Back to Journals » Neuropsychiatric Disease and Treatment » Volume 13
Cost-utility of collaborative care for the treatment of comorbid major depressive disorder in outpatients with chronic physical conditions. A randomized controlled trial in the general hospital setting (CC-DIM)
Authors Goorden M, van der Feltz-Cornelis CM , van Steenbergen-Weijenburg KM, Horn EK, Beekman ATF, Hakkaart-van Roijen L
Received 6 February 2017
Accepted for publication 11 May 2017
Published 18 July 2017 Volume 2017:13 Pages 1881—1893
DOI https://doi.org/10.2147/NDT.S134008
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Maartje Goorden,1 Christina M van der Feltz-Cornelis,2,3 Kirsten M van Steenbergen-Weijenburg,4 Eva K Horn,5 Aartjan TF Beekman,6,7 Leona Hakkaart-van Roijen1
1Institute of Health Policy and Management (iBMG)/Institute for Medical Technology Assessment (iMTA), Erasmus University Rotterdam, Rotterdam, 2Tranzo Department, Tilburg University, 3Clinical Centre of Excellence for Body, Mind and Health, GGzBreburg, Tilburg, 4Trimbos Instituut, Utrecht, 5Viersprong Institute for Studies on Personality Disorders, Halsteren, 6Department of Psychiatry, 7EMGO+ Research Institute VUmc, VU University Medical Centre, Amsterdam, the Netherlands
Purpose: Major depressive disorder (MDD) is highly prevalent in patients with a chronic physical condition, and this comorbidity has a negative influence on quality of life, health care costs, self-care, morbidity, and mortality. Research has shown that collaborative care (CC) may be a cost-effective treatment. However, its cost-effectiveness in this patient group has not yet been established. Therefore, the aim of this study was to evaluate the cost-utility of CC for the treatment of comorbid MDD in chronically ill patients in the outpatient general hospital setting. The study was conducted from a health care and societal perspective.
Patients and methods: In this randomized controlled trial, 81 patients with moderate-to-severe MDD were included; 42 were randomly assigned to the CC group and 39 to the care as usual (CAU) group. We applied the TiC-P, short-form Health-Related Quality of Life questionnaire, and EuroQol EQ-5D 3 level version, measuring the use of health care, informal care, and household work, respectively, at baseline and at 3, 6, 9, and 12 months follow-up.
Results: The mean annual direct medical costs in the CC group were €6,718 (95% confidence interval [CI]: 3,541 to 10,680) compared to €4,582 (95% CI: 2,782 to 6,740) in the CAU group. The average quality-adjusted life years (QALYs) gained were 0.07 higher in the CC group, indicating that CC is more costly but also more effective than CAU. From a societal perspective, the incremental cost-effectiveness ratio was €24,690/QALY.
Conclusion: This first cost-utility analysis in chronically ill patients with comorbid MDD shows that CC may be a cost-effective treatment depending on willingness-to-pay levels. Nevertheless, the low utility scores emphasize the need for further research to improve the cost-effectiveness of CC in this highly prevalent and costly group of patients.
Keywords: collaborative care, randomized controlled trial, chronic physical condition, major depressive disorder, cost-utility, general hospital, CC–DIM
Plain language summary
The study was undertaken to establish if collaborative care (CC) is a cost-effective treatment model when provided in the general hospital outpatient setting, rather than the primary care setting, for patients with chronic physical conditions, such as diabetes mellitus (DM), chronic heart failure (CHF), and chronic obstructive pulmonary disease (COPD), with comorbid major depressive disorder (MDD). The researchers performed a randomized controlled trial (RCT) evaluating CC provided by a consultant psychiatric nurse (CPN) as care manager; a consultation–liaison (CL) psychiatrist for diagnosis, supervision, and the prescription of antidepressant medication; and a medical specialist who provided treatment for the chronic physical condition. They found CC to be cost-effective with an incremental cost-effectiveness ratio (ICER) of €24,690 per quality-adjusted life year (QALY). Due to the high disease burden in this patient group, this may indicate that the CC model and setting may be preferable. However, the study was small, so replication in a larger study is warranted.
Introduction
Major depressive disorder (MDD) is deemed to become the leading cause of disability in 20301 and is a risk factor for a chronic physical condition.2 The prevalence of comorbid MDD in chronic physical conditions, such as chronic obstructive pulmonary disease (COPD), diabetes mellitus (DM), and congestive heart failure (CHF), is estimated to be between 7% and 16%.3 However, comorbid MDD often goes unrecognized in such cases as it may be difficult to distinguish these symptoms from the symptoms of the underlying medical condition.4
Comorbid MDD in chronic physical conditions is associated with maladaptive behavior, such as noncompliance with medical treatment recommendations. This is tripled in MDD,5 with deterioration of general functioning, lower quality of life, and higher costs over the short and long terms.6 For example, DM patients with comorbid MDD report symptoms more frequently than DM patients with a similar severity of their chronic condition but without comorbid MDD, and this leads to increased medical testing and therefore higher costs.7 In the case of DM and CHF, patients with comorbid MDD suffer greater health losses2,5 and have up to twofold higher medical costs compared to DM or CHF patients without comorbid MDD.8
It has been suggested that disease management interventions9 aimed at the treatment of MDD in patients with a chronic physical condition might increase the quality of life and decrease costs. In such programs, patients play an active role in their treatment and a care manager coordinates the treatment in collaboration with other medical specialists. A specific form of disease management is collaborative care (CC), which has been proven to be effective in the USA, the UK, and the Netherlands,2,10–14 The findings of an efficacy study15,16 showed that when CC was applied in the outpatient general hospital setting for chronic medically ill patients with comorbid MDD, there was no additional effect on the likelihood of remission and response compared to care as usual (CAU). However, it did significantly reduce the number of adverse medical events, which in turn may affect the quality of life. Since then, several systematic reviews have been undertaken exploring the effect of CC related to several chronic physical conditions, establishing its effect in terms of depressive symptoms over CAU. This has been found for cancer,17 coronary heart disease,18 and DM.19–21
The efficacy of the model as a generic approach applicable for a variety of chronic physical conditions and in the case of multimorbidity has also been explored in systematic reviews. A meta-analysis of individual participant data found CC to be effective against MDD in chronic physical conditions,22 and a systematic review found that CC is not only effective in reducing depressive symptoms, but also physical symptoms in chronic physical conditions with comorbid MDD.23
In terms of cost-effectiveness studies on CC for MDD, a review was published in 201024 showing that CC overall was more expensive, but increased the quality of life, with an incremental utility of between 0.03 and 0.12 quality-adjusted life years (QALYs). The studies in this review were mostly conducted in the USA and performed in a primary care setting. Another systematic review on studies in primary care found a dominance of CC over CAU.25 Since then, a randomized controlled trial (RCT) that explored the cost-effectiveness of CC for MDD in primary care established its dominance over CAU.26–28 Another study in the occupational health setting found that CC did reduce costs, but also the effects in that setting.29–31 Only one study has investigated the cost-effectiveness of CC for patients with a chronic medical illness with comorbid MDD, namely DM. This study showed that CC was associated with a low increment in medical health care costs, while gaining high benefits.32
There is ongoing debate from the health services perspective concerning which setting is most fitting for CC of patients with comorbid MDD in chronic physical conditions: the primary care setting or the general hospital setting.33 Cost-effectiveness may be one of the aspects taken into account in such a debate. However, so far, no cost-effectiveness studies regarding CC have been performed in the general hospital setting. This study aims to do so from a health care and a societal perspective.
The primary objective of this article is to assess the cost-utility of CC for the treatment of comorbid MDD in chronic medically ill patients in the outpatient general hospital setting from a societal perspective, taking all relevant costs and effects into account.
Patients and methods
Design
A multicenter RCT was conducted from September 2007 to October 2010 in outpatient clinics for DM, COPD, inflammatory bowel disease (IBD), and chronic heart failure (CHF) in five general hospitals in the Netherlands in Amsterdam, Almelo, Hengelo, Ede, and Maastricht. The study consisted of a two-armed randomized controlled trial, with randomization at the patient level. Patients in the participating departments who screened positive on the patient health questionnaire-9 (PHQ-9) and had an MDD according to the mini international neuropsychiatric interview (MINI) were randomly allocated to the intervention group or the CAU group within their outpatient clinic by a blinded research assistant, using a computerized method to avoid assignment bias. The patients were not blinded for their group allocation. This method of randomization is often followed in psychiatric intervention research.34 The intervention group received CC from a consultant psychiatric nurse (CPN), and in some cases antidepressant medication from the consultation–liaison (CL) psychiatrist in the department of Consultation–Liaison Psychiatry of the participating hospitals. The control group received CAU. The study protocol was approved by the Medical Ethics Committee (METC) of the VU University Medical Center and is described in greater detail elsewhere.15
Study oversight
This RCT was part of the Depression Initiative, a national initiative to improve depression management in the Netherlands.35–37 A steering group, consisting of the principal investigator (CFC), and senior investigators involved in the design, management, and analysis of the trial (ATFB, LHR), monitored the progress in quarterly meetings to oversee the project.
Participants
During the inclusion period, all patients who had visited the participating outpatient departments in the previous year and had a confirmed chronic physical condition as specified in their medical records were selected from the medical files and were invited to participate by the nurses receiving them for their regular outpatient visits. The nurses handed them an envelope containing an information letter, an informed consent form, and the screening questionnaire (depression subscale of the PHQ-9) with a return envelope. Patients who consented and screened positive for depression then received the baseline questionnaire by mail. Patients who met the inclusion criteria based on the patient files but did not visit the participating departments received the same package by mail. In the information letter, the patients were asked if they were willing to participate in a study investigating mental problems and treatment options in the general hospital setting. If they agreed to participate, they were asked to sign the informed consent form and return it together with the completed questionnaire to the researchers, who then contacted them to arrange to conduct the MINI.39
Inclusion criteria were the presence of a chronic physical condition, informed consent, age >18 years, and having a comorbid MDD as defined by a score of ≥10 on the PHQ-938 and a positive MINI.39 Exclusion criteria were insufficient knowledge of the Dutch language, dementia or delirium, alcohol or drug addiction as a main diagnosis, psychotic or bipolar disorder, suicidality, and pregnancy.
Intervention
In CC, treatment was provided by a team consisting of the patient, the CPN care manager, and the CL psychiatrist at the outpatient clinic of the general hospital according to an algorithm and monitored by a web-based tracking system that functioned as a supportive decision aid for the CPN care manager. Supervision and consultation by the CL psychiatrist was provided when the CPN care manager experienced difficulties in this process. The CC treatment encompassed guided self-help and problem-solving treatment (PST) provided by the CPN care manager in a one-to one session, antidepressants prescribed by the CL psychiatrist according to an algorithm, and consultations with the CL psychiatrist if necessary. According to the stepped care principle, treatment response was monitored biweekly with the PHQ-9. More details of the intervention are described elsewhere.15,16
Care as usual
The control group patients received usual care in the general hospital setting, which consisted of a medical specialist monitoring their medical illness and advising the patient to seek treatment for their depressive symptoms from a primary care physician if they felt the need.15,16
Measures
Data collection was performed by the Trimbos Institute in cooperation with the participating hospitals. After providing informed consent, patients received assessment questionnaires by mail at baseline (T0), and after 3 months (T1), 6 months (T2), 9 months (T3), and 12 months (T4).
Quality of life
Quality of life was assessed with the EuroQol EQ-5D 3 level version (EQ-5D-3L).40,41 This generic health index is a standardized, validated instrument that encompasses five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension consists of three levels: no problems, some problems, and extreme problems. Therefore, it defines a total of 243 different health states. The mean utility scores were estimated by applying the area-under-the-curve (AUC) method, implemented by summing the areas of the geometrical shapes obtained by linearly interpolating between utility scores over the study period.42 Dutch tariffs were used to estimate utilities.
Health care utilization costs
Part 1 of the treatment inventory cost in psychiatric patients (TiC-P) is a validated instrument that measures direct medical costs by estimating the number of contacts with health care services during the previous 3 months.42 We calculated the costs by multiplying the amount of care by the corresponding reference unit prices from 2016 (indexed to unit prices from 2014).43 The direct costs estimated by the TiC-P were as follows: costs for the general practitioner (GP), mental health care institute, psychiatrist/psychologist at an outpatient center or hospital, occupational health care, medical specialist, paramedic care provider, social worker, consultation for alcohol/drugs, alternative treatment, self-help care, admission to part-time day care, (psychiatric) hospital admission, and medication. These costs were taken into account as they are part of the validated instrument. The CPN was the care manager in the CC group and was therefore important for our analysis. The unit price estimation was based on gross wages per year, working hours, session length of 1 hour, preparation of written reports, overheads, bonuses, and training. The amount of care provided by the CPN was recorded using a separate question about resource use. The indirect costs considered were household and informal costs. The inclusion of productivity costs related to paid work is especially relevant when the intervention is targeted at patients of working age. Due to the high age of the study population, we could reasonably expect cost-effectiveness outcomes to be unaffected by productivity costs, and therefore they could be ignored even when adopting a societal perspective.44 However, the costs of household work and informal care are considered highly relevant in this study population. We therefore included these costs outside health care.
In general, travel distances in the Netherlands are small, and consequently the costs are low. To avoid further increasing the numbers of questions asked of the patients, travel costs were not considered.
Indirect costs
The second part of the TiC-P contains the short-form Health-Related Quality of Life (SF-HLQ).45 This part assesses the amount of informal care and household work.
Cost-utility
An incremental cost-effectiveness ratio (ICER) was calculated to obtain the costs per QALY, dividing the incremental costs by the incremental effects.
Statistical analyses
Analyses were conducted using the Statistical Package for the Social Sciences (SPSS) version 19.0, R (version 3.0.3.6), and Excel (2010). First, the direct costs and quality-of-life scores were calculated in SPSS. The cost-effectiveness analysis was performed from a health care and a societal perspective. Uncertainty in the analysis was assessed using bootstrapping in R, with 5,000 iterations. Bootstrapping was conducted by drawing samples from the original sample (with replacement). For each of the bootstrapped samples, a generalized estimating equation model was applied for each outcome variable (ie, quality of life or costs). Costs were adjusted for the number of chronic conditions and age.46 Quality of life was adjusted for quality of life at baseline and age. We used a multilevel model (generalized estimating equation) to adjust for imbalances between treatment arms and to allow for the correlation between measurements over time. By using this model, we could easily allow for correlation between measurements over time. We used a log link function with a gamma distribution for the costs and an identity function with a Gaussian distribution for quality of life. A correlation matrix with an autocorrelation structure was used for both costs and effects. In this way, 5,000 predicted incremental costs and 5,000 predicted incremental QALYs were generated. Each of the 5,000 ICERs was calculated as the mean of the predicted incremental costs divided by the mean of the incremental QALYs, expressed on a cost-effectiveness plane and a cost-acceptability curve. For this analysis, an “intention-to-treat” approach was used.
According to the Council for Public and Health Care (RVZ),47 the threshold in relation to the acceptability of the treatment depends on the severity of disease and uncertainty in the ICER, with a maximum of €80,000/QALY, and this is the decision rule that we applied in this study.
Results
Population
A total of 11,330 patients were approached by mail or at the desk. Of these, 43% consented to screening. Reasons for lack of consent were inability to locate the patients because of a change of address, language problems, and among the persons approached at the desk, self-reported fatigue due to their chronic physical condition, which hampered collaboration. After the MINI and checking for exclusion criteria, 81 patients with moderate-to-severe MDD could be randomized. Forty-two of these patients were randomly allocated to the CC group and 39 to the CAU group. Figure 1 provides a flowchart of the participants over the course of the study. Table 1 summarizes the baseline demographic and clinical characteristics for these patients. There were no significant differences in sociodemographic or clinical characteristics between the CC and CAU groups.
Dealing with missingness
Our analysis used a multilevel model. In this way, the skewness of the data, baseline corrections, and correlation between measurements over time could be taken into account. The advantage of a multilevel model (a generalized estimating equation model) is that the data are implicitly imputed by the model and the predictions of the model. The pattern of missingness in the data on quality of life and costs is shown in Table 2. Decisions on the variables in the model were made by plotting the residuals of the models and by using the quasi-likelihood under the independence model criterion (QIC).
Quality of life
The utility scores for quality of life were calculated per measurement moment, as shown in Table 3. The mean utility scores at baseline were low in both the groups: 0.43 (standard deviation [SD] =0.31) in the CC group and 0.45 (SD =0.28) in the CAU group. The CC group improved significantly over time. In the CAU group, the utility values gained were 0.01 (95% confidence interval [CI]: –0.04 to 0.05) and in the CC group 0.07 (95% CI: 0.02 to 0.13). The difference in effect was not significant over time: 0.07 (95% CI: –0.003 to 0.14).
  | Table 3 Mean utility scores40,41 |
Direct medical costs
The direct medical costs calculated per health care provider are shown in Table 4. The costs of the CPN (the care manager in the CC group) amounted to €291 in the CC group. The costs for the psychiatrist/psychologist were respectively €159 and €187 in the CC and CAU groups.
  | Table 4 Unit costs, average number of contacts per patient, measured by the TiC-P,40 and costs by health care providers per year (Euro’s 2016) |
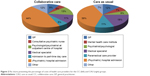
The percentage of patients who contacted the medical specialist or had a psychiatrist/psychologist consultation at an outpatient clinic was higher in the CC group compared to the CAU group. After applying the model and bootstrapping the data, the average costs per patient for the CC group were €6,490 (95% CI: 3,290 to 10,645) and the average costs per patient for the CAU group were €4,801 (95% CI: 2,878 to 7,149), resulting in a difference of €1,689 (95% CI: –2,006 to 5,974). The main costs are presented in the pie chart shown in Figure 2.
Indirect costs
As shown in Table 5, the indirect costs for informal care and household work were, respectively, €189 (407) and €302 (474) for the CC group. For the CAU group, these costs were €213 and €155.
  | Table 5 Costs per hour, number of hours, total costs, and number of missings for informal care and household work (Euro’s 2016) |
Health care perspective
The combination of higher direct medical costs and higher effects resulted in an ICER of €28,366/QALY, as shown in Table 6.
In Figure 3, the cost-effectiveness plane and the cost-acceptability curve are shown. As can be seen, 80% of the ICERs fall into the north-east quadrant, indicating a combination of higher effectiveness and higher direct medical costs for the CC group, and 17% of the ratios fall into the south-east quadrant, indicating that CC generates greater utilities and is less expensive compared to CAU.
At a threshold of €20,000/QALY, there is 40% probability that the intervention is accepted. At an ICER of €60,000/QALY, there is ~80% probability that the intervention is accepted.
Societal perspective
Again, there was a combination of higher direct medical costs and higher effect in the CC group, which resulted in an ICER of €24,690/QALY, as shown in Table 6. Although the indirect costs estimated were higher in the CC group, the model predicted otherwise, namely that the costs should actually be lower.
After bootstrapping, the ICERs were again plotted on a cost-effectiveness plane and a cost-acceptability curve (Figure 4). In this case, 77% of the ICERs fall into the north-east quadrant of the cost-effectiveness plane, indicating that CC is associated with higher costs and also higher effects compared to CAU and 20% fall in the south-east quadrant, indicating higher costs and lower effects.
  | Figure 4 Cost-effectiveness plane (left ) and cost-acceptability curve (right) from a societal perspective. |
At a threshold of €20,000/QALY, there is ~60% probability that the intervention is accepted. At an ICER of €60,000, there is ~80% probability that the intervention is accepted.
Sensitivity analysis
A sensitivity analysis was performed from a societal perspective on admission to psychiatric hospital. These costs were relatively high in the CC group, but the number of contacts was relatively low. The costs for the CC group were now €4,287 (95% CI: 2,945 to 5,923) and the costs for the CAU group were €3,155 (95% CI: 2,378 to 4,034). The difference in costs was €1,132 (95% CI: −521 to 2,939). There was only a change in the incremental costs per QALY to €18,732/QALY. In addition, the majority of the cost–effect ratios (88%) still fall into the north-east quadrant and 10% into the south-east quadrant. The cost-effectiveness plane and the cost-acceptability curve are plotted in Figure 5.
Discussion
This study is the first cost-utility study of CC for the treatment of comorbid MDD in patients with a chronic physical condition, namely DM, COPD, IBD, or CHF, in a general hospital outpatient setting. The higher costs and higher effects in the CC group lead to an ICER of €24,690/QALY from a societal perspective. We apply a decision rule of a maximum of €80,000/QALY, as explained earlier. The acceptability curve shows that at €20,000/QALY, there is a relatively low probability that the intervention is accepted. However, at a threshold of €60,000/QALY, the probability of acceptance increases to almost 80%. In this case, the ICER is €24,690/QALY, which, in view of the significant disease burden of the patients, may be acceptable. When a health care perspective was considered, the ICER decreased to €18,732/QALY. A sensitivity analysis was conducted to investigate the effect of the costs of admission to a psychiatric hospital as these costs were relatively high, but the number of patients using them was relatively low. After the sensitivity analysis, the ICER decreased and CC became more effective. The sensitivity analysis showed that the results are robust. This is a better outcome than the study on CC in MDD in the occupational health setting, which found CC to be less costly but also less effective than CAU.31 It is also a better outcome than the study on CC in MDD in primary care, which found that, taking a health care perspective, CC was less cost-effective due to higher costs compared to CAU, which led to an ICER of €53,717/QALY. Hence, in terms of cost-effectiveness, CC may be particularly promising in patients with chronic physical conditions with comorbid MDD who receive treatment in the outpatient general hospital setting. According to the Council for Public and Health Care (RVZ), the threshold in relation to the acceptability of treatment depends on the severity of disease and uncertainty in the ICER, with a maximum of €80,000/QALY.47 According to this decision rule, an innovative CC model based on the psychiatric consultation services of general hospitals may be a cost-effective intervention. However, replications of this research are necessary.
In both the groups, the largest part of the costs was due to hospital admissions for patients’ chronic physical conditions, which indicates the high disease burden in this patient group. Admission costs in the CC group were higher compared to the CAU group. However, these costs were due to a relatively small group of patients, indicated by the large SD. Apart from that, the direct medical costs in the CC group were mainly caused by visits to a psychiatrist/psychologist at an outpatient center or hospital, the CPN care manager, and admission to part-time day care. This study was conducted from a societal perspective; however, the productivity costs were negligible as the age of the patients was high, and they were consequently in general no longer part of the working population. Furthermore, we did not have data on the utilization of emergency care and therefore we could not estimate these costs. The same holds for medication for physical comorbid conditions. However, we do not expect these costs to be different between the two interventions. Hence, they are not expected to affect the ICER. With respect to occupational health care, we expect these costs not to be relevant due to the high age of the study population, meaning that they will generally be retired.
Over time, the quality of life improved in both groups, but the quality of life in the CC group increased more (significantly). In the effect study,16 there was no significant difference between the two groups in terms of total remission or of treatment response regarding depressive symptoms, as measured by the PHQ-9.38 However, the number of adverse events did significantly differ between the groups, decreasing more in the CC group, and this may subsequently have contributed to improved quality of life despite the continued presence of depressive symptoms. Further research is needed to explore the association between adverse effects, hospital admissions, and costs and quality of life in this patient group. The initial quality of life was low in both the CC and CAU groups, indicating that MDD in combination with a chronic disorder greatly affects quality of life. This finding corroborates the review of Simon,7 namely that additional impairment is experienced when depressive patients have a comorbid chronic physical condition. This study seems to show a weak trend toward increased quality of life in the CC group, contrary to the CAU group. However, the average quality of life is still remarkably low for both groups.
As CC was associated with higher costs and higher utilities, the results of this study agree with the findings of the review conducted by van Steenbergen-Weijenburg et al.24 The improvement in quality of life in CC was also substantiated in our study. Simon et al32 showed that after 1 year, medical costs for CC in patients with comorbid MDD in DM started to decline and at the end of the second year were lower than in CAU. This positive effect also extended to the benefits of intervention. This indicates that higher cost-effectiveness may be attained if a longer follow-up period is conducted, and this should be a topic for further research.
Research is warranted exploring how to lower the relatively high costs found to be associated with CC for this patient group with a high disease burden. New developments, such as E-health and M-health interventions, have been suggested as alternatives for face-to-face psychotherapeutic treatments in this patient group; however, the expectations in terms of cost-effectiveness have as yet remained unfulfilled. Standalone E-health and M-health interventions in multimorbidity have been found to be associated with patient disengagement and physician withdrawal, and with low effectiveness.48 Research attempts to develop cost-effective interventions for patients with multimorbidity should focus on patient safety,49 as a study of a tele-monitoring intervention to prevent hospitalization and emergency room visits provided evidence of higher mortality compared to CAU in elderly patients with multimorbidity.50 The outcomes of our study, showing somewhat more hospital admissions in the CC group, might be related to better monitoring of adverse somatic developments in the CC intervention requiring admission, thus resulting in better quality of life. Hence, also in terms of safety, in this patient group with chronic physical conditions, CC may be the model of choice despite the higher costs. However, this should be explored in further research. Further research might also evaluate a combination of CC and E-health, or tele-monitoring in so-called blended E-health models, in which clinical diagnostic and treatment evaluation is strongly embedded. Thus, no physician withdrawal or patient disengagement should occur. Such treatment should focus not only on the treatment of MDD, but also on better management and quality of life regarding the chronic physical condition at hand, and should also take mortality as an outcome into account.51
Limitations of the study
The first limitation of this study was the small sample size. Based on the prevalence rates indicated in the literature, this study was originally set up as a clinical trial in one hospital, but due to low inclusion rates, it was extended to a multicenter trial, thus providing sufficient participants to perform the study. Although initially response to the mail invitation was 43%, low inclusion rates were caused by patients having lower comorbid MDD rates than initially expected based on the literature; as can be seen from the flowchart, the actual number of patients fulfilling the MINI classification for MDD was only 169, of whom a further 88 had to be excluded because of acute suicidality, psychosis, addiction, and dementia, inter alia. This warrants further research into the prevalence of comorbid MDD in chronic physical conditions in clinical cohort studies. Another reason for the low inclusion rates was that patients felt too ill to participate in the study, particularly as their chronic physical condition necessitated focusing on that alone. A further limitation of the study was the high dropout rate,26 which was to be expected given the high burden of illness due to the combination of psychological and physical complaints in this patient group. This illustrates one of the reasons why few studies have yet been performed in this setting and with this population.
Conclusion
This first study has demonstrated the cost-utility of CC compared to CAU in an outpatient general hospital setting using a relatively long perspective. Despite the small patient group, it was possible to establish some clear findings on the quality of life and costs among outpatients with chronic physical conditions and comorbid MDD. According to the Council for Public and Health Care (RVZ), the threshold in relation to the acceptability of treatment depends on the severity of disease and uncertainty in the ICER, with a maximum of €80,000/QALY. According to this decision rule, an innovative CC model based on the psychiatric consultation services of general hospitals may be a cost-effective intervention. However, replications of this research are necessary.
This study showed incremental quality-of-life gains in applying a CC model for this patient group. Nevertheless, the low utility scores emphasize the need for further research to improve the (cost-)effectiveness of CC in this highly prevalent and costly group of patients.
Acknowledgments
The current affiliation of Dr Kirsten van Steenbergen-Weijenburg is Menzis, Wageningen, the Netherlands. This RCT was part of the Depression Initiative, a national initiative to improve depression management in the Netherlands that was funded by Innovatiefonds Zorgverzekeraars. Clinical Trial Register Number is NTR818.
Disclosure
CFC reports unrestricted research grants from ZonMw, Achmea, and Eli Lilly outside the submitted work. ATFB received financial support from the speakers bureaus of Lundbeck en GlaxoSmithKline. LHR reports unrestricted research grants from ZonMW, Innovatiefonds of the Dutch Health insurers, Boeringher Ingelheim, and Janssen-Cilag. The other authors report no conflicts of interest in this work.
References
World Health Organization. The Global Burden of Disease: 2004 Update; 2008. Available from: http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_part2.pdf. Accessed June 20, 2017. | ||
Katon WJ. Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues Clin Neurosci. 2011;13(1):7–23. | ||
Egede LE. Major depression in individuals with chronic medical disorders: prevalence, correlates and association with health resource utilization, lost productivity and functional disability. Gen Hosp Psychiatry. 2007;29(5):409–416. | ||
Van der Feltz-Cornelis CM, Ten Have M, Penninx BW, Beekman AT, Smit JH, De Graaf R. Presence of comorbid somatic disorders among patients referred to mental health care in the Netherlands. Psychiatr Serv. 2010;61(11):1119–1125. | ||
DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med.2000;160(14):2101–2107. | ||
Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the world health surveys. Lancet. 2007;370(9590):851–858. | ||
Simon GE. Social and economic burden of mood disorders. Biol Psychiatry. 2003;54(3):208–215. | ||
Katon W, Lin EH, Kroenke K. The association of depression and anxiety with medical symptom burden in patients with chronic medical illness. Gen Hosp Psychiatry. 2007;29(2):147–155. | ||
Neumeyer-Gromen A, Lampert T, Stark K, Kallischnigg G. Disease management programs for depression: a systematic review and meta-analysis of randomized controlled trials. Med Care. 2004;42(12): 1211–1221. | ||
Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med. 2006;166(21):2314–2321. | ||
Unutzer J, Katon W, Callahan CM, et al; IMPACT Investigators. Improving Mood-Promoting Access to Collaborative Treatment. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA.2002;288(22):2836–2845. | ||
Richards DA, Lovell K, Gilbody S, et al. Collaborative care for depression in UK primary care: a randomized controlled trial. Psychol Med. 2008;38(2):279–287. | ||
Coventry PA, Hudson JL, Kontopantelis E, et al. Characteristics of effective collaborative care for treatment of depression: a systematic review and meta-regression of 74 randomised controlled trials. PLoS One. 2014;9(9):e108114. | ||
Baumeister H, Hutter N. Collaborative care for depression in medically ill patients. Curr Opin Psychiatry.2012;25(5):405–414. | ||
Horn EK, van Benthem TB, Hakkaart-van Roijen L, et al. Cost-effectiveness of collaborative care for chronically ill patients with comorbid depressive disorder in the general hospital setting, a randomised controlled trial. BMC Health Serv Res.2007;7:28. | ||
Steenbergen-Weijenburg KM, van der Feltz-Cornelis CM, van Benthem TB, et al. Efficacy of collaborative care for comorbid major depressive disorder in chronic medically ill patients in the general hospital outpatient setting. A multi-center RCT in the Netherlands depression initiative. Tijdschr Psychiatr. 2015;57(4):248–257. Dutch. | ||
Li M, Kennedy EB, Byrne N, et al. The Management of Depression in Patients with Cancer. Toronto (ON): Cancer Care Ontario; 2015 May 11. Available from: https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=340750. Accessed June 20, 2017. | ||
Tully PJ, Baumeister H. Collaborative care for comorbid depression and coronary heart disease: a systematic review and meta-analysis of randomized controlled trials. BMJ Open. 2015;5(12):e009128. | ||
Atlantis E, Fahey P, Foster J. Collaborative care for comorbid depression and diabetes: a systematic review and meta-analysis. BMJ Open. 2014;4(4):e004706. | ||
Huang Y, Wei X, Wu T, Chen R, Guo A. Collaborative care for patients with depression and diabetes mellitus: a systematic review and meta-analysis. BMC Psychiatry. 2013;13:260. | ||
van der Feltz-Cornelis CM, Nuyen J, Stoop C, et al. Effect of interventions for major depressive disorder and significant depressive symptoms in patients with diabetes mellitus: a systematic review and meta-analysis. Gen Hosp Psychiatry. 2010;32(4):380–395. | ||
Panagioti M, Bower P, Kontopantelis E, et al. Association between chronic physical conditions and the effectiveness of collaborative care for depression: an individual participant data meta-analysis. JAMA Psychiatry. 2016;73(9):978–989. | ||
van Eck van der Sluijs J, Castelijns H, Eijsbroek V, van der Feltz-Cornelis C. Effectiveness of collaborative care for patients with a combination of physical and psychiatric problems: a review and meta-analyses. J Psychosom Res.2016;85:85. | ||
van Steenbergen-Weijenburg KM, van der Feltz-Cornelis CM, Horn EK, et al. Cost-effectiveness of collaborative care for the treatment of major depressive disorder in primary care. A systematic review. BMC Health Serv Res. 2010;10:19. | ||
Grochtdreis T, Brettschneider C, Wegener A, et al. Cost-effectiveness of collaborative care for the treatment of depressive disorders in primary care: a systematic review. PLoS One. 2015;10(5):e0123078. | ||
IJff MA, Huijbregts KM, van Marwijk HW, et al. Cost-effectiveness of collaborative care including PST and an antidepressant treatment algorithm for the treatment of major depressive disorder in primary care; a randomised clinical trial. BMC Health Serv Res. 2007;1(7):34. | ||
Huijbregts KM, de Jong FJ, van Marwijk HW, et al. A target-driven collaborative care model for major depressive disorder is effective in primary care in the Netherlands. A randomized clinical trial from the depression initiative. J Affect Disord. 2013;146(3):328–337. | ||
Goorden M, Huijbregts KM, van Marwijk HW, Beekman AT, van der Feltz-Cornelis CM, Hakkaart-van Roijen L. Cost-utility of collaborative care for major depressive disorder in primary care in the Netherlands. J Psychosom Res. 2015;79(4):316–323. | ||
Vlasveld MC, Anema JR, Beekman AT, et al. Multidisciplinary collaborative care for depressive disorder in the occupational health setting: design of a randomised controlled trial and cost-effectiveness study. BMC Health Serv Res. 2008;8:99. | ||
Vlasveld MC, van der Feltz-Cornelis CM, Adèr HJ, et al. Collaborative care for major depressive disorder in an occupational healthcare setting. Br J Psychiatry. 2012;200(6):510–511. | ||
Goorden M, Vlasveld MC, Anema JR, et al. Cost-utility analysis of a collaborative care intervention for major depressive disorder in an occupational healthcare setting. J Occup Rehabil. 2014;24(3):555–562. | ||
Simon GE, Katon WJ, Lin EH, et al. Cost-effectiveness of systematic depression treatment among people with diabetes mellitus. Arch Gen Psychiatry. 2007;64(1):65–72. | ||
Frankel SA, Bourgeois JA, Erdberg P. Comprehensive Care for Complex Patients: The Medical-Psychiatric Coordinating Physician Model. 1st ed. New York: Cambridge University Press; 2013. | ||
Van Der Feltz-Cornelis CM, Adèr HJ. Randomization in psychiatric intervention research in the general practice setting. Int J Methods Psychiatr Res. 2000;9(3):134–142. | ||
Van der Feltz-Cornelis CM. Towards integrated primary health care for depressive disorder in the Netherlands. The depression initiative. Int J Integr Care.2009;9:e83. | ||
Van der Feltz-Cornelis CM. The Depression Initiative. Description of a collaborative care model for depression in the primary care setting in the Netherlands. Clin Neuropsychiatry. 2011;8(4):260–267. | ||
de Jong FJ, van Steenbergen-Weijenburg KM, Huijbregts KM, et al. The Depression Initiative. Description of a collaborative care model for depression and of the factors influencing its implementation in the primary care setting in the Netherlands. Int J Integr Care. 2009;9:e81. | ||
Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: The PHQ primary care study. JAMA. 1999;282(18):1737–1744. | ||
van Vliet IM, Leroy H, van Megen HJM. De MINI-internationaal neuropsychiatrisch interview: Een kort gestructureerd diagnostisch interview voor DSM-IV en ICD-10 psychiatrische stoornissen [The MINI International Neuro Psychiatric Interview: A Short Structured Diagnostic interview for DSM-IV and ICD-10 mental disorders]. Tijdschrift Voor Psychiatrie. 2007;49(6):393–397. Dutch. | ||
Cheung K, Oemar M, Oppe M, Rabin R. Eq-5D User Guide, Basic Information on How to Use EQ-5D. Rotterdam: EuroQol Group; 2009. | ||
Bouwmans CAM, de Jong K, Timman R, et al. Feasibility, reliability and validity of a questionnaire on health care consumption and productivity loss in patients with a psychiatric disorder (TiC-P). BMC Health Serv Res. 2013;217(13):1–9. | ||
Matthews JNS, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300:230–235. | ||
Hakkaart-van Roijen L, Van der Linden N, Bouwmans C, Kanters T, Tan SS. Costing: methods and reference prices for economic evaluations in health care. Zorginstituut Nederland 2015. Dutch. Available from: https://www.ispor.org/PEguidelines/source/NL-Economic_Evaluation_Guidelines.pdf. Accessed June 20, 2017. | ||
Krol M, Papenburg J, Tan S, Brouwer W, Hakkaart L. A noticeable difference? Productivity costs related to paid and unpaid work in economic evaluations on expensive drugs. Eur J Health Econ. 2016;17:391–402. | ||
van Roijen L, Essink-Bot ML, Koopmanschap MA, Bonsel G, Rutten FF. Labor and health status in economic evaluation of health care. The health and labor questionnaire. Int J Technol Assess Health Care. 1996;12:405–415. | ||
Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ. 2001;10(8):779–787. | ||
RVZ. Sensible and sustainable care: Advice of the Health Council tot he Ministry of Health Welfare and Sport. Zoetermeer 2006. Available from: https://www.raadrvs.nl/uploads/docs/Advies_-_Zinnige_en_duurzame_zorg.pdf. Accessed June 20, 2017. | ||
Chavannes NH, Sont JK, van der Boog PJ Assendelft WJ. E-Health in chronic diseases: not yet feasible for everyone in every setting. Ned Tijdschr Geneeskd. 2012;156:A5345. Dutch. | ||
Car J, Huckvale K, Hermens H. Telehealth for long term conditions. BMJ. 2012;344:e4201. | ||
Takahashi PY, Pecina JL, Upatising B, et al. A randomized controlled trial of telemonitoring in older adults with multiple health issues to prevent hospitalizations and emergency department visits. Arch Intern Med. 2012;172(10):773–779. | ||
Van der Feltz-Cornelis CM. Comorbid diabetes and depression: do E-health treatments achieve better diabetes control? Diabetes Manag. 2013;3(5):379–388. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.