Simoni-Wastila L, Wei Y-J, Qian J, et al. Association of chronic obstructive pulmonary disease maintenance medication adherence with all-cause hospitalization and spending in a Medicare population. Am J Geriatr Pharmacother. 2012;10(3):201–210. doi:10.1016/j.amjopharm.2012.04.002
11. Olszanecka-Glinianowicz M, Almgren-Rachtan A. The adherence and illness perception of patients diagnosed with asthma or chronic obstructive pulmonary disease treated with polytherapy using new generation Cyclohaler. Postepy Dermatologii I Alergologii. 2014;31(4):235–246. doi:10.5114/pdia.2014.45070
12. Lacasse Y, Archibald H, Ernst P, Boulet LP. Patterns and determinants of compliance with inhaled steroids in adults with asthma. Can Respir J. 2005;12(4):211–217. doi:10.1155/2005/375454
13. Krigsman K, Moen J, Nilsson JL, Ring L. Refill adherence by the elderly for asthma/chronic obstructive pulmonary disease drugs dispensed over a 10-year period. J Clin Pharm Ther. 2007;32(6):603–611. doi:10.1111/j.1365-2710.2007.00866.x
14. Neugaard BI, Priest JL, Burch SP, Cantrell CR, Foulis PR. Quality of care for veterans with chronic diseases: performance on quality indicators, medication use and adherence, and health care utilization. Popul Health Manag. 2011;14(2):99–106. doi:10.1089/pop.2010.0020
15. Partridge MR, van der Molen T, Myrseth SE, Busse WW. Attitudes and actions of asthma patients on regular maintenance therapy: the INSPIRE study. BMC Pulm Med. 2006;6:13. doi:10.1186/1471-2466-6-13
16. Dowse R, Ehlers M. Medicine labels incorporating pictograms: do they influence understanding and adherence? Patient Educ Couns. 2005;58(1):63–70. doi:10.1016/j.pec.2004.06.012
17. Cinar S, Barlas GU, Alpar SE. Stressors and coping strategies in hemodialysis patients. Pak J Med Sci. 2009;25(3):447–452.
18. Minaiyan M, Taheri M, Mirmoghtadaee P, Marasi M. Comparative role of demographic factors and patient’s belief about prescribed medicine on adherence to drug treatment in chronic diseases. J Isfahan Med School. 2011;29:156.
19. Naqvi AA, Hassali MA, Aftab MT, Nadir MN. A qualitative study investigating perceived barriers to medication adherence in chronic illness patients of Karachi, Pakistan. Jpma. 2019;69:216.
20. Duarte-de-Araujo A, Teixeira P, Hespanhol V, Correia-de-Sousa J. COPD: understanding patients’ adherence to inhaled medications. Int J Chron Obstruct Pulmon Dis. 2018;13:2767–2773. doi:10.2147/COPD.S160982
21. Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens. 2008;10(5):348–354.
22. Plaza V, Fernandez-Rodriguez C, Melero C, et al. Validation of the ‘Test of the Adherence to Inhalers’ (TAI) for asthma and COPD patients. J Aerosol Med Pulm Drug Deliv. 2016;29(2):142–152. doi:10.1089/jamp.2015.1212
23. Ayele AA, Tegegn HG. Non adherence to inhalational medications and associated factors among patients with asthma in a referral hospital in Ethiopia, using validated tool TAI. Asthma Res Pract. 2017;3(1):7. doi:10.1186/s40733-017-0035-0
24. Aliabadi F, Borimnejad L, Kamali M, Rassafiani M, Nazi S. Perceived Maternal Parenting Self-Efficacy (PMP SE) tool: translation and face validation with Iranian mothers of hospitalized preterm neonates. Iran Rehabil J. 2013;11:7–10.
25. Lawshe CH. A quantitative approach to content validity 1. Pers Psychol. 1975;28(4):563–575. doi:10.1111/peps.1975.28.issue-4
26. LoBiondo-Wood G, Haber J. Nursing Research: Methods and Critical Appraisal for Evidence-based Practice.
27. Rodrigues IB, Adachi JD, Beattie KA, MacDermid JC. Development and validation of a new tool to measure the facilitators, barriers and preferences to exercise in people with osteoporosis. BMC Musculoskelet Disord. 2017;18(1):540. doi:10.1186/s12891-017-1624-z
28. Yaghmaie F. Content validity and its estimation. J Med Educ. 2003;3:1.
29. Naqvi AA, Hassali MA, Rizvi M, et al. Development and validation of a novel General Medication Adherence Scale (GMAS) for chronic illness patients in Pakistan. Front Pharmacol. 2018;9:1124. doi:10.3389/fphar.2018.01124
30. Naqvi AA, Hassali MA, Jahangir A, Nadir MN, Kachela B. Translation and validation of the English version of the general medication adherence scale (GMAS) in patients with chronic illnesses. J Drug Assess. 2019;8(1):36–42. doi:10.1080/21556660.2019.1579729
31. Yurdugül H. Minimum sample size for Cronbach’s coefficient alpha: a Monte-Carlo study. Hacettepe Üniversitesi Egitim Fakültesi Dergisi. 2008;35(35):1–9.
32. Fleiss JL. Design and Analysis of Clinical Experiments. Vol. 73. John Wiley & Sons; 2011.
33. de Boer MR, Moll AC, de Vet HC, Terwee CB, Volker-Dieben HJ, van Rens GH. Psychometric properties of vision-related quality of life questionnaires: a systematic review. Ophthalmic Physiol Optics. 2004;24(4):257–273. doi:10.1111/j.1475-1313.2004.00187.x
34. Polit DF, Beck CT, Owen SV. Is the CVI an acceptable indicator of content validity? Appraisal and recommendations. Res Nurs Health. 2007;30(4):459–467. doi:10.1002/nur.20199
35. Declaration H. Human experimentation: code of ethics of World Medical Association Br. Br Med J. 1964;2:18.
36. Plaza V, López-Viña A, Cosio BG. Test of adherence to inhalers. Archivos De Bronconeumología. 2017;53(7):360–361. doi:10.1016/j.arbr.2017.03.005
37. Montes de Oca M, Menezes A, Wehrmeister FC, et al. Adherence to inhaled therapies of COPD patients from seven Latin American countries: the LASSYC study. PLoS One. 2017;12(11):e0186777. doi:10.1371/journal.pone.0186777
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
