Back to Journals » ClinicoEconomics and Outcomes Research » Volume 12
Cost of Hemodialysis Treatment and Associated Factors Among End-Stage Renal Disease Patients at the Tertiary Hospitals of Addis Ababa City and Amhara Region, Ethiopia
Authors Kassa DA, Mekonnen S , Kebede A, Haile TG
Received 5 April 2020
Accepted for publication 30 June 2020
Published 27 July 2020 Volume 2020:12 Pages 399—409
DOI https://doi.org/10.2147/CEOR.S256947
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Xing Lin Feng
Daniel Asrat Kassa,1 Solomon Mekonnen,2 Adane Kebede,1 Tsegaye Gebremedhin Haile1
1Department of Health Systems and Policy, Institute of Public Health, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 2Department of Human Nutrition, Institute of Public Health, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Tsegaye Gebremedhin Haile
Department of Health Systems and Policy, Institute of Public Health, College of Medicine and Health Sciences, University of Gondar, PO Box 196, Gondar, Ethiopia
Tel +251-921279362
Email [email protected]
Purpose: Hemodialysis is a renal replacement therapy for end-stage renal disease (ESRD) patients who consume substantial healthcare resources, which increases the economic burden. Plenty of factors affects the cost of hemodialysis treatment, particularly in resource-limited settings. Moreover, the demand for hemodialysis may decrease as the cost increases, but there is limited evidence in Ethiopia. Thus, this study aimed to estimate the cost of hemodialysis treatment among ESRD patients in the tertiary hospitals of Addis Ababa City and Amhara region, Ethiopia.
Patients and Methods: An institutional-based cross-sectional study was conducted among 172 ESRD patients undergoing hemodialysis treatment. A structured questionnaire and patients’ medical chart were used to estimate the costs, and the human capital approach was applied to calculate the indirect costs. A generalized linear model (GLM) was fitted after the modified park test to identify the associated factors. In the final GLM, a p-value of < 0.05 and a 95% CI were used to declare the significant variables.
Results: The mean annual cost of hemodialysis treatment was 121,089.27ETB ($4466.59) ± 33,244.99 ($1226.29). The direct and indirect costs covered 77.0% and 23.0% of the total costs, respectively. Age (ex(b): 1.01, p-value < 0.001), highest wealth status (ex(b): 1.09, p-value: 0.008), eight (ex(b): 1.27, p-value < 0.001) and 12 visits/month (ex(b): 1.34, p-value < 0.001), anemia (ex(b): 1.13, p-value < 0.001), and comorbidity (ex(b): 1.09, p-value: 0.039) were the factors associated with the costs of hemodialysis treatment.
Conclusion: The annual cost of hemodialysis treatment among ESRD patients was high compared to the national per capita health expenditure, and two-thirds covered by the direct medical costs. Old age, high wealth status, more visits, anemia, and comorbidity were factors associated with the costs of hemodialysis. Therefore, the healthcare system must make a great effort for cost reduction and reduce the patients with kidney disease before they reach end-stages.
Keywords: burden of ESRD, direct costs, indirect costs, GLM, hemodialysis, Ethiopia
Introduction
Chronic kidney disease (CKD) is a worldwide public health threat. It is still unrecognized by many low-resource countries as a potentially devastating cause of morbidity and mortality in their population.1 Globally, it has been estimated that more than 500 million individuals have CKD, defined by either kidney damage or glomerular filtration rate (GFR) < 60 mL/min/1.73 m2 for greater or equal to 3 months, regardless of the cause.2 End-stage renal disease (ESRD) is the last stage of CKD, which needs dialysis to maintain patient’s lives. This is when kidneys become no longer support the needs of the body.3 Kidney disease imposes disproportionate, incalculable human suffering and a catastrophic economic burden in African; less than 2% of the patients with ESRD have access to renal replacement therapy (RRT), making ESRD a death sentence for most patients.4 According to the WHO data published in April 2011, kidney disease deaths in Ethiopia reached 12,038 or 1.47% of total deaths.5
Hemodialysis is the most common one in low-resource country with its provision being very challenging.6,7 Hemodialysis is a treatment modality that filter out the waste products from the blood circulates outside the body of the patient through a machine that has special filters.8 It is a renal replacement therapy for ESRD patients who consume substantial healthcare resources for management.9 Consequently, it is an economic problem that affects the ability of many consumers to access and use goods and services. So, the demand for hemodialysis may decrease when its costs increase and the nonadherence of patients remarkedly decreases.10,11 As a result, cost analysis is necessary for patients who are being chosen hemodialysis modality.
Patients with the end-stage renal disease usually have various comorbidities. The result is a continuous increase in the cost value of the resources consumption for producing hemodialysis services and a high burden of costs on patients.12,13 Thus, it means a high resource demand to every health-care system in the world. In low-income countries, hemodialysis in hospitals is the most common modality of RRT, and its provision being very challenging. Treatment for all is beyond the reach of most countries due to the lack of funds or health insurance to cover the high costs for the ever-increasing number affected.6,14
Globally, the number of patients receiving RRT in 2010 was estimated at 2.618 million, with only 7.2% living in low-and-middle-income countries. African patients with ESRD have the lowest access to RRT, with only 9–16% being treated; in central and eastern Africa, the treatment rate is estimated to be as low as 1–3%.6 A study conducted in the United States, 2011, the incidence and prevalence rate of ESRD was 357 and 1901 per million populations, respectively, with Medicare spending for ESRD at US$ 34.4 billion, and non-Medicare spending at US$14.9 billion, total ESRD costs reached US$ 49.3 billion.15 In another study, in high-income countries like that of the USA, the annual direct cost for peritoneal dialysis (PD) and hemodialysis were US$103,801.93 and US$ 95,437.93, respectively.16
In middle-income countries like that of Chile, 2007, the study has been performed a cost evaluation of PD and hemodialysis, and the annual hemodialysis costs were US$ 24,461.13.17 Brazil, 2013 evaluated the cost of hemodialysis in the treatment of ESRD, and the average annual cost per patient was US$ 30,079.00.18 Moreover, in Spain, 2012 analyzed the cost of Spanish RRT for ESRD in the year 2010. The results reveal that the average cost for hemodialysis was US$ 54,527.29 and US$37,089.70 PD.19 In the case of Greece, 2008, the study took the perspective of the health system and focused on direct costs, and the annual cost per HD patient ranged between US$ 46,649.21 for PD and US$ 57,456.90 for HD.20 Similarly, in Spain, 2010 evaluated the economics of hemodialysis, and the result showed that the cost of hemodialysis was US$ 67,432.21 In Nigeria, 2012, the cost of RRT was reported to be US$ 42,784.91 for hemodialysis and US$ 47,970.96 for PD.22 Similarly, according to the Egyptian renal registry in 2008, the prevalence of ESRD is 483 per million populations, and the cost of PD was US$ 7,974.02.23 In Sudan, 2010, conducted a cross-sectional study to estimate the costs of kidney transplantation and compared those with the costs of hemodialysis per year. The annual cost of hemodialysis two sessions per week was US$24,732.24
Assessing patients in terms of sociodemographic and economic characteristics is helped to understand which is mostly affected by CKD and progressively turn into ESRD. That will eventually necessitate RRT and the cost of dialysis to be influenced by some of these patients’ characteristics.25 Existing empirical evidence in higher-income countries showed that old age, marital status, and educational status were some of the factors associated with the cost of hemodialysis. Accordingly, a study conducted in the USA, 2011, the mean age of dialysis patients was reported 65 years. In the case of Germany, the mean age of dialysis patients was 63.9 years; the total cost reported to increase significantly with increasing age. While that of the Netherland and Germany being married and living with a partner increase the cost of hemodialysis. Besides, intermediate level education was the other factor that increases the cost of hemodialysis.2,26,27 Similarly, in middle-and-low income countries, old age and sex were significant factors for the higher cost of hemodialysis.28–30
From an economic point of view, allocate the health-care budget towards curable diseases seems rational and will be used as input for governmental agencies, non-governmental organizations, pharmaceutical industry, and continual supply of donors to improve healthcare service. However, several diseases that affect people all over the world can be related to lifelong disabilities, which can cause mental and physical impairments that bring limitations to patients’ lives. So, patients have to learn how to live with these disorders by making the best of life, even though there might be some daily activities restrictions.
Ethiopian health systems rely on disease prevention and health promotion strategies that focus on infectious diseases. This principle has been affected by the utilization of the service among those patients with chronic conditions. Besides, health insurance also did not include the treatment and care services for patients with a chronic disease like dialysis, transplantation, and other services.31 The non-coverage of dialysis by the health scheme in our setup may significantly increase the costs, which can have an impact on the individual, family, and also the health system by itself. To date, in Ethiopia, there is no evidence about the cost of hemodialysis among end-stage renal disease patients. The study analyzed the cost from the patients’ perspective, including direct medical, direct non-medical, and indirect costs, which shows the impact of hemodialysis on patients and the importance of its early treatment for policymakers. Therefore, the study aimed to measure the cost of hemodialysis treatment and associated factors among end-stage renal disease patients at the tertiary hospitals of Addis Ababa city and the Amhara region.
Materials and Methods
Study Design and Settings
A year (January 1 to end of December 2018) retrospective institutional-based cross-sectional study was conducted at the tertiary level governmental hospitals of Addis Ababa (AA) city and Amhara region, Ethiopia. In Addis Ababa city and Amhara region, five tertiary level hospitals provide hemodialysis services for end-stage renal disease patients. Those are Zewditu Memorial, St. Paulo’s Millennium Medical College, Tikur Anbessa, Bahirdar Felegehiwot, and University of Gondar comprehensive specialized hospitals, which provides preventive, curative, and other palliative services for their catchment population in the outpatients, inpatients, and emergency departments.
Study Participants
A total of 172 adult end-stage renal disease patients who started hemodialysis treatment before December 30, 2017, in the tertiary governmental hospitals of AA city and Amhara region were included in the study. All eligible patients’ medical record cards were assessed and labeled using their Medical Record Number (MRN) when they arrived at the clinics. All patients who were diagnosed with end-stage renal disease, older than 18 years, and had ambulatory care services were included in the study consecutively. Those who were seriously ill or unable to responded to the interview and came the second time during the data collection period were excluded.
Data Collection Instruments
An interviewer-administered semi-structured questionnaire that contained information on socio-demographics, economic and clinical factors, as well as direct medical, direct non-medical, and indirect costs, were used to collect retrospectively data on patients who were on hemodialysis treatment. The questionnaire was prepared from similar studies.4,6,32–42 The data were collected through face-to-face interviews, and clinical data (number of drugs prescribed, comorbidities, and complications) were collected by reviewing patients’ medical record cards. Then, information on costs was collected through chart review and patient interview.
For assuring the data quality, the questionnaire was first prepared in English and translated to the local language (Amharic) and back to English to ensure its consistency, and a pretest was done on 5% of the sample size at Dessie referral hospital before the actual data collection. Three bachelors in nursing and two health officers were recruited for data collectors and supervisors, respectively. Moreover, a 3 days training was given to data collectors and supervisors on the purpose of the study, data collection instruments, techniques and procedures.
Variables and Measurements
The cost of hemodialysis was the dependent variable measured by the direct and non-direct medical and non-medical costs. Whereas sociodemographic factors (age, sex, level of education, family size, and marital status), socioeconomic factors (wealth status, occupation), health services related factors (distance to the nearest health facility, availability of drugs, medical supplies, and laboratory services), types of vascular access for dialysis and related complication (arteriovenous fistula, Arteriovenous graft, venous catheter and frequently reported complication infections and thrombosis), comorbidity disease (hypertension, anemia, nutrition and vitamin deficiency, and others) were the independent variables.
In economic evaluation, it is crucial to describe the perspectives of a costing study because it mirrors the purpose of the study. One item may be considered a cost from one point of view, but not be considered a cost from another. Without clearly stating the perspectives of the study, it would not be possible to know the objectives of the study, and what costs should be included or excluded.43 Costs are defined as a composite sum of quantities of some activity multiplied by their respective prices,44 which described in four categories: direct medical costs, direct non-medical costs, indirect costs, and intangible costs.45 Our study was intended to measure direct medical, direct non-medical, and indirect costs.
Direct medical cost is the cost of the dialysis session, cost of drugs, cost of laboratory investigation and radiology, cost of arteriovenous fistula, cost of the central venous line (price and insertion), and others directly related to medical costs which incurred by patients.
Direct non-medical cost is the cost of transportation, cost of food, and others which are directly related to non-medical costs incurred by patients and their relatives.
Indirect cost is the cost of the absence of work (cost of lost wage) or other loss of production, which is indirectly related to being taken treatment incurred by patients.
The human capital approach is designed to estimate the value of human capital as the present value of his/her future earning under the assumption that we use future earning as a proxy for future production. It measures the indirect cost of hemodialysis by multiplying the mean salary by the number of days of work absence or by taking the value per index for informal.
All cost data were calculated as mean values per patient per year. The currency was adjusted with the United States dollar (USD), an international currency. The exchange rate between the USD and the Ethiopian Birr (ETB) at the time of data collection in 2018 was USD 1.0 = ETB 27.11.
The wealth index is based on the assumption that wealth or economic status is a latent variable. We assume that economic status is the common factor behind the ownership of the assets, such that household economic status explains the maximum variance and covariance in the asset variables. Such factors can be extracted from a set of variables by creating a set of mutually uncorrelated components or factors of the data using principal component or factor analysis.46
Data Processing and Analysis
The data were entered into Epi-Data version 3.1 and exported to STATA version 14 software for analysis. Descriptive statistics were used and presented in narrations and tabular forms to show the patients’ characteristics and costs of hemodialysis. The generalized linear model (GLM) was computed to assess the association of the independent factors with the total cost because of the non-normal distribution of the total cost.47–52 Moreover, the modified Park test was applied to check the distribution used in the GLM, and the gamma family and log link function were used. Those variables having a p-value of <0.2 in the bivariable analysis were included in the final GLM analysis. A p-value of < 0.05 and a 95% confidence level were used to declare statistically significant variables with the cost of hemodialysis. The wealth status of the participants was assessed through the principal component analysis and ranked into three (low, medium, and high) levels.
Ethical Considerations
Ethical clearance was obtained from the Institute of public health ethical review committee, University of Gondar (Ref. No. IPH/295/2018). Then, a letter of permission was secured from Zewditu Memorial, St. Paulo’s Millennium Medical College, Tikur Anbessa, Bahirdar Felgehiwot, and the University of Gondar comprehensive specialized hospitals. Patients were informed about the purpose of the study, the right to refuse and withdraw at any time. Then, written consent was obtained from each participant before the actual data collection. Confidentiality and anonymity of study participants were safeguarded throughout the entire study by using a non-personal identifier. Finally, the study was conducted in accordance with the declaration of Helsinki.
Results
Sociodemographic and Economic Characteristics
A total of 172 end-stage renal disease patients undergoing hemodialysis treatment modality participated with a response rate of 97.7%. Nearly 67% of the participants were male, and the mean age of the study participants was 46.2 ± 11.6 years, ranges from 20 to 73 years. The majority (54.1%) of the participants were married, and 46.0% attended a secondary school level. Regarding their occupation, 36.6% and 13.4% of the study participants were unemployed, and government employees, respectively. Most (93.6%) of the study participants were residing in urban. Sixty-four percent of the study participants were orthodox. Moreover, 45.3% and 26.2% were from the Amhara and Oromo ethnic groups, respectively. The mean family size among the study participants was 4.29 (±1.68), and 33.8% of the patients were in the medium wealth status (Table 1).
 |
Table 1 Sociodemographic and Economic Characteristics of Participants at the Tertiary Hospitals of AA City and Amhara Region, Ethiopia 2019 (n=172) |
Health Service-Related Characteristics
Participants averagely travel for 51.6 KM (ranges 1–280 km) to reach the hemodialysis center. Nearly 65% of the patients were used Taxi for their transportation. Only 11% of the patients’ income was enough to cover the cost of hemodialysis. The majority of the patients have support from their relatives, friends, and neighbors for cop the cost of the Hemodialysis (Table 2).
 |
Table 2 Health Services Related Characteristics and Coping Strategies for the Cost of Hemodialysis Among ESRD Patients at Tertiary Hospitals of AA City and Amhara Region, Ethiopia, 2019 (n=172) |
Clinical Characteristics and Treatment Modalities
Nearly 59% of the patients stay on hemodialysis for one to 3 years. The majority (70.3%) of the patients had three sessions of dialysis in a week, and the duration of each session ranges from three to 4 hrs. About 71.0% of the patients were on multiple treatments for the complication of chronic kidney disease. Of these, 63.4% were taken Erythropoietin. Of the total ESRD patients, 124 (72.1%) of the had treatment for a complication related to vascular access they used. Arteriovenous fistula and catheter were the predominant vascular access used for hemodialysis (Table 3).
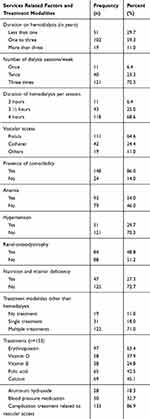 |
Table 3 Service-Related Characteristics and Treatment Modalities for ESRD Participants at the Tertiary Hospitals of AA City and Amhara Region, Ethiopia 2019 (n=172) |
Cost of Hemodialysis
The overall mean annual cost of hemodialysis treatment for end-stage renal disease patients was 121,089.27 ($4466.59) ± 33,244.99 ($1226.29) Ethiopian birr ($1: 27.11 Ethiopian birr). The direct medical costs (hemodialysis, drugs, and laboratory investigation costs) have the largest percentage to the total hemodialysis treatment costs on patients (65.2%), 11.8% of hemodialysis costs on patients are direct non-medical costs, while 23.0% of hemodialysis costs are indirect costs (Table 4).
 |
Table 4 Annual Mean Cost of Hemodialysis Treatment Among ESRD Patients at the Tertiary Hospitals of AA City and Amhara Region, Ethiopia 2019 (n=172) |
Factors Associated with the Cost of Hemodialysis Treatment
Generalized Linear Model (GLM) was fitted to identify the factors associated with the overall annual mean cost of hemodialysis among end-stage renal disease patients at the tertiary hospitals of AA city and Amhara region. The modified Park test result showed that the overall annual mean cost of hemodialysis was within the gamma distribution (coefficient 2.0002, 95% CI: 2.00018–2.00024, SE: 0.0000129, p-value < 0.001). In the final multivariable analysis, age, wealth status, visits per month, anemia, and comorbidity were significantly associated with the cost of hemodialysis treatment among ESRD patients.
Accordingly, as age increase by a year, the cost of hemodialysis increased by 1.011 times the mean cost (ex(b): 1.01, 95% CI: 1.00–1.01, p-value <0.001). Being in the highest wealth status has incur the cost of hemodialysis by 1.09 times more compared to the lowest wealth status (ex(b): 1.09, 95% CI: 1.02–1.16, p-value: 0.008). Having eight and 12 visits per month incur the cost of hemodialysis by 1.27 (ex(b): 1.27, 95% CI: 1.13–1.42, p-value < 0.001) and 1.34 times (ex(b): 1.34, 95% CI: 1.19–1.50, p-value < 0.001) compared to those who have a visit per month, respectively. The presence of anemia was incurring the cost of hemodialysis by 1.13 times the mean cost (ex(b): 1.13, 95% CI: 1.06–1.20, p-value < 0.001) compared to those who do not have anemia. The presence of comorbidity incurs the cost of hemodialysis by 1.09 times the mean costs (ex(b): 1.09, 95% CI: 1.00–1.20, p-value: 0.039) compared to those who do not have comorbidity. Sex, education, presence of hypertension, renal-osteodystrophy, number of drugs, and complications did not have a significant impact on the total cost of hemodialysis for those end-stage renal disease patients in the final GLM analysis (Table 5).
Discussion
A prevalence-based cost of hemodialysis treatment study was carried out retrospectively among end-stage renal disease patients at the tertiary hospitals of Addis Ababa city and Amhara region. The study revealed that the annual cost of the hemodialysis treatment was ETB 121,089.27 ($4466.59) per patient per year, in which 65.2%, 11.8%, and 23.0% of the costs covered by the direct medical, direct non-medical, and indirect costs, respectively.
The finding is slightly higher than that of a study conducted in Indonesia.53 Our higher cost of hemodialysis treatment might be due to the difference in time, the perspectives of cost measurements, the currency exchange rate, and the difference in study participants in which our included only ESRD patients. Our finding is nearly in line with a study conducted in Sudan, 2010, the cost of hemodialysis was US$ 6847.00.24 This comparable finding might be explained by the similar use of perspective to measure the cost of hemodialysis and also similar hospital level. Our finding is lower than a study conducted in Chile in 2007, and showed the cost of hemodialysis treatment was US$ 24,461.13,17 Nigeria in 2012; the cost of hemodialysis was $42,784.91,22 in Brazil the average annual total cost of hemodialysis was $30,079.18 Moreover, it has remained considerably less than that of a study conducted in the USA in 1998 which was US$ 95,437.93,16 Greek in 2008 is US$ 57,456.9,20 and Spain in 2012 is ranged US$ 54,527.29 to $67,432,19 and India showed that the annual health system costs of hemodialysis were $858 873.33 This significant variation of hemodialysis cost might be due to the difference in the socioeconomic status, types of hospitals in which our study included only governmental hospitals but others included the private hospitals. The other possible reason might be the differences in the study perspective (patients’ perspective) and the items of costs involved.
Our study finding showed that 65.2%, 11.8%, and 23.0% of the total annual hemodialysis treatment cost were covered by direct medical, direct non-medical, and indirect costs, respectively. This result is consistent with a study conducted in Sudan, 2010 which showed that 67.6%, 10.8%, and 21.6% of the total cost of the hemodialysis was covered by the direct medical, direct non-medical, and indirect costs, respectively,24 and a study conducted in Germany showed that the largest part of the costs (55%) was caused by the dialysis procedures.26 But the finding is inconsistent with a study conducted in Spain, 2010 (drugs and consumables cost) constituted 51–78% of the overall expenses of dialysis.21 In Brazil, 82.3% of the total cost of hemodialysis is accounted for direct medical cost, 5.3% direct non-medical cost and 12.4% indirect cost of hemodialysis.18 The possible explanation for the discrepancy could be the accessibility of health services in that of Spain and Brazil which might decrease the direct non-medical and indirect costs. Moreover, the variations in the socioeconomic status of the study participants might be the other possible explanation.
The study finding showed that multiple sociodemographic and economic factors, old age, higher wealth status, having more visits per month, presence of anemia, and comorbidity were factors significantly increase the cost of hemodialysis treatment among ESRD patients. As the age increase by a year, the cost of incurring hemodialysis treatment increases by 1.01 times the mean cost. This finding is supported by studies conducted in France,54 UK,25 Brazil,18 and Nigeria,22 which showed the cost varies depending on the age and dominantly higher among the older age group. This might be explained by the age increase the complication of chronic kidney disease will increase, and older ages are prone to comorbidity, which increases the cost of hemodialysis treatment.
Being in the higher wealth status increases the cost of hemodialysis treatment by 1.09 times compared to the lower wealth status. Moreover, having eight and twelve visits per month increases the cost of hemodialysis treatment by 1.27 and 1.34 times more compared to those who have once visit per month. This finding is supported by a study conducted in India showed that patients in the higher income were more likely to incur the cost of hemodialysis treatment.33 This might be explained by those who were wealthier can afford the cost of the hemodialysis treatment; particularly the direct non-medical costs. The other possible justification might be since the services have been provided in the tertiary hospitals level of the country in our study, which has been located in the central, patients might incur more costs as the visits increase. So, the Ethiopian health system should be revised to increase the hemodialysis treatment services by providing at the general and primary hospital levels.
Having anemia and other comorbidity increases the cost of incurring for hemodialysis treatment by 1.13 and 1.09 times the mean annual costs, respectively, which is supported by studies conducted in France54 and Calgary,55 in which comorbidity significantly increases the cost of hemodialysis treatment. For those end-stage renal disease patients with comorbidity, the probability of ordering multiple drugs and treatment is higher, which increases the costs simultaneously. But, the level of cost incurring differs between ours and other studies. This difference may be heterogeneity in underlying comorbidities among patients in the study participated and their study perspective and methodology they used. Other studies included the cost of hemodialysis with an aviary of economic perspectives.
Finally, this study gives an insight into the establishment of a national end-stage renal disease registry, universal access to ESRD treatment, and the implementation of targeted-screening programs of chronic kidney disease that offer an opportunity for enhancing the prevention and control of ESRD in Ethiopia.
Strengths and Limitations of the Study
Our study included all the tertiary level hospitals of the Addis Ababa city and Amhara region that might give a representative sample for end-stage renal disease patients. Moreover, the data collection techniques used multiple data sources, both face-to-face interviews and patient medical records reviews. But our work might not be free from recall bias since it uses a retrospective of cost estimation. Moreover, the cost of hemodialysis was limited to the patients’ perspective and does not incorporate other costs of the health system. Besides, the intangible costs were not estimated in this study, which might affect the total cost of hemodialysis treatments.
Conclusion
The annual cost of hemodialysis treatment among end-stage renal disease patients was significantly high compared to the national per capita health expenditure, and two-thirds of the total cost was covered by the direct medical cost. The study showed that the economic burden of hemodialysis treatment on end-stage renal disease patients and their family, like many families’ monthly income is not enough to cover the hemodialysis cost. Old age, high wealth status, having more visits per month, presence of anemia, and comorbidity were the factors that significantly increased the cost of hemodialysis treatment among end-stage renal disease patients. Therefore, to reduce the significant burden of hemodialysis treatment costs on patients, the health-care system must make a great effort for cost reduction and reduce the number of patients with kidney disease before they reach end-stage through early detection. Besides, it should increase the treatment availability by opening a hemodialysis unit in the district hospitals to decrease the direct non-medical costs. The Ethiopian health insurance system should also revise its services packages to include the dialysis treatments for the reduction of direct medical costs.
Furthermore, future studies should conduct cost estimation including the intangible costs and use of other perspectives to capture a wider range of adverse events of ESRD in the country, thereby providing a broader assessment of costs.
Data Sharing Statements
All the data supporting the findings are within the manuscript. Additional detailed information and raw data are available from the corresponding author on reasonable request.
Acknowledgments
We are very grateful to the Institute of Public Health, University of Gondar, and all the Chief Executive Officers (CEO) of the five hospitals for their cooperation. We would also like to thank all the urology units’ coordinators and all of the study participants who participated in this study. Our appreciation also goes to the data collectors and supervisors for their unreserved contribution.
Author Contributions
All authors substantially contributed to the design, acquisition, analysis and interpretation of data, drafting, revising and final approval. Besides, all authors agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yeates K, Ghosh S, Kilonzo K. Developing nephrology programs in very low-resource settings: challenges in sustainability. Kid Int Suppl. 2013;3(2):202–205. doi:10.1038/kisup.2013.14
2. Ssystem URD. USRDS 2008 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2008.
3. Gerogianni S, Babatsikou F, Gerogianni G, et al. Concerns of patients on dialysis: a research study. Health Sci J. 2014;8(4):423.
4. Naicker S, Ashuntantang G. End stage renal disease in sub-Saharan Africa. In: Chronic Kidney Disease in Disadvantaged Populations. Elsevier; 2017:125–137.
5. Fiseha T, Kassim M, Yemane T. Prevalence of chronic kidney disease and associated risk factors among diabetic patients in southern Ethiopia. Am J Health Res. 2014;2(4):216–221. doi:10.11648/j.ajhr.20140204.28
6. Liyanage T, Ninomiya T, Jha V, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385(9981):1975–1982. doi:10.1016/S0140-6736(14)61601-9
7. McDonald SP, Russ GR. Burden of end-stage renal disease among indigenous peoples in Australia and New Zealand. Kidney Int. 2003;63:S123–S127. doi:10.1046/j.1523-1755.63.s83.26.x
8. Lakshmi K, Nagesh Y, Krishna MV. Performance comparison of three data mining techniques for predicting kidney dialysis survivability. Int J Adv Eng Technol. 2014;7(1):242.
9. Chang Y-T, Hwang J-S, Hung S-Y, et al. Cost-effectiveness of hemodialysis and peritoneal dialysis: a national cohort study with 14 years follow-up and matched for comorbidities and propensity score. Sci Rep. 2016;6(1):1–12. doi:10.1038/s41598-016-0001-8
10. Ghimire S, Castelino RL, Lioufas NM, Peterson GM, Zaidi STR, Chilcot J. Nonadherence to medication therapy in haemodialysis patients: a systematic review. PLoS One. 2015;10(12):12. doi:10.1371/journal.pone.0144119
11. Ranasinghe P, Perera YS, Makarim MF, Wijesinghe A, Wanigasuriya K. The costs in provision of haemodialysis in a developing country: a multi-centered study. BMC Nephrol. 2011;12(1):42. doi:10.1186/1471-2369-12-42
12. Just PM, Riella MC, Tschosik EA, Noe LL, Bhattacharyya SK, de Charro F. Economic evaluations of dialysis treatment modalities. Health Policy. 2008;86(2–3):163–180. doi:10.1016/j.healthpol.2007.12.004
13. Prabahar M, Chandrasekaran V, Soundararajan P. Epidemic of chronic kidney disease in India -what can be done? Saudi J Kidney Dis Transpl. 2008;19(5):847–853.
14. Grassmann A, Gioberge S, Moeller S, Brown G. ESRD patients in 2004: global overview of patient numbers, treatment modalities and associated trends. Nephrol Dial Transplant. 2005;20(12):2587–2593. doi:10.1093/ndt/gfi159
15. Collins AJ, Foley RN, Chavers B, et al. United States renal data system 2011 annual data report: atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis. 2012;59(1Suppl 1):A7, e1–420.
16. Bruns FJ, Seddon P, Saul M, Zeidel ML. The cost of caring for end-stage kidney disease patients: an analysis based on hospital financial transaction records. J Am Soc Nephrol. 1998;9(5):884–890.
17. Pacheco A, Saffie A, Torres R, et al. Cost/utility study of peritoneal dialysis and hemodialysis in Chile. Perit Dial Int. 2007;27(3):359–363. doi:10.1177/089686080702700328
18. de Abreu MM, Walker DR, Sesso RC, Ferraz MB. A cost evaluation of peritoneal dialysis and hemodialysis in the treatment of end-stage renal disease in Sao Paulo, Brazil. Perit Dial Int. 2013;33(3):304–315. doi:10.3747/pdi.2011.00138
19. Villa G, Fernández–Ortiz L, Cuervo J, et al. Cost-effectiveness analysis of the Spanish renal replacement therapy program. Perit Dial Int. 2012;32(2):192–199. doi:10.3747/pdi.2011.00037
20. Kontodimopoulos N, Niakas D. An estimate of lifelong costs and QALYs in renal replacement therapy based on patients’ life expectancy. Health Policy. 2008;86(1):85–96. doi:10.1016/j.healthpol.2007.10.002
21. Lorenzo V, Perestelo L, Barroso M, Torres A, Nazco J. Economic evaluation of haemodialysis. Analysis of cost components based on patient-specific data. Nefrología. 2010;30(4):403–412.
22. Okafor C, Kankam C. Future options for the management of chronic kidney disease in Nigeria. Gend Med. 2012;9(1):S86–S93. doi:10.1016/j.genm.2011.10.002
23. Matri A, Elhassan E, Abu-Aisha H. Renal replacement therapy resources in Africa. Arab J Nephrol Transplant. 2008;1(1):9–14.
24. Elsharif ME, Gariballa EE, Gadour M. Costs of hemodialysis and kidney transplantation in Sudan A single center experience. 2010.
25. Grün RP, Constantinovici N, Normand C, Lamping DL. Costs of dialysis for elderly people in the UK. Nephrol Dial Transplant. 2003;18(10):2122–2127. doi:10.1093/ndt/gfg354
26. Icks A, Haastert B, Gandjour A, et al. Costs of dialysis—a regional population-based analysis. Nephrol Dial Transplant. 2010;25(5):1647–1652. doi:10.1093/ndt/gfp672
27. de Wit GA, Merkus MP, Krediet RT, de Charro FT. Health profiles and health preferences of dialysis patients. Nephrol Dial Transplant. 2002;17(1):86–92. doi:10.1093/ndt/17.1.86
28. Suja A, Anju R, Anju V, Neethu J, Peeyush P, Saraswathy R. Economic evaluation of end stage renal disease patients undergoing hemodialysis. J Pharm Bioallied Sci. 2012;4(2):107. doi:10.4103/0975-7406.94810
29. Ekrikpo UE, Udo AI, Ikpeme EE, Effa EE. Haemodialysis in an emerging centre in a developing country: a two year review and predictors of mortality. BMC Nephrol. 2011;12(1):50. doi:10.1186/1471-2369-12-50
30. Cherchiglia ML, Machado EL, Szuster DAC, et al. Epidemiological profile of patients on renal replacement therapy in Brazil, 2000–2004. Rev Saude Publica. 2010;44:639–649. doi:10.1590/S0034-89102010000400007
31. Birara D. Reflections on the Health Insurance Strategy of Ethiopia. 2018. Available from: https://www.researchgate.net/publication/323551458_Reflections_on_the_Health_Insurance_Strategy_of_Ethiopia.
32. Shaikh M, Woodward M, John O, et al. Utilization, costs, and outcomes for patients receiving publicly funded hemodialysis in India. Kidney Int. 2018;94(3):440–445. doi:10.1016/j.kint.2018.03.028
33. Kaur G, Prinja S, Ramachandran R, Malhotra P, Gupta KL, Jha V. Cost of hemodialysis in a public sector tertiary hospital of India. Clin Kidney J. 2018;11(5):726–733. doi:10.1093/ckj/sfx152
34. Gilardino RE, Gonzalez-Pier E, Brabata C. End-stage renal disease models in the Americas: optimizing resources to achieve better health outcomes. Value Health Reg Issues. 2018;17:115–118. doi:10.1016/j.vhri.2018.04.001
35. Golestaneh L, Alvarez PJ, Reaven NL, et al. All-cause costs increase exponentially with increased chronic kidney disease stage. Am J Manag Care. 2017;23(10 Suppl):S163–S172.
36. Gao A, Osgood ND, Jiang Y, Dyck RF. Projecting prevalence, costs and evaluating simulated interventions for diabetic end stage renal disease in a Canadian population of aboriginal and non-aboriginal people: an agent based approach. BMC Nephrol. 2017;18(1):283. doi:10.1186/s12882-017-0699-y
37. Etheredge H, Fabian J Challenges in expanding access to dialysis in South Africa—expensive modalities, cost constraints and human rights.
38. Yang F, Lau T, Luo N. Cost-effectiveness of haemodialysis and peritoneal dialysis for patients with end-stage renal disease in Singapore. Nephrology (Carlton). 2016;21(8):669–677. doi:10.1111/nep.12668
39. Naoum P, Topkaroglou I, Kitsonis D, et al. Cost calculations during “dire straits”: a cost-of-illness analysis of regular hemodialysis for end-stage renal disease in Greece. Int J Artif Organs. 2016;39(2):87–89. doi:10.5301/ijao.5000477
40. Takura T. Cost-effectiveness of hemodialysis in Japan. Contrib Nephrol. 2015;185:124–131.
41. Mushi L, Marschall P, Fleßa S. The cost of dialysis in low and middle-income countries: a systematic review. BMC Health Serv Res. 2015;15(1):506. doi:10.1186/s12913-015-1166-8
42. Chang Y-K, Hsu -C-C, Chen P-C, et al. Trends of cost and mortality of patients on haemodialysis with end stage renal disease. Nephrology (Carlton). 2015;20(4):243–249. doi:10.1111/nep.12380
43. Holtgrave DR. Handbook of Economic Evaluation of HIV Prevention Programs. Springer Science & Business Media; 1998.
44. Peeters P, Rublee D, Just PM, Joseph A. Analysis and interpretation of cost data in dialysis: review of Western European literature. Health Policy. 2000;54(3):209–227. doi:10.1016/S0168-8510(00)00112-3
45. Letsios A. The effect of the expenditure increase in the morbidity and the mortality of patients with end stage renal disease: the USA case. Hippokratia. 2011;15(Suppl 1):16.
46. Rutstein SO The DHS wealth index: approaches for rural and urban areas. DHS working papers; 2008.
47. Malehi AS, Pourmotahari F, Angali KA. Statistical models for the analysis of skewed healthcare cost data: a simulation study. Health Econ Rev. 2015;5(1):11. doi:10.1186/s13561-015-0045-7
48. Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford university press; 2015.
49. Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. OUP Oxford; 2014.
50. Jones AM. Models for Health Care. University of York, Centre for Health Economics; 2010.
51. Moran JL, Solomon PJ, Peisach AR, Martin J. New models for old questions: generalized linear models for cost prediction. J Eval Clin Pract. 2007;13(3):381–389. doi:10.1111/j.1365-2753.2006.00711.x
52. Blough DK, Ramsey SD. Using generalized linear models to assess medical care costs. Health Serv Outcomes Res Methodol. 2000;1(2):185–202. doi:10.1023/A:1012597123667
53. Prodjosudjadi W. Incidence, prevalence, treatment and cost of end-stage renal disease in Indonesia. Ethn Dis. 2006;16(2):S2.
54. Zambrowski -J-J. Cost of dialysis in France. Nephrol Ther. 2016;12(Suppl 1):S95–S97. doi:10.1016/j.nephro.2016.02.002
55. Manns B, Lee H, Taub K, Dean S, Johnson D, Donaldson C. Cost Analysis of Ongoing Care of Patients with End-Stage Renal Disease: What are the Important Determinants? Edmonton (AB): Alberta Centre for Health Services Utilization Research; 2003.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

