Back to Journals » Journal of Inflammation Research » Volume 13
Creation of a Novel Inflammation-Based Score for Operable Colorectal Cancer Patients
Authors Huang Q, Cao Y, Wang S, Zhu R
Received 20 July 2020
Accepted for publication 9 September 2020
Published 6 October 2020 Volume 2020:13 Pages 659—671
DOI https://doi.org/10.2147/JIR.S271541
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Ning Quan
Qian Huang,1 Yinghao Cao,2 Shouyi Wang,1 Rui Zhu1
1Department of Pediatric, Zhongnan Hospital of Wuhan University, Wuhan, People’s Republic of China; 2Department of Colorectal Surgery and Gastroenterology, Wuhan Union Hospital, Tongji Medical College of Huazhong University of Science and Technology, Wuhan, People’s Republic of China
Correspondence: Rui Zhu
Department of Pediatric, Zhongnan Hospital of Wuhan University, No. 169 Donghu Road, Wuhan 430071, People’s Republic of China
Email [email protected]
Aim: Systemic inflammation has been implicated in the progression of patients with colorectal cancer (CRC). We evaluated the prognostic ability of a comprehensive score based on several inflammatory indexes in operable CRC patients.
Patients and Methods: Between July 2013 and September 2017, this study retrospectively identified 1279 CRC patients receiving radical surgery in Wuhan Union Hospital and randomly assigned them into training (N=921) and validation (N=358) sets. A novel score, the CRC-specific inflammatory index (CSII), was developed from a series of inflammatory indexes significantly associated with survival in patients with CRC. This novel score was then divided into three categories and compared to the well-known systematic inflammatory index (SII) and TNM stage. Finally, a survival nomogram was generated by combining the CSII and other informative clinical features.
Results: The CSII-OS was calculated as 1.110×lg ALRI + 1.082×CAR + 0.792×PI, while CSII-DFS was 1.709×lg ALRI + 1.033×CAR based on multivariable Cox regression analysis. Patients with high CSII experienced a worse OS (HR=23.72, 95% CI, 11.30– 49.78, P < 0.001) and worse DFS (HR=15.62, 95% CI, 6.95– 35.08, P < 0.001) compared to those in CRC patients with low CSII. Moreover, ROC analyses showed that the CSII possessed excellent performance (AUC=0.859) in predicting OS and DFS. The AUC of the OS nomogram based on CSII, TNM stage, and chemotherapy was 0.897, while that of the DFS nomogram based on CSII, T stage, and TNM stage was 0.873. High-quality calibration curves in both OS and DFS nomograms were observed. Verification in the validation dataset showed results consistent with those in the training dataset.
Conclusion: The CSII is a CRC-specific prognostic score based on the combination of available inflammatory indexes. High CSII is a strong predictor of worse survival outcomes. The CSII also exhibits better predictive performance compared to SII or TNM stage in operable CRC patients.
Keywords: colorectal cancer, systemic inflammation, prognosis, CRC-specific inflammatory index, survival nomogram
Introduction
Colorectal cancer (CRC) is the second most common cause of cancer-related death in the United States.1 Approximately 147,950 new-onset cases and 53,200 deaths due to CRC are predicted the year of 2020.1 The genetic pathogenesis of CRC is complicated, with various gene mutations occurring in early-stage disease.2 Both KRAS and APC have high mutation rates among CRC patients, mutations that signify reduced survival times.3,4 Despite advances in surgical treatment and chemoradiotherapy, the long-term prognosis of patients with CRC is still not optimistic.5 Therefore, the identification of a novel prognostic biomarker for better risk stratification is imperative.
The systematic inflammatory response is mainly generated by innate immune cells and contributes to CRC induction and progression.6,7 This inflammatory response can be reflected by a series of hematological indexes, including platelets, monocytes, basophils,8 neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), platelet-to-lymphocyte ratio (PLR), and C-reactive protein (CRP)/prealbumin,9 some of which are closely associated with survival in patients with CRC.10,11 As the interplay between tumor, inflammation, and immunity depends on a complex network,12,13 the clinical usefulness of a single biomarker is more or less limited by low predictive efficiency. Hence, the combination of immune-inflammatory indexes could reflect systematic inflammation status to refine prognostic performance.
However, no combined inflammatory score has been specifically designed for prognostic stratification in patients with operable CRC. Moreover, previous studies14,15 assessing the prognostic values of inflammatory markers were generally based on small sample size, with limited confidence in their conclusions. Hence, the present study initially created and validated a novel CRC-specific inflammatory index (CSII) from a sufficient sample of patients with operable CRC. We then compared the predictive value of the CSII to those of the SII and TNM staging system for the prediction of overall survival (OS) and disease-free survival (DFS). Finally, we combined the novel index with other common clinical variables to create and verify a survival nomogram for the prediction of OS and DFS in patients with operable CRC.
Patients and Methods
Study Population
We retrospectively reviewed a CRC database16 of 3500 patients treated at Wuhan Union Hospital from July 2013 to September 2017.16 The patients included in our study were staged by computed tomography (CT) before surgery, with additional information obtained from pathological reports issued after surgery. The inclusion criteria were (1) CRC confirmed by pathology; (2) CRC patients without distant metastasis; and (3) patients treated with surgical therapy. The exclusion criteria were (1) patients with CRC administered anti-inflammatory agents before surgical excision; (2) CRC patients complicated with acute infection, chronic infection or hematologic diseases; and (3) CRC patients lacking critical clinical or follow-up data.
Finally, a total of 1279 operable CRC patients with intact clinical and follow-up data were included in this study and randomly classified into the training (N=921) and validation (N=358) groups. All patients included in this study provided informed consent, in accordance with the Declaration of Helsinki. The study plan was reviewed and successfully approved before research initiation by the Ethics Administration Office of Wuhan Union Hospital (No. 2018-S377).
Data Collection
The clinical information of operable CRC patients was retrospectively extracted from the “Biological big data platform for individualized diagnosis and treatment of colorectal cancer” (No.2019SR1267841). We obtained blood routine and biochemistry test findings from the first admission day. The following clinical along with pathological information was automatically collected from this platform: age, gender, tumor site, family history of cancer, histological grade, tumor size, vascular invasion, circumferential resection margin, T stage, N stage, TNM stage, adjuvant radiotherapy, recurrence, death, and a series of inflammatory indexes. After surgical resection, follow-up was implemented every 3 months for the first 2 years and every 6 months in the third to fifth years. The primary endpoint of this study was OS and the secondary endpoint was disease-free survival (DFS). OS was defined as the period from the data of operation to the date of death by any cause. DFS was calculated from date of operation until date of death from recurrent or progression.
Evaluation of the Systematic Inflammatory Indexes
Blood routine and chemistry profiles were routinely measured for each CRC patient on admission. The systematic inflammatory indexes in the present study comprised the NLR (neutrophil-lymphocyte ratio), PLR (platelet-lymphocyte ratio), MLR (monocyte-lymphocyte ratio), SII (systemic immune-inflammation index), ALRI (aspartate aminotransferase to lymphocyte ratio), CAR (C-reactive protein-albumin ratio), GPS (Glasgow prognostic score), mGPS (modified Glasgow prognostic score) and PI (prognostic index). NLR was defined as the ratio of neutrophil to lymphocyte ratio; PLR was defined as the ratio of platelet to lymphocyte; MLR was calculated by dividing monocyte by lymphocyte; SII was platelet counts × neutrophil counts/lymphocyte counts; ALRI referred to the ratio of aspartate aminotransferase to lymphocyte; CAR was calculated by C-reactive/protein-albumin. The cut-off values of the above inflammatory indexes were identified by X-tile software.17 Moreover, GPS, mGPS and PI were defined and categorized according to the previous studies.18,19
Statistical Analyses
SPSS 23.0, X-tile 3.6.1, and R 3.3.1 were used to perform the statistical analyses. Continuous variables were summarized as means together with standard deviations or interquartile ranges depending on the distribution of the data, and as frequencies along with percentages for binary variables. To construct a novel inflammatory score specific to CRC, multivariate Cox regression was employed to select single inflammatory indexes significantly associated with OS or DFS (P<0.05). The training set was applied to develop the novel inflammatory score based on the β-coefficients from the multivariable Cox analysis. The CSII was divided into three risk groups (low risk, intermediate risk, and high risk) using the 50th and 85th percentiles as cutoffs.20 Receiver operating characteristic (ROC) analyses were then performed to assess the predictive abilities of CSII, SII, and TNM stage for the prediction of survival outcomes. Moreover, we combined CSII with other clinical features with P<0.05 generated by the multivariate Cox regression to construct a survival nomogram. Td-ROC curves were drawn to assess the prognostic performance of survival nomogram for 1-year, 3-year and 5-year prediction. Calibration ability refers to the predictive accuracy of event probabilities and was assessed with calibration curves in which predicted outcomes versus observed outcomes. P <0.05 on both sides represented statistically significant differences.
Results
Patient Clinical Features
A total of 1279 patients with CRC (763 men and 516 women) met the inclusion criterion and were enrolled in this study. The detailed flow of patient selection is shown in Figure 1. According to the TNM stage 8th edition, 197, 502, and 580 cases were stage I, II, and III, respectively. Adjuvant chemotherapy was administered to 650 patients and 57 cases experienced systemic radiotherapy. The mean OS and DFS were 29.7 (22.5, 40.3) months and 20.8 (13.4, 30.6) months, respectively. The cutoff values for systemic inflammatory indicators were determined by the X-tile software. Based on these cutoff values, all systemic inflammatory indicators included in this study were statistically associated with OS (Figure S1) and DFS (Figure S2) in CRC patients.
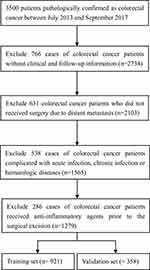 |
Figure 1 Flow chart of patient selection. |
Creation of the Novel Inflammatory Index
To establish and further validate the novel CSII CRC-specific inflammatory index, we randomly split the whole study population into training (N=921) and validation (N=358) sets. As clearly exhibited in Table S1, we observed no obvious differences in clinical features between the datasets. For the creation of the CSII, we selected only informative inflammatory indexes significantly related to OS or DFS by Cox regression. As shown in Table S2, higher ALRI, CAR, and PI were independent risk factors for poorer OS and ALRI with CAR was an independent risk factor for unfavorable DFS. Hence, based on the β coefficient, the CSII for OS (CSII-OS) was generated: 1.110×lg ALRI + 1.082×CAR + 0.792×PI. The CSII for DFS (CSII-DFS) was 1.709×lg ALRI+ 1.033×CAR. As illustrated in Figure S3, CRC patients with high ALRI, CAR, or PI showed higher CSII-OS or CSII-DFS scores compared to those in the low group. In addition, analysis of variance was utilized to investigate the association between CSII-OS or CSII-DFS and clinical features among groups showed that both CSII-OS and CSII-DFS were significantly related to tumor differentiation (PCSII-OS=0.0434, PCSII-DFS=0.0422), TNM stage (PCSII-OS=0.0213, PCSII-DFS=0.0223), and T stage (PCSII-OS=0.0012, PCSII-DFS=0.0005) (Figure S4)
Comparisons of the CSII to the SII and TNM Stage
The 50th and 85th percentiles were used as cutoffs to classify the CSII, CSII-OS (2.24, 5.32), and CSII-DFS (2.63, 5.08) into three categories in the training and validation datasets. As reflected by the Kaplan–Meier survival curves, CRC patients with high or intermediate CSII-OS exhibited worse OS both in the training (Figure 2A) and validation (Figure 2B) datasets. Similarly, CRC patients with high or intermediate CSII-DFS showed more unfavorable DFS in both in the training (Figure 2C) and validation (Figure 2D) datasets. ROC analyses were further applied to assess the predictive significance of the CSII in predicting survival and comparing to both SII and TNM stage. The predictive ability of CSII as measured by AUC in predicting OS was 0.859 in the training set (Figure 3A) and 0.859 in the validation set (Figure 3B). Similarly, the CSII also showed remarkable performance in the prediction of DFS among CRC patients, as reflected by the AUC of 0.862 in the training set (Figure 3C) and 0.781 in the validation set (Figure 3D). The AUCs of SII and TNM for OS were 0.592 and 0.656 in the training set and 0.614 and 0.660 in the validation set, respectively (Figure 3). The AUCs of the SII and TNM for DFS were 0.559 and 0.574 in the training set and 0.555 and 0.561 in the validation set, respectively. Thus, we concluded that the performance of the CSII in predicting both OS and DFS was superior to those of the SII and DFS.
Construction and Validation of a Survival Nomogram
Based on the multivariate Cox results for OS (Table 1), three variables were eventually included in the OS nomogram: CSII, TNM stage, and chemotherapy (Figure 4A). The overall ability of the survival nomogram to predict OS among operable CRC patients was 0.897 in the training set (Figure 3A) and 0.873 in the validation set (Figure 3B). Furthermore, time-dependent (td)-ROC analyses were also exploited to estimate the efficiency of the nomogram in predicting 1-year, 3-year and 5-year OS. In the evaluation of the 1-, 3-, and 5-year survival rates, the predictive power of the survival nomogram as measured by AUCs were 0.854, 0.857, and 0.883 in the training set (Figure 5A) and 0.879, 0.891 and 0.910 in the validation set (Figure 5B), respectively. Moreover, the calibration curves (Figure 6A–F) of the survival nomogram showed that the predicted survival rates calculated by the survival nomogram were highly comparable to the actual values, indicating the reliable repeatability of the survival nomogram for OS.
 |
Table 1 Univariate and Multivariate Analyses of Factors Associated with OS |
For DFS (Table 2), the nomogram incorporated three informative variables (T stage, TNM stage, and CSII) (Figure 4B). The overall predictability of the survival nomogram in predicting DFS among operable CRC patients was 0.871 in the training set (Figure 3B) and 0.788 in the validation set (Figure 3D). The td-ROC curves (Figure 5C and D) demonstrated the good accuracy of the survival nomogram for 1-, 3- and 5-year DFS prediction in both the training (AUC 0.878, 0.855, and 0.895, respectively) and validation (AUC 0.870, 0.855, and 0.818, respectively) sets. The DFS nomogram showed good calibration, as reflected by the calibration curves (Figure 6G–L). The survival nomogram based on CSII and other significant features showed better discriminatory ability in terms of OS and DFS between high- and low-risk patients with CRC.
 |
Table 2 Univariate and Multivariate Analyses of Factors Associated with DFS |
Discussion
The major fraction of CRC cases is related to environmental factors rather than genetic changes.21 Recent work has emphasized the role of chronic inflammation in colon tumorigenesis, including initiation, progression, and metastasis.22 Systematic inflammatory response is involved in CRC occurrence and progression; however, the prognostic significance of inflammatory indexes in CRC patients has not been fully elucidated. To avoid fragmented knowledge regarding systemic inflammation, we aimed to create a novel inflammation-based score incorporating significant hematological indexes. To our knowledge, this study is the first to demonstrate the prognostic significance of a combined score (CSII) was specific to CRC. The CSII showed the highest relative influence on both OS and DFS, as proven by multivariate Cox regression. Moreover, this novel biomarker was superior to the SII and TNM stage for the prediction of OS and DFS in patients with CRC. More importantly, the survival nomogram incorporating CSII and other significant clinical variables showed a higher predictive ability for OS and DFS in CRC patients than any other single index. Hence, we concluded that the CSII is a reliable prognostic factor in patients with operable CRC.
Cancer-associated inflammation refers to the infiltration of inflammatory cells and the production of inflammatory mediators in cancerous tissues.23 Cancer-associated inflammation is evaluated based on the morphological characteristics of inflammatory cells in hematoxylin and eosin (H&E)-stained slides.24 Although reliable, this method is inconvenient and invasive. Moreover, this method is performed postoperatively, while measuring noninvasive biomarkers before surgery is of great significance. Clinicians have increasingly focused their attention on the association between inflammatory indexes and prognosis in patients with CRC.25–30 Among these indexes, the SII is reportedly the most relevant biomarker for this purpose.
The association between SII and prognosis in patients with CRC has been well studied. Chen et al15 reported that an SII cutoff value of 340 was an independent risk factor for worse OS and DFS in patients with CRC. Saori et al31 divided the SII into three categories and reported more unfavorable survival in CRC patients with high or intermediate SII compared to that in patients with low CRC. Moreover, Xie et al32 demonstrated that the high SII was independently correlated with unfavorable OS in patients with metastatic CRC. However, two clinical studies33,34 investigating the prognostic significance of the SII in CRC patients receiving neoadjuvant chemoradiotherapy showed that the SII was not an independent predictor for OS or DFS. In addition, a recent study7 with 408 CRC patients reported that SII was not associated with OS or DFS in multivariate Cox regression analyses. Our study also assessed the prognostic value of the SII in patients with CRC, finding that the predictive ability as reflected by the AUC for OS was 0.592 and 0.614 in the training and validation sets, and was 0.559 and 0.555, respectively, for DFS. Thus, the SII, which was derived from liver cancer, was not a reliable prognostic factor in patients with CRC. In contrast, the CSII was specially designed for CRC based on 1279 cases and was a powerful prognostic index both for OS and DFS. Encouragingly, CSII exhibited more excellent performance in predicting OS and DFS compared to the SII in patients with CRC in both the training and validation sets.
The TNM staging system has been widely utilized for prognostic prediction in patients with CRC based on the invasion extent of the primary tumor, lymph node status, and distant metastasis.18 As the TNM stage mainly represents the biological behavior of CRC, it might not accurately identify patients at high risk of cancer death or tumor recurrence. The prognosis of patients with CRC is associated not only with the clinicopathological features and interventional therapies but also with host inflammatory response.35–37 The CSII is a combined score based on a series of informative inflammation indexes that reflect tumor immune response and host inflammation status. The ROC analyses showed a better predictive performance for the prediction of OS or DFS for CSII than for TNM stage, indicating that the CSII could serve as a complementary biomarker to TNM stage for risk stratification. In addition, the CSII combined with other significant variables to create a survival nomogram showed a more powerful ability for the prediction of OS or DFS compared to CSII or TNM stage alone, implying that the combination of indexes reflecting host inflammatory status and important clinical features might be useful to accurately predict prognosis in patients with CRC.
Despite the relatively large sample size, this clinical research study has three obvious limitations. First, this retrospective study was carried out in a single center and lacked external validation. Second, although we collected hematological information for each patient on admission prior to surgical treatment, the levels of inflammatory indexes varied over time and dynamic monitoring of these inflammatory indexes may be more precise. Lastly, we did not evaluate the impact of gene profiling related to inflammatory pathways due to the lack of related medical records. Large-scale clinical studies with more clinical and genetic features performed in multiple centers are needed to validate our conclusions.
Conclusions
We developed a novel inflammatory score based on a series of single inflammatory indexes significantly associated with the survival of operable CRC patients. The CSII outperformed the well-known SII inflammatory index and could be an effective biomarker for predicting the prognosis of operable CRC patients. Moreover, the survival nomogram combining CSII with significant clinical features provided added prognostic value for predicting survival outcomes among these patients. However, larger-scale prospective trials are warranted to validate our results.
Abbreviations
CSII, CRC-specific inflammatory index; CRC, colorectal cancer; OS, overall survival; DFS, disease-free survival; NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; LMR, monocyte-lymphocyte ratio; SII, systemic immune-inflammation index; ALRI, aspartate aminotransferase to lymphocyte ratio; CAR, C-reactive protein-albumin ratio; GPS, Glasgow prognostic score; mGPS, modified glasgow prognostic score; PI, prognostic index.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Goding SA, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145–164. doi:10.3322/caac.21601
2. Cho A, Jo K, Hwang SH, et al. Correlation between KRAS mutation and (18)F-FDG uptake in stage IV colorectal cancer. Abdom Radiol (NY). 2017;42(6):1621–1626. doi:10.1007/s00261-017-1054-2
3. Allievi N, Goffredo P, Utria AF, et al. The association of KRAS mutation with primary tumor location and survival in patients undergoing resection of colorectal cancers and synchronous liver metastases. Chin Clin Oncol. 2019;8(5):46. doi:10.21037/cco.2019.08.10
4. Corsini EM, Mitchell KG, Mehran RJ, et al. Colorectal cancer mutations are associated with survival and recurrence after pulmonary metastasectomy. J Surg Oncol. 2019;120(4):729–735. doi:10.1002/jso.25630
5. Wang W, Kandimalla R, Huang H, et al. Molecular subtyping of colorectal cancer: recent progress, new challenges and emerging opportunities. Semin Cancer Biol. 2019;55:37–52. doi:10.1016/j.semcancer.2018.05.002
6. Shalapour S, Karin M. Pas de Deux. Control of Anti-tumor Immunity by Cancer-Associated Inflammation. Immunity. 2019;51(1):15–26. doi:10.1016/j.immuni.2019.06.021
7. Fuca G, Guarini V, Antoniotti C, et al. The Pan-Immune-Inflammation Value is a new prognostic biomarker in metastatic colorectal cancer: results from a pooled-analysis of the Valentino and TRIBE first-line trials. Br J Cancer. 2020. doi:10.1038/s41416-020-0894-7
8. Liu Q, Luo D, Cai S, Li Q, Li X. Circulating basophil count as a prognostic marker of tumor aggressiveness and survival outcomes in colorectal cancer. Clin Transl Med. 2020;9(1):6. doi:10.1186/s40169-019-0255-4
9. Lu J, Xu BB, Zheng ZF, et al. CRP/prealbumin, a novel inflammatory index for predicting recurrence after radical resection in gastric cancer patients: post hoc analysis of a randomized Phase III trial. Gastric Cancer. 2019;22(3):536–545. doi:10.1007/s10120-018-0892-0
10. Maeda K, Shibutani M, Otani H, et al. Inflammation-based factors and prognosis in patients with colorectal cancer. World J Gastrointest Oncol. 2015;7(8):111–117. doi:10.4251/wjgo.v7.i8.111
11. Dolan RD, Lim J, McSorley ST, Horgan PG, McMillan DC. The role of the systemic inflammatory response in predicting outcomes in patients with operable cancer: systematic review and meta-analysis. Sci Rep. 2017;7(1):16717. doi:10.1038/s41598-017-16955-5
12. Markman JL, Shiao SL. Impact of the immune system and immunotherapy in colorectal cancer. J Gastrointest Oncol. 2015;6(2):208–223. doi:10.3978/j.issn.2078-6891.2014.077
13. Riley JM, Cross AW, Paulos CM, Rubinstein MP, Wrangle J, Camp ER. The clinical implications of immunogenomics in colorectal cancer: A path for precision medicine. Cancer-Am Cancer Soc. 2018;124(8):1650–1659. doi:10.1002/cncr.31214
14. Choi KW, Hong SW, Chang YG, et al. Inflammation-based score (Glasgow prognostic score) as an independent prognostic factor in colorectal cancer patients. Ann Surg Treat Res. 2014;86(6):309–313. doi:10.4174/astr.2014.86.6.309
15. Chen JH, Zhai ET, Yuan YJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. 2017;23(34):6261–6272. doi:10.3748/wjg.v23.i34.6261
16. Cao Y, Deng S, Yan L, et al. Perineural invasion is associated with poor prognosis of colorectal cancer: a retrospective cohort study. Int J Colorectal Dis. 2020;35(6):1067–1075. doi:10.1007/s00384-020-03566-2
17. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259. doi:10.1158/1078-0432.CCR-04-0713
18. Park JH, Watt DG, Roxburgh CS, Horgan PG, McMillan DC. Colorectal Cancer, Systemic Inflammation, and Outcome: staging the Tumor and Staging the Host. Ann Surg. 2016;263(2):326–336. doi:10.1097/SLA.0000000000001122
19. Li Z, Li S, Ying X, et al. The clinical value and usage of inflammatory and nutritional markers in survival prediction for gastric cancer patients with neoadjuvant chemotherapy and D2 lymphadenectomy. Gastric Cancer. 2020;23(3):540–549. doi:10.1007/s10120-019-01027-6
20. Royston P, Altman DG. External validation of a Cox prognostic model: principles and methods. BMC Med Res Methodol. 2013;13:33. doi:10.1186/1471-2288-13-33
21. Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138(6):2101–2114. doi:10.1053/j.gastro.2010.01.058
22. Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. doi:10.1155/2014/149185
23. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi:10.1038/nature07205
24. van Verschuer VM, Hooning MJ, van Baare-georgieva RD, et al. Tumor-associated inflammation as a potential prognostic tool in BRCA1/2-associated breast cancer. Hum Pathol. 2015;46(2):182–190. doi:10.1016/j.humpath.2014.10.020
25. Mei Z, Shi L, Wang B, et al. Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: A systematic review and meta-analysis of 66 cohort studies. Cancer Treat Rev. 2017;58:1–13. doi:10.1016/j.ctrv.2017.05.005
26. Omichi K, Cloyd JM, Yamashita S, et al. Neutrophil-to-lymphocyte ratio predicts prognosis after neoadjuvant chemotherapy and resection of intrahepatic cholangiocarcinoma. Surgery. 2017;162(4):752–765. doi:10.1016/j.surg.2017.05.015
27. Ratjen I, Shivappa N, Schafmayer C, et al. Association between the dietary inflammatory index and all-cause mortality in colorectal cancer long-term survivors. Int J Cancer. 2019;144(6):1292–1301. doi:10.1002/ijc.31919
28. Stojkovic LM, Pavlovic MA, Stankovic S, et al. Combined Diagnostic Efficacy of Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), and Mean Platelet Volume (MPV) as Biomarkers of Systemic Inflammation in the Diagnosis of Colorectal Cancer. Dis Markers. 2019;2019:6036979. doi:10.1155/2019/6036979
29. Feliciano E, Kroenke CH, Meyerhardt JA, et al. Association of Systemic Inflammation and Sarcopenia With Survival in Nonmetastatic Colorectal Cancer: results From the C SCANS Study. JAMA Oncol. 2017;3(12):e172319. doi:10.1001/jamaoncol.2017.2319
30. Deng Q, Geng Y, Zhao L, et al. NLRP3 inflammasomes in macrophages drive colorectal cancer metastasis to the liver. Cancer Lett. 2019;442:21–30. doi:10.1016/j.canlet.2018.10.030
31. Yatabe S, Eto K, Haruki K, et al. Signification of Systemic Immune-Inflammation Index for prediction of prognosis after resecting in patients with colorectal cancer. Int J Colorectal Dis. 2020. doi:10.1007/s00384-020-03615-w
32. Xie QK, Chen P, Hu WM, et al. The systemic immune-inflammation index is an independent predictor of survival for metastatic colorectal cancer and its association with the lymphocytic response to the tumor. J Transl Med. 2018;16(1):273. doi:10.1186/s12967-018-1638-9
33. Yang J, Xu H, Guo X, et al. Pretreatment Inflammatory Indexes as Prognostic Predictors for Survival in Colorectal Cancer Patients Receiving Neoadjuvant Chemoradiotherapy. Sci Rep. 2018;8(1):3044. doi:10.1038/s41598-018-21093-7
34. Zhang Y, Liu X, Xu M, Chen K, Li S, Guan G. Prognostic value of pretreatment systemic inflammatory markers in patients with locally advanced rectal cancer following neoadjuvant chemoradiotherapy. Sci Rep. 2020;10(1):8017. doi:10.1038/s41598-020-64684-z
35. Sreekumar R, Harris S, Moutasim K, et al. Assessment of Nuclear ZEB2 as a Biomarker for Colorectal Cancer Outcome and TNM Risk Stratification. JAMA Netw Open. 2018;1(6):e183115. doi:10.1001/jamanetworkopen.2018.3115
36. Ahmed FA, Elbarmelgi MY, Azim HA, Abozeid AA, Mashhour AN. TNMF versus TNM in staging of colorectal cancer. Int J Surg. 2016;27:147–150. doi:10.1016/j.ijsu.2016.01.087
37. McSorley ST, Black DH, Horgan PG, McMillan DC. The relationship between tumour stage, systemic inflammation, body composition and survival in patients with colorectal cancer. Clin Nutr. 2018;37(4):1279–1285. doi:10.1016/j.clnu.2017.05.017
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





