Back to Journals » ClinicoEconomics and Outcomes Research » Volume 9
Cost per care of the first year of direct antiviral agents in the Liguria Region: a multicenter analysis
Authors Cenderello G, Fanizza C, Marenco S, Nicolini LA, Artioli S, Baldissarro I, Dentone C, De Leo P, Di Biagio A
Received 10 December 2016
Accepted for publication 31 March 2017
Published 22 May 2017 Volume 2017:9 Pages 281—293
DOI https://doi.org/10.2147/CEOR.S129859
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Dean Smith
Giovanni Cenderello,1 Caterina Fanizza,2 Simona Marenco,3 Laura Ambra Nicolini,4 Stefania Artioli,5 Isabella Baldissarro,3 Chiara Dentone,6 Pasqualina De Leo,7 Antonio Di Biagio4
1SC Malattie Infettive, EO Ospedali Galliera, 2Rete ligure HIV, 3SSD Epatologia, AOU S. Martino, 4Clinica Malattie Infettive, AOU S. Martino, Genoa, 5SC Malattie Infettive ASL5 La Spezia, 6SC Malattie Infettive, ASL‑1, Sanremo, 7SC Malattie Infettive ASL2, San Paolo, Savona, Italy
Aims: Despite the remarkable efficacy shown in clinical practice, concerns have been raised about the costs associated with direct antiviral agent (DAA) therapy. This article presents the real-life costs for DAA treatment sustained by the Italian National Health Service in the Liguria Region (Northern Italy).
Methods: A retrospective analysis of the cost per care sustained for DAA treatment, relating to the period from January 1 to December 31, 2015 in five centers in Liguria was performed. All patients undergoing DAA-based treatments for hepatitis C virus (HCV) infection were enrolled. On-treatment costs included: HCV treatment, laboratory test, outpatient services, attended visits, drugs used for the management of adverse events (erythropoietin, albumin or red blood cell packs) and inpatient service admissions.
Results: In total, 327 patients were enrolled. No difference in terms of sustained virologic response (SVR) rate among different treatments was reported. The majority (85.0%) of patients did not report any side effects and only 15 (4.6%) required hospital admission. Forty-two patients (12.8%) required high-cost drugs for the management of adverse events. The overall cost sustained was €14,744,433. DAA±ribavirin (RBV) accounted for the wide majority of this cost (98.9%; €14,585,123). Genotype (GT) 1, the most commonly treated GT, was associated with an average cost of €43,445 per patient. Detailed analysis of the costs for GT 1 showed the treatment based on ritonavir boosted paritaprevir/ombitasvir + dasabuvir±RBV with an average cost of €24,978 (RBV+) and €25,448 (RBV−) per patient was the most cost-effective. The average cost per SVR was €48,184. Once again, the ritonavir boosted paritaprevir/ombitasvir + dasabuvir regimen was associated with the lowest cost/SVR (€25,448/SVR [GT 1b] and similar results for other GTs).
Conclusion: Antiviral regimen is the major contributor to costs in the treatment of HCV infection. Appropriate regimen selection could result in a major cost saving, which can be reinvested to allow more patients to be treated.
Keywords: HCV treatment, HCV costs, cost efficacy
Introduction
Infection by hepatitis C virus (HCV) represents a major health issue. HCV is estimated to infect 180 million people worldwide and it is the seventh cause of death globally.1 More than 2 million Italian individuals have contracted the HCV virus; of note, 20%–50% of infections are reported in intravenous drug abusers.2–4 The predominant genotype (GT) of HCV is 1b, with a higher prevalence in women and elderly, followed by GT 2.5
The main target of antiviral treatment is to reduce the onset of disease complications, including liver cirrhosis and hepatocellular carcinoma. To this end, antiviral therapy is aimed at achieving eradication of HCV infection, that is, a sustained virologic response (SVR).
Interferon-based antiviral therapy resulted in long-term success and a reduced mortality.6,7 However, the recent introduction of interferon-free regimens based on second-generation direct antiviral agents (DAAs) led to a dramatic improvement in clinical outcomes, with SVR rates >90%. In 2015, the interferon-free regimens available were: 1) sofosbuvir + ledipasvir (SOF+LDP),8,9 2) daclatasvir + sofosbuvir (SOF+DCV),10–12 3) ombitasvir + paritaprevir/ritonavir (PTVr/OBV) + dasabuvir (DSV),13–17 and 4) SOF+simeprevir (SOF+SIM).18 All these regimens can be associated, or not, with ribavirin (RBV). The treatment schedules defined by the Italian Society for Liver Diseases according to the European Association for Liver Diseases are included in Table S1. Despite their high efficacy, concerns have been raised about the costs associated with DAA therapy. With respect to the Italian scenario, different budget impact analyses have been published, however, considering only the drug cost19 or the complete treatment path.20 To date, no studies have investigated the actual cost sustained by the Italian National Health Service (NHS) during the first year of clinical use of second-generation DAAs. This manuscript aims to present the real-life costs for DAA treatment sustained by the NHS in the Liguria Region.
Methods
Study design
In order to achieve the primary objective, a retrospective analysis of the cost per care sustained for DAA treatment, in the period from January 1 to December 31, 2015 (Northern Italy), was conducted in the Liguria Region. Six centers, linked in a network, were involved: Infectious Disease Unit, San Remo; ASL 2 Infectious Disease Unit, San Paolo Hospital, Savona; Infectio us Disease Unit, Galliera Hospital, Genoa; Infectious Disease Clinic, San Martino Hospital, Genoa; Liver Unit, San Martino Hospital, Genoa and Infectious Disease Unit, ASL 5, La Spezia. The study was approved by the Regione Liguria ethical committee (approval ID 268REG2016). An informed consent form was signed by each patient.
All patients undergoing DAA-based regimens for HCV infection and reaching the 12-week posttreatment evaluation by March 31, 2016 (end of treatment by December 31, 2015) were enrolled. Patients belonging to special categories (e.g., those in hemodialysis, organ transplant recipients or thalassemia major carriers) were excluded due to the high costs involved in their underlying disease.
Health care providers generated a list of the subjects with HCV who started treatment in the study period, with all relevant demographic and treatment details. An automated system collected all data from laboratory and administrative services on an online platform, in order to obtain data on hospitalizations, medical visits and laboratory tests for each patient.21,22 The clinicians involved in the study provided the final data about the treatment outcome at 12 weeks after treatment.
Additional data were recorded on the use of special drugs characterized by high cost, such as erythropoietin, albumin or red blood cell packs.
Use of resources and costs
On-treatment costs included HCV treatment, laboratory tests, outpatient services, attended visits, drugs used for the management of adverse events (erythropoietin, albumin or red blood cell packs) and inpatient service admissions. Hospitalizations were classified by ICD -9 codes, and the financial costs considered were those established by the Italian NHS.
The drug-related costs of SOF and SOF/LDV are linked to a price/volume payback scheme covered by confidential agreement.23 However, the Emilia-Romagna Region periodically reports an update of the average costs of each drug on its institutional website.24 Therefore, this source at the moment represents the most reliable one for the economic analysis, and hence was used in this study.25–27 The cost of the treatment was defined on the basis of the month of the first prescription. Given the contract existing between the Italian NHS and pharmaceutical companies, 24 weeks of treatment have the same cost as 12 weeks (Table S2).28
For patients who discontinued treatment for any reason before the scheduled end, the following costs were attributed: 1) within 4 weeks, one-third of 12-week treatment; 2) within 8 weeks, two-thirds of 12-week treatment and 3) >8 weeks, the same as 12-week treatment.
The costs attributed to each additional procedure, including treatment with RBV and erythropoietin, were those defined by the Liguria Region for the year 2015 (Table S3).29–31 However, albumin and red cell blood pack-related costs were not included because they were administered during hospital admission and hence were already considered in diagnosis related group reimbursement for the hospital stay from patients’ perspective (Tables S4 and S5).32
The cost per SVR was defined as the total cost for each regimen divided by the number of SVR observed for that specific regimen.
According to the Italian Drug Agency (AIFA), DAAs are fully reimbursable only for subjects affected by METAVIR fibrosis at F3 or F4 stage or those with lower fibrosis but presenting an extrahepatic disease (malignant lymphoma, HCV-related vasculitis and symptomatic cryoglobulinemia).28,33 These rules have been summarized in the six criteria defined by AIFA as follows: 1) patients with liver cirrhosis Child Pugh A or B and/or HCC with complete treatment; 2) patients with recurrence of HCV infection after liver transplantation with a METAVIR score >2; 3) patients with chronic hepatitis and serious extrahepatic liver diseases (malignant lymphoma, HCV-related vasculitis and symptomatic cryoglobulinemia); 4) patients with chronic hepatitis C and METAVIR F3; 5) patients on the waiting list for liver transplantation and 6) patients with chronic hepatitis C and solid organ transplantation (different from liver) or bone with fibrosis METAVIR >2.
Statistical analysis
Data were analyzed by descriptive statistics. Demographic and clinical characteristics were also reported according to the HCV treatment. Differences in categorical variables were compared with the chi-squared test or Fisher’s exact test, whereas continuous variables were compared with analysis of variance or Kruskal–Wallis test, as appropriate. Normality of continuous variables was tested using Kolmogorov–Smirnov test.
To account for the different prices of HCV treatments during the year (depending on treatment initiation) and to control simultaneously for the possible confounding effect of different variables, a multivariate linear regression analysis was performed. Only the variables with a univariate p<0.05 were introduced in the model.
A univariate sensitivity analysis was conducted to determine the impact of the SVR rate, HCV treatment prices, and direct cost on the cost-per SVR. The rate of SVR varied over a range of 2%–5%, the anti-HCV treatment prices varied over a range of 10%–50%, and direct costs varied over a range of 10%–30%.
A p-value <0.05 was considered significant. Analyses were performed by R software.
Results
Population
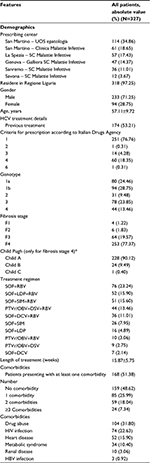
In total, 327 patients were enrolled, covering 49% of all DAA-based regimens started in 2015 in the involved center (cut-off: December 31, 2015). The remaining 51% were those not meeting the inclusion criteria, mainly the patients who were still undergoing treatment or did not reach the 12-week posttreatment evaluation point by March 31, 2016. The demographic and clinical details are presented in Table 1. The majority of patients were males (71%) living in Liguria (97%) and had been already treated for HCV (53%; Table 1). The rate of SVR at 12 weeks posttreatment was 93.5% (n=306). Among the 21 patients who did not reach this goal, a relapse was observed in 11 (52%), a vital breakthrough in 2 (9%), treatment interruption in 5 (24%), death (between the end of treatment and the 12 weeks time point) in 2 (9%) and failure in 1 (5%) patient. Reasons for treatment interruption were: two deceased, toxicity in two patients and personal decision in the last one. The main clinical features according to the treatment prescribed are shown in Table S6.
SVR rates
No differences in terms of SVR rate among different treatments were reported (Table 2). A detailed analysis of the SVR according to HCV GT showed a significant difference between GT 3 (SVR 85.9%) and other GTs. GT 1 is the most susceptible to treatment with DAA, with an overall SVR rate of 95.9%, while GT 2 and GT 4 were associated with an overall SVR rate of 96.7% and 95.4%, respectively. A combined analysis of treatment and regimen confirms these findings. In GT 1b-infected patients, the SVR rate ranged from 90.9% to 100%, according to the regimen prescribed. Very similar rates were observed in GT 1a (from 93.7% to 100%), GT 2 (96.7%) and GT 4 (from 92.3% to 100%; Table 2). On the other hand, GT 3 was associated with an SVR rate ranging from 82.2% to 100%, according to therapy. DCV was available in Italy later than in other European countries, and therefore, our study (aimed at the description of the first year of treatment in Liguria) included only a small quote of patients affected by GT 3 treated with this molecule; the only combination available was SOF and RBV.
Adverse events
The majority (85.0%) of patients did not present any side effects, and only 15 (4.6%) patients required hospital admission; the median length of hospital stay was 11 days (interquartile range: 3–18 days). Forty-two (12.8%) patients required high-cost drugs for the management of adverse events: erythropoietin was necessary in 39 (11.9%) patients, albumin in 5 (1.5%) patients and red blood cell packs in 3 (1%) patients; and 3 patients required 2 of these treatments and 1 patient required all of them.
Pharmacoeconomic analysis
The overall cost sustained for the treatment of 327 patients was €14,744,433. DAA±RBV accounted for the highest proportion of cost (98.9%; €14,585,123), followed by the management of adverse events (hospital admissions and high-cost drugs for treatment support; €89,078; 0.6%). A marginal impact was attributed to laboratory analysis and outpatient service visits (€38,180, 0.26% and €32,053, 0.22%, respectively).
When the average costs per care were evaluated according to the GT, GT 2 was the one absorbing the lowest resources, while GT 3 and GT 4 were those with higher costs per patient (Table 3). GT 1, the most commonly treated GT (174 patients), was associated with an average cost of €43,445 per patient. A detailed analysis of the costs of GT 1 showed the treatment based on PTVr/OBV+DSV±RBV with an average cost of €24,978 (with RBV) and €25,448 (without RBV) per patient was the most economical; on the other hand, SOF+SIM+RBV regimen was the most expensive, with an average cost of €61,027. Between GT 1a and GT 1b, an important difference in costs was reported (Table 4). In GT 1a treatment, the PTVr/OBV+DSV±RBV regimen was associated with the lowest cost, although it was prescribed only in 17.5% of cases. On the other hand, the PTVr/OBV+DSV±RBV regimen was the most frequently prescribed for GT 1b (31.9%/9.6%), and it was associated with the lowest cost (Table 5).
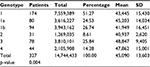  | Table 3 Total cost and average cost (in €) per patient according to genotype Abbreviation: SD, standard deviation. |
  | Table 4 Total and mean costs for different DAA treatments in patients according to HCV genotype |
Multivariate linear regression model confirmed a significant difference in costs of care according to HCV treatment, even after adjusting for the clinical characteristics at treatment initiation (Table S7).
The average cost per SVR was €48,184, with negligible differences between pretreated patients and naïve subjects. Also, the PTVr/OBV+DSV regimen was associated with lowest cost per SVR (€25,448 per SVR [GT 1b] and similar results for other GTs; Table 6).
Sensitivity analysis (Table 7) confirms the antiviral drug cost as the major contributor to the total cost. A second contributor was the achievement of SVR: an increase in SVR rate led to a major reduction in cost per SVR.
Discussion
We have reported a detailed analysis of all costs related to the first year of use of all oral DAAs for the treatment of HCV infection. Our study allowed us to identify the less-costly options, with possible indications on how to optimize the treatment schedule and, hence, treat more patients at the same global cost. In our opinion, in a condition of limited resources available, this is the only strategy that could increase the number of treated patients. It is worth nothing that our analysis was conducted in a “field-practice” cohort of consecutive unselected patients, regardless of comorbidities, coinfections, age, gender or prior therapies.
Our results highlight some critical issues: safety, efficacy and costs. Overall, SVR was reached in 93.8% of the patients treated, a rate that is well-aligned with the current clinical experience. The majority of our population was composed of patients with more severe fibrosis, as regulated by AIFA that allows the prescription of DAAs only to METAVIR F3 or F4 fibrosis stage.28 Nevertheless, DAA-based regimens were safe and effective, with SVR rates in line or even better compared with those reported in other real-life studies.34,35 In our study, hospital admissions were limited to a small proportion of patients (12.4%). This finding is in contrast with those existing for the first-generation DAAS – as recorded, for instance, in the CUPIC study, where 40% of patients reported a serious adverse event requiring hospital admission.36
Anemia requiring support with high-cost drugs was observed in 42 patients (12.4%). The PAN study, a large German study based on first-generation DAAs, reported the same side effect only in 2.4%,37 but in that study, only 17.3% of the cohort presented an advanced fibrosis or liver cirrhosis (determined by AST to platelet ratio index). In our cohort, 77.3% of patients had a METAVIR score F4 defined by fibroscan or liver biopsy.37 Therefore, the more advanced the liver fibrosis, the higher the risk of anemia, as already shown by other studies.38 However, given the current trend in reducing the use of RBV,39 the impact of this element will be further reduced.
When the evaluation of costs was correlated with SVR rate, the two GTs with the lowest SVR rates, namely, GT 3 and GT 4, were those associated with the highest costs. On the other hand, GT 1b was the most frequently reported in our cohort – and all over Italy – and therefore, a treatment associated with a low total cost, such as PTVr/OBV+DSV±RBV (€24,978/25,448), could represent an interesting option for the reduction of total expense. Moreover, the notable difference in average cost reported between GT 1a and GT 1b could be attributed to the different pattern of treatment. In fact, the most frequently prescribed drug for GT 1b was PTVr/OBV/DSV±RBV, that is, the less-expensive option. Of note, in GT 1, the regimen based on PTVr+OBV+DSV±RBV demonstrated the lowest cost per SVR, granting a considerable saving of about 37%–38% with the major comparator LDP+SOF±RBV. In a scenario of limited health care resources, this option would allow treatment for more patients while maintaining the same SVR rate.
The average cost per SVR is distinctly less than those observed in previous studies on first-generation DAAs, which ranged from €70,163 to €110,156 in the PAN study.39 Noteworthy, first-generation DAAs were associated with a higher incidence of costs due to the management of adverse events (up to 8%),40 compared with the costs reported in our analysis (0.6%). These findings may lend support to the more favorable safety profile of second-generation DAAs, compared with previous regimens.41
All treatment regimens with RBV are far more expensive than the same regimen without this drug. In addition, multivariate analysis showed a significant difference in costs of care according to the HCV treatment, even after adjusting for the clinical characteristics at treatment initiation.
Our analysis confirms indirectly that the direct cost of the drug and – above all – RBV-associated toxicities with necessary laboratory analysis increase the total expenditure. The only exception was GT 1b; however, three patients infected by this GT started treatment and stopped it after 2 weeks (two for toxicity) and 8 weeks (personal decision in one patient), therefore reducing the total cost.
Given the observed differences in SVR rates among different GTs, the correct determination of GT is of paramount importance in order to avoid errors in treatment selection42 and failure derived from inadequate therapy (about 15%–40% of the estimated reduction in SVR attainment). In agreement with this, a recent Italian study has shown the cost-effectiveness of retesting HCV GT.43
The sensitivity analysis conducted on the cost per SVR clearly shows that the more pronounced the reduction in the cost of HCV treatment, the higher the savings for the NHS. On the other hand, the same decrease in costs for laboratory and visits would only have a minimal influence. The foreseeable reduction in RBV use will likely minimize the impact of laboratory-driven and erythropoietin-associated costs.36 Furthermore, the potential increase in SVR rate (2%–5%) with next-generation drugs might further decrease the cost for treatment by diminishing the failures, provided that the cost per SVR remains stable. On the other hand, sensitivity analysis suggests that, should the drug cost be cut by 50%, the cost per SVR would be €24,353. This cost corresponds to 5 years of management for one HCV-infected patient according to a recent study by Perrone et al.44
Moreover, in the context of price reduction, the drug-related cost (SOF+LDP €6,715±RBV; PTVr/OBV+DSV±RBV €12,833 PTVr/OBV+RBV €11,807±RBV) observed at the time of the drafting of this article (November 2016)45 was aligned with the sensitivity analysis of 50% of reduction. Therefore, considering these drug-related costs, the total cost is very similar to that of interferon-based therapy, but the latter is associated with a much lower SVR rate (ranging from 46% to 80%).46–51
By taking into consideration all the above, this dramatic cost reduction and its impact on the NHS budget could probably allow to expand the treatment criteria.
Our study is not without its limitations. For instance, treatment costs for other antiviral agents in coinfected patients were not considered. Similarly, costs for radiology/ultrasound examination or fibroscan examinations before treatment were not taken into account, as well as those costs necessary during follow-up after SVR achievement. Even more importantly, our estimations were based according to the only available data, that is, those published by the Emilia-Romagna on its institutional website. However, Liguria benefits from a higher discount from total costs than Emilia-Romagna (more HCV patients have been treated in Liguria than in Emilia-Romagna), and therefore, the costs might result in an overestimation of those actually sustained in Liguria.
Despite these limitations, our analysis shows that the leading contributor to costs in the treatment of HCV infection is the antiviral regimen; an appropriate regimen selection could result in a great cost saving which can be reinvested to allow more patients to be treated.
Acknowledgments
The authors are deeply grateful to Claudio Viscoli, Giovanni Cassola, Giuseppe Ferrea, Marco Anselmo and Antonio Picciotto for the assistance in developing the study and in the useful discussion.
Disclosure
The authors report no conflicts of interest in this work.
References
Stanaway JD, Flaxman AD, Naghavi M, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016;388(10049):1081–1088. | ||
Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61(Suppl 1):S45–S57. | ||
Sacchini D, Craxì L, Refolo P, et al; WEF Study Group. Ethical assessment of hepatitis C virus treatment: the lesson from first generation protease inhibitors. Dig Liver Dis. 2015;47(5):351–355. | ||
Davis GL, Rodrigue JR. Treatment of chronic hepatitis C in active drug users. N Engl J Med. 2001;345(3):215–217. | ||
Petruzziello A, Coppola N, Diodato AM, et al. Age and gender distribution of hepatitis C virus genotypes in the metropolitan area of Naples. Intervirology. 2013;56(3):206–212. | ||
Hill AM, Saleem J, Heath KA, Simmons B. Effects of Sustained Virological Response (SVR) on the risk of liver transplant, hepatocellular carcinoma, death and re-infection: meta-analysis of 129 studies in 23,309 patients with Hepatitis C infection. Hepatology 2014;60:55A. | ||
van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308(24):2584–2593. | ||
Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889–1898. | ||
Afdhal N, Reddy R, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370(16):1483–1493. | ||
Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al; AI444040 Study Group. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370(15):211–221. | ||
Wyles DL, Ruane P, Sulkowski M, et al. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med. 2015;373(8):714–772. | ||
Nelson, Cooper JN, Lalezari JP, et al. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase 3 study. Hepatology. 2015;61(4):1127–1135. | ||
Hézode C, Asselah T, Reddy KR, et al. Ombitasvir plus paritaprevir plus ritonavir with or without ribavirin in treatment-naive and treatment-experienced patients with genotype 4 chronic hepatitis C virus infection (PEARL-I): a randomised, open-label trial. Lancet. 2015;385(9986):2502–2509. | ||
Ferenci P, Bernstein D, Lalezari J, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370(21):1983–1992. | ||
Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/rombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370(17):1594–1603. | ||
Zeuzem S, Jacobson IM, Baykal T, et al. Retreatment of HCV with ABT-450/rombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370(17):1604–1614. | ||
Wyles D, Sulkowski MS, Eron JJ, et al. TURQUOISE-I: 94% SVR12 in HCV/HIV-1. Coinfected patients treated with ABT- 450/r/ombitasvir dasabuvir and ribavirin. 65th Annual meeting of the American Association for the Study of Liver Diseases. Nov 7–11; 2014; Boston, MA, US. | ||
Lawitz E, Sulkowski MS, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naïve patients: the COSMOS randomised study. Lancet. 2014;384(9956):1756–1765. | ||
Cenderello G, Artioli S, Viscoli C, et al. Budget impact analysis of sofosbuvir-based regimens for the treatment of HIV/HCV-coinfected patients in northern Italy: a multicenter regional simulation. Clinicoecon Outcomes Res. 2015;8:15–21. | ||
Garagiola E, Ferrario L, Croce D, et al. HCV novel therapeutic regimens in Wonderland: a budget impact analysis in the Lombardy Region. Dig Liver Dis. 2016;48(10):1200–1207. | ||
Fraccaro P, Dentone C, Fenoglio D, Giacomini M. Multicentre clinical trials’ data management: a hybrid solution to exploit the strengths of electronic data capture and electronic health records systems. Inform Health Soc Care. 2013;38(4):313–329. | ||
Fraccaro P, Pupella V, Gazzarata R, et al. The Ligurian Human Immunodeficiency Virus Clinical Network: a web tool to manage patients with human immunodeficiency virus in primary care and multicenter clinical trials. Med 2 0. 2013;2(2):e5. | ||
Minerva D, Epatite C. Il farmaco c’è ma costa troppo. Available from: http://espresso.repubblica.it/visioni/scienze/2015/02/27/news/quel- farmaco-costa-troppo-1.201536. Accessed June 27, 2015. | ||
salute.regione.emilia-romagna.it [homepage on the internet]. Documenti. Trattamento epatite c (Documents. HCV treatment). Available from: http://www.salute.regione.emilia-romagna.it/documenti. Accessed November 29, 2016 | ||
Regione Emilia Romagna Direzione Generale Sanità e Politiche Sociali. Documento di indirizzo per la definizione delle strategie terapeutiche da applicare sul breve termine per “Nuovi antivirali diretti nella terapia dell’epatite C cronica” aggiornamento 14 Maggio 2015. Available from: http://salute.regione.emilia-romagna.it/documentazione/ptr/ptr/archivio/PTR_229_maggio_2015.pdf. Accessed October 26, 2016. | ||
Regione Emilia Romagna Direzione Generale Sanità e Politiche Sociali. Documento di indirizzo per la definizione delle strategie terapeutiche da applicare sul breve termine per “Nuovi antivirali diretti nella terapia dell’epatite C cronica” aggiornamento 16 Luglio 2015. Available from: http://salute.regione.emilia-romagna.it/documentazione/ptr/ptr/archivio/229_nuovi-antivirali-epatiteC-luglio-2015. Accessed October 26, 2016. | ||
Regione Emilia Romagna Direzione Generale Sanità e Politiche Sociali. Documento di indirizzo per la definizione delle strategie terapeutiche da applicare sul breve termine per “Nuovi antivirali diretti nella terapia dell’epatite C cronica” aggiornamento Dicembre 2015 Available from: http://salute.regione.emilia-romagna.it/documentazione/ptr/ptr/archivio/229_nuovi-antivirali-epatiteC-dicembre-2015. Accessed October 26, 2016. | ||
Official Italian Gazette. General Series n 283–05/12/2014. DETERMINA 12 novembre 2014. Regime di rimborsabilità e prezzo del medicinale per uso umano “Sovaldi (sofosbuvir)” [Price and reimbursement process of drug for human use named “Sovaldi” (sofosbuvir)]. (Determina 1353/2014). | ||
Data on file. Regione Liguria. Deliberazione della Giunta Regionale n. 957 del 30/07/2013 con oggetto “Remunerazione delle prestazioni di assistenza specialistica ambulatoriale. Adeguamento delle tariffe al D.M. 18 ottobre 2012.” | ||
Data on file. Regione Liguria. Gara farmaci 2014 Lotto Gara 2014 1095 SUB A Ribavirina TEVA 200 mg. | ||
Data on file. Regione Liguria. Gara Farmaci 2014 Lotto Gara 2A NeoRecormon. | ||
Ministero del Lavoro, della Salute e delle Politiche Sociali. Classificazione Diagnosis Related Groups versione 14. Available from: http://www.salute.gov.it/imgs/C_17_pubblicazioni_1094_allegato.pdf. Accessed October 26, 2016. | ||
Official Italian Gazette. General Series n 109–13/05/2015 DETERMINA 8 maggio 2015. Regime di rimborsabilità e prezzo del medicinale per uso umano “Harvoni (ledipasvir/sofosbuvir)” [Price and reimbursement process of drug for human use named “Harvoni”(ledipasvir/sofosbuvir)]. (Determina 544/2015). | ||
Ioannou GN, Beste LA, Chang MF, et al. Effectiveness of Sofosbuvir, Ledipasvir/Sofosbuvir, or Paritaprevir/Ritonavir/Ombitasvir and Dasabuvir Regimens for Treatment of Patients With Hepatitis C in the Veterans Affairs National Health Care System. Gastroenterology. 2016;151(3):457–471. | ||
Ingiliz P, Christensen S, Kimhofer T, et al. Sofosbuvir and ledipasvir for 8 weeks for the treatment of chronic hepatitis C virus (HCV) Infection in HCV-monoinfected and HIV-HCV-coinfected individuals: results from the German hepatitis C cohort (GECCO-01). Clin Infect Dis. 2016;63(10):1320–1324. | ||
Hézode C, Fontaine H, Dorival C, et al; CUPIC Study Group. Triple therapy intreatment experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC) – NCT01514890. J Hepatol. 2013;59(3):434–441. | ||
Stahmeyer JT, Rossol S, Bert F, et al. Outcomes and costs of treating hepatitis C patients in the era of first generation protease inhibitors – Results from the PAN Study. PLoS One. 2016;11(7):e0159976. | ||
Lashin AH, Shaheen YA, Metwally MA, El-Feky HM, Hegab MF, Abbas SM. Incidence and predictors of hematological side effects in chronic HCV Egyptian patientstreated with PEGylated interferon and ribavirin. Indian J Gastroenterol. 2013;32(5):316–323. | ||
Feld JJ, Moreno C, Trinh R, et al. Sustained virologic response of 100% in HCVgenotype 1b patients with cirrhosis receiving ombitasvir/paritaprevir/r and dasabuvir for 12 weeks. J Hepatol. 2016;64(2):301–307. | ||
Bichoupan K, Martel-Laferriere V, Sachs D, et al. Costs of telaprevir-based triple therapy for hepatitis C: $189,000 per sustained virological response. Hepatology. 2014;60(4):1187–1195. | ||
Maan R, van Tilborg M, Deterding K, et al. Safety and effectiveness of direct-acting antiviral agents for treatment of patients with chronic hepatitis C virus infection and cirrhosis. Clin Gastroenterol Hepatol. 2016;14(12):1821–1830. | ||
Polilli E, Cento V, Restelli U, et al. Consequences of inaccurate hepatitis C virus genotyping on the costs of prescription of direct antiviral agents in an Italian district. Clinicoecon Outcomes Res. 2016;8:467–473. | ||
Gane E, Kowdley KV, Pound D, et al. Efficacy of sofosbuvir, velpatasvir, and GS-9857 in patients with hepatitis C virus genotype 2, 3, 4, or 6 infections in an open-label, phase 2 trial. Gastroenterology. 2016;151(5):902–909. | ||
Perrone V, Sangiorgi D, Buda S, Degli Esposti L. Diseases progression and healthcare resource consumption in patients affected by hepatitis C virus in real practice setting. Clinicoecon Outcomes Res. 2016:8:591–597. | ||
Regione Emilia Romagna Direzione Generale Sanità e Politiche Sociali. Documento di indirizzo per la definizione delle strategie terapeutiche da applicare sul breve termine per “Nuovi antivirali diretti nella terapia dell’epatite C cronica” aggiornamento Settembre 2016. Available from: http://salute.regione.emilia-romagna.it/documentazione/ptr/elaborati/229-epatite-c-cronica-settembre-2016. Accessed October 26, 2016. | ||
De Compardi P, Koleva D, Mangia A, Motterlini N, Garattini L. Analisi costo minimizzazione della terapia di durata 12 o 24 settimane di peg-inteferferone alfa 2b e di ribavirina nell’infezione da da virus dell’epatite C. Quaderni di Farmacoeconomia. Numero 10 Ottobre 2009. Available from: http://www.quadernidifarmacoeconomia.com/component/content/article/118-archivio-qf-preview/611-qf10.html. Accessed October 26, 2016. | ||
Ravasio R, Sacchi P, Maiocchi L, et al. Costo efficacia di peginteferone α2a+ribavirina verso peginterferone α2b+ribavirina nel trattamento dell’epatite cronica di tipo C in pazienti non pretrattati. [Cost-effectiveness of peginterferon α-2a plus ribavirin versus peginterferon α-2b plus ribavirin as initial therapy for patients with Chronic Hepatitis C]. Pharmacoeconomics Ital-Res-Articles. 2005;7(3):207. | ||
Shepherd J, Brodin HF, Cave CB, Waugh NR, Price A, Gabbay J. Clinical- and cost-effectiveness of pegylated interferon alfa in the treatment of chronic hepatitis C: a systematic review and economic evaluation. Int J Technol Assess Health Care. 2005;21(1):47–54. | ||
Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975–982. | ||
Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958–965. | ||
Hadziyannis SJ, Sette H Jr, Morgan TR, et al; PEGASYS International Study Group. Peginterferon-alpha2a andribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140(5):346–355. |
Supplementary materials
  | Table S1 Italian guidelines for the treatment of HCV at the time of study (Documento Società Italiana Studio Fegato-AISF December 17, 2014)1 Abbreviations: DCV, daclatasvir; DSV, dasabuvir; HCV, hepatitis C virus; LDP, ledipasvir; OBV, ombitasvir; PTVr, ritonavir boosted paritaprevir; RBV, ribavirin; SIM, simeprevir; SOF, sofosbuvir. |
  | Table S4 Other costs (indicated in Euros) Abbreviation: DRG, diagnosis related group. |
  | Table S5 Cost of drugs |
References
AISF. Documento di indirizzo dell’Associazione Italiana per lo Studio del Fegato per l’uso razionale di antivirali diretti di seconda generazione nelle categorie di pazienti affetti da epatite C cronica ammesse alla rimborsabilità in Italia AISF [released 17 December 2014, updated December 2016]. Available from: http://www.webaisf.org/pubblicazioni/documento-aisf-hcv-2016.aspx. Accessed November 29, 2016. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.