Back to Journals » Journal of Multidisciplinary Healthcare » Volume 13
Critical Evaluation of Drug Advertisements in a Medical College in Lalitpur, Nepal
Authors Jha N , Sapkota Y, Shankar PR
Received 29 April 2020
Accepted for publication 15 July 2020
Published 29 July 2020 Volume 2020:13 Pages 717—725
DOI https://doi.org/10.2147/JMDH.S259708
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Video abstract presented by Yunima Sapkota.
Views: 414
Nisha Jha,1 Yunima Sapkota,2 Pathiyil Ravi Shankar3
1Department of Pharmacology, KIST Medical College, Gwarko, Lalitpur, Nepal; 2Department of Pharmacy, Central Institute of Science and Technology, Baneshwor, Nepal; 3Department of Basic Sciences, Oceania University of Medicine, Apia, Samoa
Correspondence: Nisha Jha Email [email protected]
Purpose: The information provided in drug advertisements (DAs) often do not follow the recommended criteria and may promote irrational prescribing behaviors. Recently Health Action International (HAI) formulated detailed criteria to evaluate DAs which further develop and expand on the World Health Organization (WHO) criteria. This study was done to evaluate DAs using both criteria.
Methods: The study was carried out from October 2019 to January 2020 in the Department of Pharmacology of KIST Medical College, Lalitpur, Nepal. A structured proforma was used to collect data.
Results: Altogether 100 DAs were analyzed. Maximum (85%) were having pictorial presentations. Majority (89%) were found to have authentic information and 3% were found to have exaggerated information. All DAs mentioned generic name, brand name, active drug per dosage form and approved therapeutic uses. Only 4% of DAs mentioned about the adverse effects that can be caused by the use of these medicines. The DAs evaluated as per the HAI criteria for pictures and images showed that people portrayed did not seem to be Nepalese. Females and males were portrayed differently with females being laypersons and males being healthcare professionals. Nineteen DAs contained 33 references to scientific literature. Thirty references contained adequate citation information to be identified and were retrievable. Retrieved references were of high methodological quality and from peer-reviewed journals. There was only one graph in the DAs and it contained the number needed to treat (NNT) information. The graph was not having statistical calculations and was not obscured by other visual material.
Conclusion: Using both HAI and WHO criteria for assessing the DAs was the strength of this study. None of the DAs fulfilled all the criteria. Additionally, lack of any information on harm in the large majority of DAs, and very limited backing of claims with references was also seen.
Keywords: drug advertisements, ethical criteria, evaluation, health action international, Nepal
Introduction
Different methods for promoting medicines are adopted by pharmaceutical industries. These include a combination of visual aids, drug advertisements (DAs), leaflets and various audio-visual resources.1
Medical professionals also use these industry-produced sources for updating their current knowledge about new drugs for treating diseases.2,3 Pharmaceutical promotion can influence prescribing behavior.4 Different methods of promotion were addressed in the article and the influence of personal gifts to the doctor was also mentioned. Medical representatives are hired for providing information to the medical professionals and marketing the products in person.5 World Health Organization (WHO) defines drug promotion as all informational and persuasive activities by manufacturers and distributors, the effect of which is to induce the prescription, supply, purchase, and/or use of medicinal drugs.6 The WHO has developed ethical criteria for medicinal drug promotion.7 DDA is the national drug regulatory authority in Nepal. Guidelines have been developed by DDA for the ethical promotion of medicine in 2007. This was developed to encourage the rational use of medicine to prevent unethical drug promotion practices in the country.8 The drug regulatory authority of Nepal, Department of Drug Administration (DDA) has the power to screen drug advertisements as per the drug act of 1978.9 The DAs may not contain information about the adverse effects drug interactions and contraindications caused while using the medicine.10–12
Ethical drug promotion and authenticity of the DAs are important to promote the rational use of medicines. It has been found that the information provided in the DAs may not follow the code of ethics and can promote irrational prescribing behaviors.11,13 Few studies have been done in this area in Nepal. These studies used WHO criteria for assessing the DAs.4,11,12 WHO has developed eleven different criteria which a drug advertisement should contain and these are shown in Table 1. Some of the Health Action International (HAI) criteria were also used to analyze the DAs.14 These were regarding the types of references cited and details about the graphical and pictorial presentations in the DAs (Table 1). In addition, the presence of ‘catchy terms’ was also noted.
 |
Table 1 WHO and HAI Criteria Used to Assess the Drug Advertisements |
Hence, this study was done with an objective of evaluating the DAs in a scientific and critical manner for their adherence towards the mentioned HAI and WHO ethical criteria.
Method
A descriptive cross-sectional analysis of advertisements was carried out from October 2019 to January 2020 in the Department of Pharmacology of KIST Medical College, Lalitpur, Nepal. A total of 100 printed drug advertisements were analyzed. DAs were collected from all medical representatives visiting doctors in the out-patient departments during the specified duration of the study. There were 120 advertisements available during the study period, out of which 100 were selected as they met the inclusion criteria.
The inclusion criterion was companies registered with DDA on 1st of October 2019 and exclusion criteria were drug advertisements related to herbal products for human use, advertisements related to medical devices and reminder advertisements. A structured proforma was used to collect data. This proforma has been developed on the basis of the Health Action International (HAI) publication, understanding and responding to pharmaceutical promotion and the WHO ethical criteria for medicinal drug promotion.7,14 The proforma had different sections. These were section-1: presence of catchy terms, section-2: therapeutic category of the drugs promoted, section-3: type of claims, section-4: information about advertisements as per WHO criteria, section-5: evaluation of references, section-6: evaluation of data, graphs and pictures presented in pharmaceutical advertisements (as per HAI criteria). Catchy terms were the terms/phrases used for easy grasping and remembering therapeutic category and use of the drugs promoted in DAs. Table 2 shows some examples of the different types of catchy terms present in the DAs assessed.
 |
Table 2 Examples of Catchy Slogans/Terms Used |
In section 3, the claims from DAs were assessed. A claim was considered “justifiable” when the information was supported by suitable references, and “false” when the cited reference did not match the claim. Similarly, the term “exaggerated” claim was mentioned when the DAs were referring to unnecessary application/information in the advertisements that was not supported by the cited studies. Likewise, a claim was regarded as ‘ambiguous’ when there was no clear relation between a claim and the evidence provided by references.14 These criteria are in accordance with the HAI criteria and also with a recent article.15
A standard approach was used to code the advertisements. The DAs were independently analyzed by the first and second authors who then met, compared notes and arrived at a consensus, if required.
Then, the consensus statement was examined by the third author. If there was any discrepancy, the opinion of the third author was also obtained.
Ethical approval to conduct this study was obtained from the Institutional Review Committee (IRC), KIST Medical College Teaching Hospital (KISTMCTH) Reference number: 2076/77/26.
Results
There was a total of 120 DAs available, among which 100 were chosen as per inclusion criteria. The list of selected DAs with the manufacturing companies and drugs involved has been provided in the Appendix 1. Appendix 2 mentions the generic names of the drugs and their categories.
Figure 1 describes the therapeutic categories and their percentage in the assessed DAs. A maximum number of DAs were promoting antimicrobials 22 (22%) and the least number were promoting antiallergic drugs 2(2%).
 |
Figure 1 Deals with common therapeutic classes of drugs depicted. Antimicrobials, cardiovascular and anti-diabetic drugs were most commonly advertised. |
It was observed 86 (86%) DAs were promoting single drugs whereas 14 (14%) were promoting fixed-dose combinations as listed in the WHO Model Formulary-Essential Medicines, 20th edition.16 Among the DAs assessed, 81 were from national pharmaceutical companies and 19 were from multinational companies. Catchy terms or phrases were present in 68% of DAs and absent in 32%. Catchy terms are defined as short and sweet slogans developed as per the medicine’s therapeutic uses and name. Table 2 provides examples of different types of catchy terms present in the DAs assessed.
Figure 2 describes the types of visual depictions used in DAs. Maximum DAs were having pictures 85 (85%) and the least number of DAs were having graphs 1(1%), and scientific Table (2%). Graphical and pictorial presentations were analysed as per the HAI criteria mentioned in Table 1. Tables 3 and 4 depict the analyses as per the HAI criteria.
 |
Table 3 Evaluation of Pictures and Images in Drug Advertisements as per HAI Criteria (n=85) |
 |
Table 4 Evaluation of References in Drug Advertisements as per HAI Criteria (n=33) |
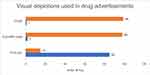 |
Figure 2 Provides information about the types of visual depictions used in the drug advertisements. Pictures were most commonly used while graphs and tables were used rarely. |
Figure 3 describes the types of pictures presented in DAs. The DAs also included the figures of medical instruments, doctors, and miscellaneous pictures like leaves, sunlight etc.
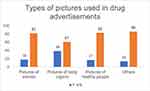 |
Figure 3 Shows types of pictures used in the advertisements. Pictures of body organs, women and healthy people were used. Among other images used were of nature, sunlight and medical instruments. |
References were present in 19 out of 100 DAs. The 19 DAs cited a total of 33 references. Six DAs had randomized clinical trial (RCT) articles and three DAs had cited review articles as their references. Randomized placebo control trial, in-vitro research study and websites were present as reference only in one DA each. Three of the references given in the DAs were not retrievable. The retrievable references provided scientific backup to the claims made by the advertisements. DAs citing only one reference were 15, whereas 4 of the DAs cited two or more references. The references in these DAs were a combination of Randomized Control Trials, Review Article and Websites.
None of the DAs cited non-randomized controlled trial, case-control studies, case report, editorial and books as references. Eighty-nine percent of DAs were found to have authentic information and 3% were found to have exaggerated information. None of the DAs made any controversial and false claims. All retrieved references were of high methodological quality and all references were from peer-reviewed medical or pharmacy journals. None of the studies were financed by the companies who had used the reference in their DAs. There were no retrieved references from supplement issues of journals.
The DAs evaluated as per the HAI criteria for pictures and images showed that they did not represent the racial and ethnic composition of the people of Nepal. Images of women were present in DAs as patients. Only males were portrayed as surgeons and physicians in the DAs. Elderly people were not portrayed and there were no symbols and metaphors used. Other images were of leaves, sunlight and medical instruments like sphygmomanometer and glucometer.
Only one DA had a graph and the number needed to treat (NNT) information was present in the graph. The graph was not obscured by other visual material and the information was meaningful.
The results from the analysis of DAs as per WHO’s ethical criteria are shown in Table 5. Only, 26 (26%) DAs had two therapeutic uses for the advertised drug, 4 (4%) DAs had only one use and 15 (15%) DAs had five or more approved therapeutic uses. Side effects and major adverse drug reactions were mentioned by only 4 (4%) DAs.
 |
Table 5 WHO Ethical Criteria for Medicinal Drug Promotion Met by Drug Advertisements (n =100) |
Discussion
DAs are an important source of drug information for healthcare professionals. There are many new and old drugs marketed for treating diseases. Besides single-drug formulations, many FDCs are also available in the market.3 A huge amount of money is spent each year on drug promotion and marketing activities.17 This study has shown that none of the DAs met the WHO ethical criteria for medicinal drug promotion in its entirety. Similar findings were observed in other studies conducted in various countries.1,3,11 The present study showed that 22% of DAs promoted the antimicrobial group of drugs. This is similar to other studies done elsewhere.12,18 Though these are prescription-only drugs, their irrational use is commonly seen around the globe.18–23
Thirty-nine DAs were having pictorial presentations of body organs followed by 18 DAs with pictures of women and 17 DAs with pictures of healthy people. Eighty-four DAs had pictures in our study, among which only 14 had irrelevant pictures which was very less as compared to another study having 78.6% of irrelevant pictures.24
The evaluation of pictures and images as per the HAI criteria showed that the racial and ethnic composition did not correspond to the people of Nepal. Only females were portrayed in drug advertisements as patients. This study has a greater number of images portraying females, which is different compared to a study from India, where males were portrayed more often than females.24 Pictures of women were used to create an attraction for the particular medicinal product.24 Pictures and images can help shape the way doctors use the product. Images can also be a means for drug advertisers to promote their medicines. A study from the US has showed that picture of a man was used as a doctor and picture of a woman was used as a patient. Commonly, males were shown as employers, and females were shown as employees doing typical female jobs like office assistants and food servers in the restaurants.24,25
Data and graphical presentations are commonly used to the support the claims shown in DAs. An analysis of the graphs from 64 advertisements in another study showed that 8% had errors and only 36% were self-explanatory.25
A study showed that DAs tended to promote antidepressants as focused on the female gender and depression as detached from any social context.26 Although depression was diagnosed twice as commonly in women than in men, some DAs were showing biased information mentioning the ratio of 5:1 and 10:0. This was done purposefully to promote gender-biased treatments.26
Eighty-nine percent of DAs were found to have authentic information and 3% of DAs were found to have exaggerated information on comparing the information provided with standard textbooks and the current edition of the Nepalese national formulary.27
None of the DAs made any controversial and false claims. This finding contrasted with a study done in Bangladesh, which showed that there were 21.5% of overstated claims and ambiguous and exaggerated claims were 16.312%, respectively.28 The majority (>80%) of DAs had no references cited. This is not good as DAs should have authentic references which can help doctors verify the authenticity of the claims made, if they so desire. Forty-three percent of DAs were not supported by appropriate references in a study from Nepal,12 whereas 50% of the claims made in DAs were not supported in a study done in Bangladesh.28
DAs citing references of non-placebo controlled randomized control trials were maximum (n=6) followed by review articles (n=3). These findings were different compared to other studies where a higher percentage of claims made by DAs were supported by appropriate references.1,15,25 A Spanish review showed that in 44% of the claims for which a reference was cited, the referenced studies did not support the claim.13
Analysis as per WHO ethical criteria showed that all DAs had mentioned the generic names, brand names, active drug per dosage forms and their approved therapeutic uses. This finding was better as compared to other studies, which mentions that only 87%11 and 88% of DAs18 mentioned about the presence of active ingredients in DAs. Similarly, the presence of the manufacturer’s name and address was present in 94 DAs in our study which is consistent with these studies. Adverse effects and major adverse drug reactions were mentioned by only 4 (4%) DAs. This was much lower as compared to studies from India which showed a much greater percentage of drug advertisements contained information about adverse effects.1,29 Adverse effects were reported by more DAs in another study done in Nepal, where the percentage was 33%. None of the DAs were consistently following all the eleven ethical criteria as given by WHO. This finding was consistent with the findings from previous studies done in Nepal and Brazil.11,12,30 Another study also highlighted the fact that the information for adverse reactions and warnings about drug interactions were missing in all the drug advertisements analyzed.11 Regarding the references, other studies have also found that only 60% of the DAs had citable/retrievable references.31 Only 56% of DAs were supported by citable references according to another study done in Nepal.
Limitations of the Study
The number of DAs analyzed was low and were from obtained from only one teaching hospital. It could have been better if DAs from other centers and hospitals could have been collected for the same purpose. We analyzed DAs to which we could obtain access which may not provide a representative picture about DAs in Nepal.
Conclusion
The DAs were assessed using the HAI and WHO’s ethical criteria for medicinal drug promotion. Using both criteria for assessing the DAs was the strength of this study. None of the DAs fulfilled all the WHO ethical criteria. The drug regulatory authority should make it mandatory for the industry to follow the said guidelines for providing the physicians with complete information about the drug. There have been very few studies done in Nepal on this issue and this study may help frame the necessary guidelines for improving the standards of drug advertisements. For critically appraising DAs, data and graphs, texts and references and images should be analysed. Often social dimensions are relevant as in the representation of the relationship of doctors and their patients or how females, elderly and the children are represented in DAs. Additionally, lack of any information on harm in the large majority of advertisements, and the very limited backing of claims with scientific evidence were also areas of concern.
Abbreviations
DA, drug advertisement; DDA, Department of Drug Administration; FDC, fixed dose combination; HAI, Health Action National; IRC, Institutional Review Committee; KISTMCTH, KIST Medical College and Teaching Hospital; WHO, World Health Organization.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mali SN, Dudhgaonkar S, Bachewar NP. Evaluation of rationality of promotional drug literature using World Health Organization guidelines. Indian J Pharmacol. 2010;42(5):
2. Phoolgen S, Kumar SA, Kumar RJ. Evaluation of the rationality of psychotropic drug promotional literatures in Nepal. J Drug Discov Ther. 2012;2:6–8.
3. Gopalakrishnan S, Murali R. India: Campaign to tackle unethical promotion. Essential drugs monitor. [Online]; 2002. Available from: http://www.apps.who.int/medicinedocs/pdf/s4937e/s4937e.pdf.
4. Giri BR, Shankar PR. Learning how drug companies promote medicines in Nepal. PLoS Med. 2005;2(8):e256. doi:10.1371/journal.pmed.0020256
5. Kornfield R, Donohue J, Berndt ER, Alexander GC. Promotion of prescription drugs to consumers and providers, 20012010. PLoS One. 2013;8:e55504. doi:10.1371/journal.pone.0055504
6. Rivera SM, Gilman AG. Drug invention and the pharmaceutical industry. In: Knollman B, Chabner B, Brunton L, editors. Goodman & Gilman’s the Pharmacological Basis of Therapeutics. New York: McGraw-Hill Medical; 2011:3–16.
7. Ethical criteria for medicinal drug promotion. World Health Organization [Online]; 1988 [
8. Department of Drug Administration, Kathmandu, Nepal. Guidelines on Ethical Promotion of Medicine. 2007. Unofficial draft document.
9. Department of Drug Administration (NP). Drug Act, 2035 [statute on the internet] Nepal: ministry of Health; 1978; [cited 2017]. Available from: http://www.lawcommission.gov.np/en/?s=Drug±act±1978.
10. Jadav SS, Dumatar CB, Dikshit RK. Drug promotional literatures (DPLs) evaluation as per World Health Organisation (WHO) criteria. J App Pharm Sci. 2014;4:84–88.
11. Alam K, Shah AK, Ojha P, Palaian S, Shankar PR. Evaluation of drug promotional materials in a hospital setting in Nepal. South Med Rev. 2009;2:2–6.
12. Prasad P, Bajracharya SR, Deo S, et al. Adherence of drug promotional literatures distributed by pharmaceutical companies to World Health Organization ethical criteria for medicinal drug promotion. J Nepal Health Res Counc. 2019;17(44):345–350. doi:10.33314/jnhrc.v17i3.1840
13. Santiago MG, Bucher HC, Nordmann AJ. Accuracy of drug advertisements in medical journals under new law regulating the marketing of pharmaceutical products in Switzerland. BMC Med Inform Decis Mak. 2008;8:16. doi:10.1186/1472-6947-8-61
14. Understanding and responding pharmaceutical promotion. A practical guide. World Health Organization/Health Action International collaborative project. Available from: https://haiweb.org/wp-content/uploads/2015/05/Pharma-Promotion-Guide-English.pdf.
15. Al-Tuhaifi TM, Awad AM, Abu-Zaid A, et al. Assessment of consistency between claims and references referred to in pharmaceutical advertising brochures in the Kingdom of Saudi Arabia. Cureus. 2019;11(1):e3907. doi:10.7759/cureus.3907
16. World Health Organization. WHO model list of essential medicines; 2017. Available from: https://www.who.int/medicines/publications/essentialmedicines/en/.
17. Rohra DK, Gilani AH, Memon IK, et al. Critical evaluation of claims made by pharmaceutical companies in drug promotional material in Pakistan. J Pharm Pharm Sci. 2006;9:50–59.
18. Angsulee NK. A participatory evaluation of the implementation of WHO’s ethical criteria for medical drug promotion in multiple countries. Final report; 2004. Available from: https://scholar.google.com/scholar_lookup?journal=Final±Report&title=A±participatory±evaluation±of±the±implementation±of±WHO’s±ethical±criteria±for±medical±drug±promotion±in±multiple±countries&author=NK±Angsulee&publication_year=2004&.
19. Othman N, Vitry A, Roughead EE. Quality of pharmaceutical advertisements in medical journals: a systematic review. PLoS One. 2009;4(7:e6350. doi:10.1371/journal.pone.0006350.
20. Harper I, Rawal N, Subedi M. Disputing distribution: ethics and pharmaceutical regulation in Nepal. Stud Nepali Hist Soc. 2011;16(1):1–39.
21. Khakhkhar T, Mehta M, Shah R, Sharma D. Evaluation of drug promotional literatures using WHO guidelines. J Pharm Negative Results. 2013;4(1):33–38. doi:10.4103/0976-9234.116770
22. Department of Drug Administration (NP). Drug Category Rules, 2043 [statute on the internet] Nepal: ministry of Health; 1986 [
23. Bbosa GS, Mwebaza N, Odda J, Kyegombe DB, Ntale M. Antibiotics/antibacterial drug use, their marketing and promotion during the post-antibiotic golden age and their role in emergence of bacterial resistance. Health. 2014;6(05):410–425. doi:10.4236/health.2014.65059
24. Jaykaran YP, Kantharia ND, Saxena D. Gender and racial bias in drug promotional material distributed by pharmaceutical companies. J Pharmacol Pharmacother. 2012;3(1):
25. Cooper RJ, Schriger DL. The availability of references and the sponsorship of original research cited in pharmaceutical advertisements. CMAJ. 2005;172:487–491. doi:10.1503/cmaj.1031940
26. Thunander Sundbom L, Bingefors K, Hedborg K, Isacson D. Are men under-treated and women over-treated with antidepressants? Findings from a cross-sectional survey in Sweden. B J Psych Bull. 2017;41(3):
27. Nepalese National Formulary.
28. Islam MS, Farah SS. Misleading promotion of drugs in Bangladesh: evidence from drug promotional brochures distributed to general practitioners by the pharmaceutical companies. J Public Health. 2007;29(2):
29. Vyas N, Shahani S. Analysis of drug promotional literatures in a tertiary care hospital: a cross sectional study. Int J Basic Clin Pharmacol. 2019;8(5):1102–1105. doi:10.18203/2319-2003.ijbcp20191608
30. Wzorek LF, Correr CJ, Trindade ACB, Pontarolo R. Analysis of medicine advertisement produced in Brazil. Pharm Pract. 2007;5(3):
31. Mejía R, Avalos A. Printed material distributed by pharmaceutical propaganda agents. Medicina. 2001;61(3):
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
