Back to Journals » Drug Design, Development and Therapy » Volume 16
cRGD Urokinase Liposomes for Thrombolysis in Rat Model of Acute Pulmonary Microthromboembolism
Authors Liang C, Huang T , Zhang X , Rao H, Jin Z , Pan X, Li J , Mo Y, Cai Y , Wu J
Received 24 November 2021
Accepted for publication 8 March 2022
Published 25 March 2022 Volume 2022:16 Pages 801—816
DOI https://doi.org/10.2147/DDDT.S351021
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Chunting Liang *, Tongtong Huang *, Xiaofeng Zhang, Huaqing Rao, Zhiru Jin, Xiaoxiong Pan, Jingtao Li, Yingying Mo, Yongzhi Cai, Ji Wu
Department of Ultrasonic Medicine, The First Hospital Affiliated to Guangxi Medical University, Nanning, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ji Wu, Department of Ultrasonic Medicine, The First Hospital Affiliated to Guangxi Medical University, Nanning, 530021, People’s Republic of China, Email [email protected]
Purpose: To study the thrombolytic effect and safety of cRGD urokinase liposomes (cRGD-UK-LIP) in rats with acute pulmonary microthromboembolism (APMTE), and explore the application value of echocardiography (ECHO) in animal models.
Patients and Methods: Ninety-six SD rats were randomized into 6 groups (16/group): normal control, sham operation, APMTE, normal saline (NS), free urokinase (UK), cRGD-UK-LIP. Four groups (APMTE, NS, UK, cRGD-UK-LIP) of rats were injected with autologous thrombus to induce APMTE. Samples were injected into 3 groups (NS, UK, cRGD-UK-LIP) of rats after modeling. Echocardiography was used to assess right ventricle (RV) function and morphology in rats. Six rats in each group were randomly selected and pulmonary artery pressure (PAP) of them was measured through ECHO-guided transthoracic puncture. Finally, the rats were killed and their tissues were taken for pathological examination.
Results: Compared with normal control or sham operation group, rats in APMTE group had enlarged RV, decreased RV function, increased PAP, and lung tissue of them showed postthromboembolic appearance. There was no significant difference between NS group and APMTE group. RV morphology and function of rats in the UK group and cRGD-UK-LIP group were better and vessels with residual thrombus in these 2 groups were less than APMTE group, especially in the cRGD-UK-LIP group. In terms of PAP, only cRGD-UK-LIP group was significantly lower than APMTE group. No hyperemia, bleeding and swelling were observed in heart, liver and kidney of rats in each group.
Conclusion: A rat model of APMTE was successfully established. cRGD-UK-LIP has better thrombolytic effect than free urokinase and it is safe. Echocardiography is not merely an important way to evaluate the morphology and function of RV, transthoracic puncture measurement under the guidance of it can be an effective way to monitor PAP in animal models.
Keywords: cRGD urokinase liposomes, acute pulmonary microthromboembolism, targeted thrombolysis, right ventricle, echocardiography, pulmonary artery pressure
Introduction
Pulmonary thromboembolism (PTE), a syndrome with pulmonary and cardiac dysfunction, caused by thrombi blocking major pulmonary artery and its branches, is a frequently encountered phenomenon and an important cause of high morbidity and mortality in lung diseases.1 Thrombi include detached thrombi from deep vein or right heart and in situ thrombus, in which detached thrombi from deep vein was dominant.2 Symptoms and prognosis of PTE depend on the site of embolism. Embolism of main pulmonary artery and important branches can be treated with timely and effective reperfusion therapy because it starts and progresses rapidly and easily observed. Microthromboembolism, however, is often ignored by clinicians because of no specific clinical manifestations, hidden thrombus and slow disease progression, which leads to long term existence and recurrence of thromboembolism, and may develop into persistent pulmonary hypertension and chronic pulmonary heart disease, causes irreversible damage to the lungs and heart.3,4 Accurate examination and effectual treatment are the cornerstones to curb the adverse outcome of microthromboembolism.5
The examination of pulmonary thromboembolism includes serum D-dimer, ventilation-perfusion scintigraphy, pulmonary angiography, spiral/helical computed tomography, and magnetic resonance imaging.6 Echocardiography, a convenient, economical and non-invasive inspection method, characterized by directly visualizing the embolus of main pulmonary artery and its important branches, providing evidence of its hemodynamic sequelae, is one of the important methods for diagnosis, treatment and prognosis evaluation of pulmonary embolism.7 In addition, echocardiography, used to observe the heart structure in real time and noninvasively, is an important auxiliary method in cardiac interventional operation.8 Whereas the research and application of echocardiography in animal models related to pulmonary embolism are insufficient.
Reperfusion of lung tissue is the focus of pulmonary thromboembolism therapy.9 Thrombolytic drugs, such as urokinase, activating plasminogen into plasmin to dissolve fibrin and then breaking down thrombosis, are the first choice for reperfusion therapy. However, these thrombolytic drugs have side effects, such as systemic fibrinolysis, hemorrhage, short half-life et al.10 Incomplete thrombolysis, involved in side effects, which leads to distal vascular embolism, is also one of the causes of pulmonary microthromboembolism. Therefore, upgrading thrombolytic drugs is one of the keys to optimize the treatment of pulmonary thromboembolism. Liposome is a fitting carrier for drugs because it releases contents slowly and its surface structure is easily modificated.11 RGD peptides, which specifically binds GPIIb/IIIa on activated platelets, is an ideal ligand for targeting thrombus.12 Hence, RGD functionalized liposome encapsulating urokinase has the advantages of targeting thrombus and prolonging half-life of urokinase.13 Its potential as improved thrombolytic drug was also proved by Zhang et al,13 as well as the earlier experiment of our research group.14,15
Previous studies on pulmonary embolism mostly focused on the main pulmonary artery and larger branches. These types of acute pulmonary embolism are often fatal, and the key to their treatment is timely and effective thrombolytic therapy.16 Thrombus-targeted liposome for slow and smooth drug release is more suitable for pulmonary microthromboembolism. Nevertheless, its application in the treatment of acute pulmonary microthromboembolism is still blank, and whether its therapeutic effect is better than traditional thrombolytic drugs in acute pulmonary microthromboembolism is still unknown.
Thus, based on the successful preparation of cRGD-UK-LIP and the proven thrombolytic effect of it in vitro, this study intended to establish a rat acute pulmonary microthromboembolism model, observe the morphology and function of RV through echocardiography, measure PAP through ECHO-guided transthoracic puncture, and make pathological observation of the hearts, lungs and other tissues of rats.14,15 In conclusion, this investigation researched the thrombolytic effect and safety of cRGD-UK-LIP in rats with APMTE, which could provide basic research evidence and new ideas for APMTE thrombolytic therapy, introduce RV morphology and function and hemodynamic parameters to enrich the basic data of cRGD-UK-LIP thrombolytic test, and explored the application and value of echocardiography in animal models of pulmonary embolism.
Materials and Methods
Materials
Ninety-six healthy 8-week-old male SD rats were provided by the Animal Experiment Center of Guangxi Medical University (license number: SCXK GUI 2020–0003, SYXK GUI 2020–0004). Animal feeding environment in line with China’s national standard, Laboratory Animal-Requirements of Environment and Housing Facilities (GB14925-2010). Animal management and experiment meeted the standard requirements, Animal management regulations. The research plan was approved by the Experimental Animal Ethics Committee of Guangxi Medical University. Cholesterol and 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) were purchased from Beijing Solarbio Science & Technology Co., Ltd. 1, 2-distearoyl-sn-phosphoethanolamine N- [methoxy (polyethylene glycol)-2000] (DSPE-mPEG2000) and DSPE-mPEG2000-cRGD were synthesized by Hunan Huateng Pharmaceutical Co., Ltd. Urokinase for injection (250,000U, Guangdong Tianpu Biochemical Pharmaceutical Co., Ltd) was obtained from Central Pharmacy of the First Affiliated Hospital of Guangxi Medical University. Chloral hydrate used in anesthesia was purchased from Tianjin Damao Chemical Reagent Factory. BL-420F biological signal acquisition and analysis system was purchased from Chengdu Taimeng Software Co., Ltd. Color Doppler ultrasound system (GE Vivid E95, USA), equipped with probe L8-18i-D, probe frequency 18MHz. The needle of a 5mL syringe is used for puncture.
Methods
Preparation of cRGD-UK-LIP
After mixing 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine N- [succinimidyl (polyethylene glycol)-2000] (DSPE-PEG2000-NHS), 1,2-distearoyl-sn-phosphoethanolamine N-[methoxy (polyethylene glycol)-2000] (DSPE mPEG2000) and cholesterol in a certain proportion, thin film hydration method was used to prepare cyclic RGD functionalized liposomes loaded with urokinase. The detailed preparation process refers to the research results of Rao Huaqing, a researcher in our team, and Nengpan Zhang, a researcher from University of Science and Technology of China.13,14
Preparation of Autologous Thrombus
The inner diameter of pulmonary small vessels in 5 normal SD rats were observed and measured, and found that the inner diameter was about 100um. Take 0.5mL blood from the tail vein of a rat, use a capillary pipet (about 100um in diameter) to quickly inhale the blood, and place it in a 37°C constant temperature water bath box overnight. Flushing the thrombus with normal saline to prepare thrombus particles autogenous thrombotic particles (about 100um×100um×100um) and suspending them in normal saline.
Establishment of Rat Models of APMTE
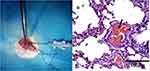
The rats were anesthetized by intraperitoneal injection of 10% chloral hydrate solution (0.3mL/100g). After anesthesia, the rats were fixed on the experimental table, and the right common jugular vein was exposed. About 0.5mL saline suspension containing 30 autogenous thrombotic particles was injected at a constant and slow speed (0.5mL/min, 1 embolus was inserted in about 2s, as far as possible to ensure that the emboli did not cluster) through the right common jugular vein with a 1mL syringe. And then 0.2mL saline was used to flush the tube (Figure 1A). The blood volume of rat was 60mL/kg, and each rat used in the experiment was about 250–300g (blood volume 15–18mL). The amount of fluid injected into the rats during modeling accounted for 4.7–4% of the total blood volume. After injection, the general condition of heart rate and respiration were observed and the thrombus were confirmed in pulmonary arterioles by pathological observation (Figure 1B).
Grouping and Administration
Ninety-six SD rats were randomized into 6 groups: normal control, sham operation, acute pulmonary microthromboembolism (APMTE), normal saline (NS), free urokinase (UK), cRGD urokinase liposomes (cRGD-UK-LIP), each group had 16 rats. Rats in the normal control group received no treatment, rats in the sham operation group were injected with 0.7mL saline through the right common jugular vein, and rats in the other 4 groups were injected with autogenous thrombi (0.5mL autogenous thrombotic suspension+0.2mL saline) through the right common jugular vein to induce acute pulmonary microthromboembolism.
After modeling, all rats were observed for 1 hour. Then RV structure and function of all rats in the normal control group, sham operation group and APMTE group were assessed by ECHO, 6 rats in each group were randomly selected for transthoracic puncture measurement of PAP under the guidance of ECHO. Rats in NS group, UK group (100U/g), cRGD-UK-LIP group (100U/g) were administrated with appropriate samples via tail vein and then observed for 9 hours.
After observing for 9 hours, RV structure and function of all rats in NS group, UK group and cRGD-UK-LIP group were assessed by ECHO, 6 rats in each group were randomly selected for transthoracic puncture measurement of PAP under the guidance of ECHO.
Finally, only lung tissues of unpunctured rats were collected for pathological observation, because of the possibility of accidental injury to both lungs during puncture process (Figure 2).
Echocardiography for Right Ventricular Function and Morphology
The echocardiographic studies were performed on Vivid E95 machines (GE Vivid E95, USA). M-mode, 2D and Doppler ultrasonography were used to detect right heart morphological and functional parameters. Each index was measured 3 times and averaged. Right ventricular transverse diameter (RVTD) and left ventricular transverse diameter (LVTD) were measured at the end of diastole in apical four-chamber view, and the ratio of RVTD to LVTD (RV/LV) was calculated. These indicators were used to assess RV morphology. Tricuspid annular plane systolic excursion (TAPSE) and right ventricular outflow tract systolic excursion (RVOT SE) were determined by M-mode echocardiography in apical four-chamber view and short-axis view. In the apical four-chamber view, the sampling volume was placed on lateral tricuspid annulus to obtain tissue Doppler images, and isovolumic contraction time (ICT), isovolumic relaxation time (IRT) and ejection time (ET) were measured. Right ventricular myocardial performance index (Tei index) = (ICT+IRT)/ET. TAPSE, RVOT SE and Tei index were used to evaluate RV systolic function. Ratio of pulsed Doppler of the tricuspid inflow to tissue Doppler of the lateral tricuspid annulus (E/e’) was used to assess RV diastolic function (Figure 3).
Echocardiography-Guided Transthoracic Puncture Measurement of Pulmonary Artery Pressure
After anesthesia, the puncture needle was connected to BL-420F system, and prefilled with heparin normal saline (20U/mL). Under the guidance of ECHO, the surgeon carefully inserted the puncture needle into the right ventricular outflow tract (RVOT) in parasternal short-axis section (Figure 4). After the RV pressure curve appeared, the surgeon continued to slowly advance the puncture needle to reach the main pulmonary artery and recorded the PAP curve.
Pathological Observation
Before the rats were sacrificed under excessive anesthesia, heparin (10U/100g) was injected through the tail vein to prevent blood coagulation. Tissues of the heart, lung, liver and kidney were removed quickly and cleaned with normal saline, and then observed in general. In SD rats, the blood flow rate of the main pulmonary artery was about 0.5m/s. We injected saline steadily and slowly (<0.5m/s) from the RV to clean the pulmonary vessels. Once the saline was clean, the entire lung tissue and other tissues were fixed in 10% formalin solution for 48h, dehydrated with alcohol, transparent with xylene, embedded in paraffin and sliced. Sections were stained with hematoxylin and eosin (H&E). Due to the small size of the thrombus, the infarct site of the lung was not obvious in general view, the lung was sliced from the sagittal position. With the help of experienced pathologists, arterioles and residual thrombus were searched at pulmonary lobule level. At high magnification (×200/×400), tissues of the heart, lung, liver and kidney were observed for signs of hyperemia, swelling, bleeding and inflammation, 60 pulmonary arterioles with diameters ranging from 80μm to 120μm were randomly observed and the number of vessels with residual thrombus was counted.
Statistical Analysis
SPSS version 22.0 was used for statistical analysis. Parametric data were expressed as mean ± standard deviation (Mean±SD). The measurement data with normal distribution and homogeneity of variance were compared by ANOVA, and LSD test was used for further pairwise comparison. Otherwise, they were compared by nonparametric test (Kruskal–Wallis test), and Bonferroni correction was used for further pairwise comparison. Two-sided values of P<0.05 was considered statistically significant.
Results
Characteristics of Rat
During modeling process, a certain proportion of rats in each group died (mortality rate 6–25%), most of which were due to anesthesia accidents, only one rat in the APMTE group and one rat in the NS group died of right heart failure (Table 1). None of rats died after administration as planned. RV structure and function of surviving rats were observed by echocardiography. Six rats in each group were successfully punctured to measure PAP under echocardiography guidance, and the remaining rats were sacrificed according to the norms and pathological specimens of them were taken.
 |
Table 1 Characteristics of Rats in Every Group |
RV Morphology and Function
The results are presented in Table 2. There was no significant difference in RV structure and function between normal control group and sham operation group. Compared with them, APMTE group had enlarged RV (RVTD:4.32±0.22 vs 3.81±0.19/3.83±0.31, P<0.05) and decreased RV systolic and diastolic functions (TAPSE: 1.66±0.15 vs 2.10±0.20/2.11±0.19, P<0.05; E/e’: 8.58±1.32 vs 6.43±1.51 or /6.30±0.79, P<0.05; Tei index: 0.83±0.13 vs 0.65±0.11/0.63±0.17, P<0.05, Figure 5A and B). RV morphology and function in NS group were not significantly different from that in APMTE group. Tei index of UK group was lower than that of APMTE group (0.68±0.07 vs 0.83±0.13, P<0.05). Significant differences were noted in RVTD, TAPSE, E/e ‘and Tei index between cRGD-UK-LIP group and APMTE group (P < 0.05). When compared with the UK group, RVTD was decreased (3.87±0.29 vs 4.15±0.27, P < 0.05) and TAPSE was increased in the cRGD-UK-LIP group (2.02±0.20 vs 1.78±0.16, P<0.05) (Figure 5C and D).
 |
Table 2 Echocardiographic Characteristics of RV Morphology and Function |
Pulmonary Artery Pressure
The results are presented in Table 3. PAP in normal control group were not significantly different from that in sham operation group. PAP was significantly higher in the APMTE group than in the normal control or sham group (sPAP, dPAP, mPAP: APMTE group vs normal control/sham operation group, P<0.05). There was no significant difference in PAP between NS/UK group and APMTE group. PAP in cRGD-UK-LIP group was significantly lower than that in APMTE/NS group (sPAP, dPAP, mPAP: cRGD-UK-LIP group vs APMTE/NS group, P<0.05) (Figures 6 and 7).
 |
Table 3 Pulmonary Artery Pressure in Every Group |
Gross Observation
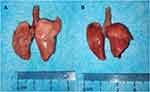
There were no obvious changes in heart, lung, liver and kidney tissues of rats in normal control group and sham operation group. In APMTE and NS group, hyperemia, swelling and bleeding were observed on the surface of lung tissues. Slight hyperemia, swelling and patchy hemorrhage were still observed on the surface of lung tissue of rats in UK group. Compared with the APMTE group, hyperemia and swelling on the surface of lung tissues were significantly improved in cRGD-UK-LIP group, some part of surface returned to pink with only sporadic speckled bleeding (Figure 8).
Histological Observation
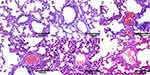
Except for the normal control group and the sham operation group, thrombi were found in pulmonary arterioles of the other groups, with different shapes and sizes. Large emboli with diameters from 80μm to 120μm were found in the arterioles, similar in size to injected autologous thrombus. Emboli with diameters of about 50μm or smaller could be seen in arterioles. Due to the large number and unfixed location, these vessels and thrombus were not counted. In APMTE and NS group, the pulmonary thrombus structure was relatively intact and arteriole lumens were almost occluded. The alveoli around the embolism site collapsed and many erythrocytes exuded. Thrombus structure in UK group was loose, and arteriole lumens were slightly clear. Alveoli around the embolism still collapsed and there were still many erythrocytes exudate. In cRGD-UK-LIP group, structure of pulmonary arteriole thrombus was looser and lumen patency was higher. The collapse degree of alveoli around embolism decreased and erythrocyte exudation was reduced (Figure 9).
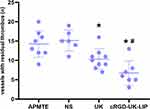
The number of vessels with residual thrombus in 60 arterioles (80–120μm) of each rat in the 4 groups was counted 9 hours after administration (APMTE, NS, UK, cRGD-UK-LIP group: 14.3±3.5, 15.2±2.7, 10.3±2.6, 6.7±3.2, Table 4). Between APMTE and NS group, there was no significant difference in the number of residual thrombus vessels. Vessels with residual thrombus in the UK group and the cRGD-UK-LIP group were less than that in the APMTE/NS group (P<0.05), and the cRGD-UK-LIP group was the least (Figure 10). No hyperemia, bleeding and swelling were observed in heart, liver and kidney of rats in each group.
 |
Table 4 The Number of Vessels with Residual Thrombus |
Discussion
Rat Model of Acute Pulmonary Microthromboembolism
Fragmentation of large thromboembolus and in situ thrombosis in small vessels are two dominating causes of pulmonary microthromboembolism. The formation of microthrombus in situ, related to endothelial dysfunction, is a chronic process.17 Acute pulmonary microthromboembolism is associated with large thrombus disruption.18 After large thromboembolus formation, most clots undergo fibrinolysis and organization that dissipates the thrombus and recanalizes the vessel.19 Thromboemboli may break up into numerous tiny fragments, either in the process of endogenous thrombolysis or passing through the heart, thus blocking small ramifications of pulmonary arteries. After the recanalization of the vessel, the fibrin and platelets covering the surface of thrombus are carried to a distance because of blood flow. Then the coagulation process is reactivated to produce microthrombus to jam the micro branches of pulmonary vessel.20
When APMTE occurs, pulmonary vascular resistance, pulmonary arterial pressure and right ventricular afterload increase, because the total cross-sectional area of pulmonary vessels decreases.21 Neuro-modulation, humoral regulation, such as vasoactive mediators et al and mechanical obstruction of the pulmonary vasculature by the embolic thrombus attribute to that cross-sectional area decrease. In order to adapt to the sudden rise of afterload, morphology and function of RV changed through neuromodulation and humoral regulation, such as Frank-Starling mechanism, to maintain pulmonary blood flow. When the afterload exceeds compensatory capacity of right ventricle, dilatation and dysfunction of right ventricular eventually occur.22
There are two common methods to establish animal models of pulmonary thromboembolism, in vivo and in vitro embolus formation. The usually used animals for modeling are large animals such as pigs, sheep and dogs, and small animals such as rats, mice and rabbits.23 In vivo embolus formation is to induce in situ thrombosis by physical/chemical means in blood vessels. The method of in vitro embolus formation is to prepare emboli outside the body and inject them into the venous system or right atrium, and then emboli travel to the lung and cause pulmonary embolism. Different methods and animals have their own advantages, disadvantages and applicable scope. Because the rats’ blood pressure was similar to that of humans, their tolerance was strong and they were easy to feed, and their pulmonary artery pressure had been measured successfully in other research, rats were chosen as experimental animals in this study.
In order to simulate the natural process of acute pulmonary microembolism, the method of in vitro embolus formation was used to establish animal models. Through the microscopic observation and measurement of pulmonary arteriole in SD rats, it was found that the inner diameter of pulmonary arteriole in rats was about 100um, so the blood of rat tail vein was made into autogenous thrombus particles with a size of about 100um×100um×100um and injected into rats’ lungs through jugular vein. However, a sharp reduction in pulmonary blood flow over a short time can be fatal, and that’s why acute pulmonary embolism is lethal. Theoretically, autogenous thrombus particles (100μm) will stay in our target, pulmonary arterioles. But it is undeniable that some of the particles do stay in the blood vessels above the level of pulmonary arterioles, and some of the thrombus would break into smaller emboli and embolize further branches on the way. The method of in vitro embolus formation can control the number and speed of embolus injection. So as to find the balance between successful modeling and animal death, before the formal experiment, a series of preliminary experiments were conducted through the method of in vitro embolus formation by comparing the influence of different embolus injection quantity and velocity on modeling, then it was corroborated by pathology.
In the formal experiment, suspension (0.5mL NS+30autogenous thrombus particles) was injected into rats at a certain velocity (0.5mL/min). Accidental death due to anesthesia was not counted. Among modeled 4 groups, only 1 rat in APMTE group and 1 rat in NS group died of right heart failure (modeling success rate > 90%). Compared with normal control/sham operation group, rats in APMTE group had enlarged RV, decreased RV function, increased PAP, and lung tissue of them showed post-thromboembolic appearance. The number of vessels with thrombus in 60 arterioles (80–120μm) of each rat in APMTE group was 14.3±3.5. Based on the results above, this study established a rat model of APMTE with strong control and high success rate, which not only provided a solid experimental basis for the thrombolytic experiment of cRGD-UK-LIP, but also provided an experimental platform for the in-depth study of pulmonary thromboembolism.
Thrombolysis Efficacy and Safety of cRGD-UK-LIP
Thrombus-targeted liposomes have potential as new thrombolytic drugs. Zhang et al produced liposomes, cRGD-UK-LIP, with long half-life and target ability towards thrombus. The ability of cRGD liposomes to target platelets analyzed by flow cytometry and fluorescent microscopy study. Furthermore, mouse mesenteric thrombosis model was established to verify the thrombolytic effect of cRGD-UK-LIP in vivo.13
Our research was standing on the shoulders of giants to further study the thrombolytic effect of cRGD-UK-LIP in APMTE. Based on Zhang Nengpan’s experiment, cRGD-UK-LIP, with 176.8nm hydrodynamic size, was successfully prepared in our previous research. It can be stable when stored in PBS buffer. Besides, it will not cause rupture of red blood cells and there is no cytotoxicity. Entrapment efficiency of cRGD-UK-LIP was about 58.83%, drug loading rate was about 40.64%, and most of the encapsulated urokinase could be released stably and continuously within 8h14. In vitro thrombolytic experiments, cRGD-UK-LIP could effectively dissolve thrombi and its thrombolytic ability was stronger than that of urokinase liposomes without cRGD modification (UK-LIP).24 For the purpose of further studying the thrombolytic effect and safety of cRGD-UK-LIP in rats with APMTE, we observed for 9 hours after appropriate drug treatment, so as to ensure the local drug concentration reached the highest level. Then the morphology and function of the right heart, PAP were evaluated and heart, lung, liver, kidney, etc were observed microscopely for comparison of thrombolysis effect between APMTE and free urokinase groups.
Urokinase dissolves thrombus by catalyzing plasminogen into plasmin to degrades fibrin, which can restore pulmonary vascular perfusion, reduce pulmonary vascular resistance and PAP, improve RV function.9 RGD peptides are known to bind to integrins, including GPIIb/IIIa on activated platelets and integrin αVβ3.12 Integrin αVβ3 was highly expressed on the surface of tumor cells and tumor neovascularization endothelial cells, but low in normal tissues and mature vascular endothelial cells.25 Autologous thrombus from rat tail vein blood was used to establish APMTE model, in which activated platelets play a central role. Moreover, cyclic RGD can better target activated platelets than linear RGD.26 Putatively, cRGD is an ideal targeting ligand in treatment of thromboembolism. Further, cRGD-UK-LIP not only releases urokinase gradually, but also has potential to evade mononuclear phagocyte system because it is decorated with polyethylene glycol (PEG), thus lengthening its circulation in bloodstream and prolonging its action time.27 cRGD-UK-LIP performs fibrinolytic ability more fully and accurately than free urokinase theoretically.
Compared with free urokinase group, rats in cRGD-UK-LIP group showed better right ventricular function, lower PAP, less thrombus in lung tissue and more unobstructed pulmonary microvascular lumen. Although some results failed to meet expectations, for example, PAP in the cRGD-UK-LIP group was not significantly lower than that in the UK group, and comparison of RV/LV and RVOT SE in each group was not statistically significant. Nonetheless, other indirect indicators such as RV morphology and function, direct indicators such as lung tissue structure and the number of residual thrombus vessels were also observed and compared to evaluate the thrombolytic effect of cRGD-UK-LIP. Ergo, the experimental results are reliable and agree with the theory.
cRGD-UK-LIP with target ability towards thrombus overcomed systemic fibrinolysis induced by indiscriminate activation of plasminogen by free urokinase, thereby reducing bleeding risk.13 Although the encapsulation of liposomes prolonged the residence time of urokinase in the body, the urokinase inside the liposomes was isolated from the blood and did not affect vascular function and white blood cells. Moreover, liposomes with PEG coating have been shown to be biocompatible in clinical trials.28 In order to control the variables in this study, samples were injected immediately through the tail vein in all groups. Free urokinase in the blood was rapidly metabolized by the liver. In this experiment, no hyperemia, hemorrhage and swelling were observed in the lung, heart, liver and kidney tissues of rats in all groups. The contents above indicated the safety of cRGD-UK-LIP application in vivo.
Application Value of Echocardiography in Animal Model
The European Society of Cardiology suggested in 2019 ESC guidelines that patients with pulmonary embolism should be risk stratified based on hemodynamic status, RV function and myocardial injury, so as to accurately assess the severity of the patient’s condition, and take timely and effective measures to improve patient prognosis and reduce mortality.5 Echocardiography is an accurate, non-invasive and convenient way to evaluate the morphology, function and hemodynamic status of the RV, which is widely used in clinical patients and animal models. Right ventricular transverse diameter (RVTD), ratio of RVTD to LVTD (RV/LV), tricuspid annular plane systolic excursion (TAPSE), right ventricular outflow tract systolic Excursion (RVOT SE), right ventricular index of myocardial performance (Tei index), ratio of early diastolic tricuspid inflow velocity to tricuspid annulus velocity (E/e’) et al are the classic echocardiography indicators to evaluate the morphology, systolic and diastolic functions of the RV, which have been widely studied and applied in clinical or scientific researches.29 In this study, changes in these indicators were sensitively observed in rats before and after modeling and thrombolytic therapy, and morphology and function of RV in rats were evaluated accordingly, which was indirectly confirmed by pathological examination and was in accordance with the expected results. Therefore, echocardiography can be used as an effective means to access the morphology and function of the RV in rats.
Cardiac catheterization, right ventricular puncture and peak tricuspid regurgitation velocity (TRV) or pulmonary regurgitant velocity are commonly used in the measurement of pulmonary pressure in experimental animals.30 Cardiac catheterization, the “gold standard” for the measurement of PAP, is to insert a cardiac catheter through the jugular vein under the guidance of X-ray to the right heart and the pulmonary artery, which reflects the pressure of the pulmonary artery under standard atmospheric pressure.31 However, it is not only invasive, but also complicated to operate and expensive. Right ventricular puncture is a non-visual method, which reflects the pressure of the ventricle. Its success rate is limited because it is easy to cause tissue damage and death. Although it is non-invasive and convenient to estimate PAP by valvular regurgitation velocity, first of all, not every experimental animal has reflux, and secondly, this method is not suitable for test objects with structural abnormalities of RV, valve and pulmonary artery.32
In this research, under the guidance of ECHO, transthoracic puncture was conducted and puncture needles were placed in the main pulmonary artery to measure pulmonary arterial pressure. Pulmonary hypertension was observed in the APMTE group, and changes in pulmonary arterial pressure were also monitored in the other groups, which were obliquely confirmed by pathological inspection of the lung tissue. This method directly and truly reflects the PAP of rats, as well as visualizes the puncture process, which not only reduces the difficulty of operation and boosts the success rate, but also decreases the unpredictable influence of complex manometric procedures on PAP measurement. Accordingly, this study provides an innovative and valid way for measuring PAP in the rat model of APMTE, as well as in other animal models.
Limitation
In this study, the function, morphology of RV and hemodynamic parameters in rats were observed and compared at a single time point. There are several reasons. First, rats could not tolerate repeated anesthesia. After modeling, rats showed accelerated heart rate and tachypnea, even slight blows were fatal, but both echocardiography and pulmonary artery pressure measurement required anesthesia. Second, similar to the traditional pressure measurement method, ECHO-guided transthoracic puncture is invasive, which leads to high mortality after pressure measurement is completed, even though it has merits of visual operation and high success rate. Depend on our team’s previous research, most of the encapsulated urokinase in cRGD-UK-LIP could be released stably and continuously within 8h. Hemodynamic parameters and tissues of rats were collected at a single time point (9h, after most of the drugs were released). Though, untreated modeling group, APMTE group, was added for comparison, observation at multiple time points in same animal which may increase the power of statistics is better.
Most of the data in this experiment were consistent with theory and expectations, but there were still some parameters that did not have suppositional performance. The mean value of PAP in the cRGD-UK-LIP group was lower than that in the UK group, although it could reflect the trend of PAP after thrombolytic therapy to some extent, there was no significant difference between these two groups. Primely, it was due to the insufficient number of rats. Secondarily, PAP measurement by transthoracic puncture is a new method combined with echocardiography, which is not perfect and mature. Length and inner diameter of the puncture needle affected the measurement results. Even if this method was highly sensitive, it could only reflect the relative changes in the pressures of each group and failed to accurately measure the PAP under standard atmospheric pressure. Safer and more accurate pressure measurement methods are still worth further exploration.
Albeit cRGD-UK-LIP was proved to dissolve thrombus better than free urokinase in this experiment, a few thrombi in pulmonary small vessels were observed microscopely after 9 hours of administration, which may be related to the mode and time of administration or the stability and release rate of cRGD-UK-LIP in rats. Hence, more efficient ways to use cRGD-UK-LIP and preferable production process are worthy of further study.
This study is a preliminary exploration of the thrombolytic effect of cRGD-UK-LIP in rats. Each group has a small number of animals, and only compared the thrombolytic effect of cRGD-UK-LIP and free urokinase in rats. The comparison of the thrombolytic effect of cRGD-UK-LIP and the new drugs put into use clinically, its thrombolytic effect and drug metabolism in the human body are still blank. More in-depth research is urgently needed.
Conclusion
A rat model of acute pulmonary microthromboembolism was successfully established by injecting autologous thrombus via jugular vein. cRGD-UK-LIP has potential to become an innovative thrombolytic drug because it has better thrombolytic effect than free urokinase and it is safe in rats with APMTE. Apart from using ECHO to evaluate the morphology and function of the RV, ECHO-guided transthoracic puncture measurement can be a practical way to monitor PAP in animal models.
Acknowledgments
This study was supported in part by National Natural Science Foundation of China (Grant No. 81760314) and Guangxi medical “139” Project for Training High-level Backbone Talents (Grant No. G201903014). Chunting Liang and Tongtong Huang are co-first authors for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Raskob GE, Angchaisuksiri P, Blanco AN. Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol. 2014;34(11):2363–2371. doi:10.1161/ATVBAHA.114.304488
2. Wendelboe AM, Raskob GE. Global burden of thrombosis: epidemiologic aspects. Circ Res. 2016;118(9):1340–1347. doi:10.1161/CIRCRESAHA.115.306841
3. Pengo V, Lensing A, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350(22):2257–2264. doi:10.1056/NEJMoa032274
4. Kim NH. Group 4 pulmonary hypertension: chronic thromboembolic pulmonary hypertension: epidemiology, pathophysiology, and treatment. Cardiol Clin. 2016;34(3):435–441. doi:10.1016/j.ccl.2016.04.011
5. Konstantinides SV. 2019 ESC Guidelines for the diagnosis and management of acute pulmonaryembolism developed in collaboration with the European Respiratory Society (ERS). Russ J Cardiol. 2020;25(8):3848. doi:10.15829/1560-4071-2020-3848
6. Fink C, Henzler T, Shirinova A, Apfaltrer P, Wasser K. Thoracic magnetic resonance imaging. J Thorac Imaging. 2013;28(3):171–177. doi:10.1097/RTI.0b013e31828d40ee
7. Hockstein MA, Haycock K, Wiepking M, Lentz S, Dugar S, Siuba M. Transthoracic right heart echocardiography for the intensivist. J Intensive Care Med. 2021;36(9):1098–1109. doi:10.1177/08850666211003475
8. Davidson LJ, Davidson CJ. Transcatheter treatment of valvular heart disease: a review. JAMA. 2021;325(24):2480–2494. doi:10.1001/jama.2021.2133
9. Kun L, Mingzhe C, Kewei Z, Kai L, Heng L, Shuiting Z. Treatment of acute pulmonary embolism using rheolytic thrombectomy. EuroIntervention. 2021;17(2):e158–e166.
10. Altaf F, Wu S, Kasim V. Role of fibrinolytic enzymes in anti-thrombosis therapy. Front Mol Biosci. 2021;8:680397. doi:10.3389/fmolb.2021.680397
11. Koudelka S, Mikulik R, Mašek J, et al. Liposomal nanocarriers for plasminogen activators. J Control Release. 2016;227:45–57. doi:10.1016/j.jconrel.2016.02.019
12. Pawlowski CL, Li W, Sun M, et al. Platelet microparticle-inspired clot-responsive nanoMedicine for targeted fibrinolysis. Biomaterials. 2017;128:94–108. doi:10.1016/j.biomaterials.2017.03.012
13. Zhang N, Li C, Zhou D, et al. Cyclic RGD functionalized liposomes encapsulating urokinase for thrombolysis. Acta Biometer. 2018;70:227–236. doi:10.1016/j.actbio.2018.01.038
14. Rao H. Experimental Study on the Preparation and Physicochemical Properties of Urokinase-Loaded Crgd-Modified Liposomes. Guangxi Medical University; 2020.
15. Rao H, Che X, Pan X, Huang G, Chen X, Wu J. A comparative study on physicochemical properties of two kinds of targeting thrombus urokinase-loaded microbubbles. Chongqing Med. 2021;50(5):5.
16. Jiménez D, de Miguel-díez J, Guijarro R, et al. Trends in the management and outcomes of acute pulmonary embolism: analysis from the RIETE registry. J Am Coll Cardiol. 2016;67(2):162–170. doi:10.1016/j.jacc.2015.10.060
17. Haworth SG. Role of the endothelium in pulmonary arterial hypertension. Vascul Pharmacol. 2006;45(5):317–325. doi:10.1016/j.vph.2006.08.006
18. Wagenvoort CA. Pathology of pulmonary thromboembolism. Chest. 1995;107(1):10S–17S. doi:10.1378/chest.107.1_Supplement.10S
19. Mukhopadhyay S, Johnson TA, Duru N, Buzza MS, Antalis TM. Fibrinolysis and inflammation in venous thrombus resolution. Front Immunol. 2019;10. doi:10.3389/fimmu.2019.00010
20. Wang Z. Thrombosis&Hemostasis Basic Principles&Clinical Practice.
21. Zhai Z, Wang D, Lei J, et al. Trends in risk stratification, in-hospital management and mortality of patients with acute pulmonary embolism: an analysis from China pUlmonary thromboembolism REgistry Study (CURES). Eur Respir J. 2021;58(4):2002963. doi:10.1183/13993003.02963-2020
22. Kosta S, Dauby PC, Marsden AL. Frank-Starling mechanism, fluid responsiveness, and length-dependent activation: unravelling the multiscale behaviors with an in silico analysis. PLoS Comput Biol. 2021;17(10):e1009469. doi:10.1371/journal.pcbi.1009469
23. Miu R, Wang J, Wang C, Pang B. Advances in animal models of pulmonary thromboembolism. Int J Respir. 2009;29(22):4.
24. Pan X. Experimental Study on Thrombolysis of Urokinase-Loaded Cyclic RGD Modified Liposomes. Guangxi Medical University; 2020.
25. Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119(19):3901–3903. doi:10.1242/jcs.03098
26. Huang G, Zhou Z, Srinivasan R, et al. Affinity manipulation of surface-conjugated RGD peptide to modulate binding of liposomes to activated platelets. Biomaterials. 2008;29(11):1676–1685. doi:10.1016/j.biomaterials.2007.12.015
27. Kim JY, Kim JK, Park JS, Byun Y, Kim CK. The use of PEGylated liposomes to prolong circulation lifetimes of tissue plasminogen activator. Biomaterials. 2009;30(29):5751–5756. doi:10.1016/j.biomaterials.2009.07.021
28. Uster PS, Allen TM, Daniel BE, et al. Insertion of poly(ethylene glycol) derivatized phospholipid into pre-formed liposomes results in prolonged in vivo circulation time. FEBS Lett. 1996;386(2–3):243–246. doi:10.1016/0014-5793(96)00452-8
29. Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. doi:10.1016/j.echo.2010.05.010
30. Horton KD, Meece RW, Hill JC. Assessment of the right ventricle by echocardiography: a primer for cardiac sonographers. J Am Soc Echocardiogr. 2009;22(7):776–792. doi:10.1016/j.echo.2009.04.027
31. Ristow B, Schiller NB. Stepping away from ritual right heart catheterization into the era of noninvasively measured pulmonary artery pressure. J Am Soc Echocardiogr. 2009;22(7):820–822. doi:10.1016/j.echo.2009.05.023
32. Aduen JF, Castello R, Lozano MM, et al. An alternative echocardiographic method to estimate mean pulmonary artery pressure: diagnostic and clinical implications. J Am Soc Echocardiogr. 2009;22(7):814–819. doi:10.1016/j.echo.2009.04.007
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.