Back to Journals » ClinicoEconomics and Outcomes Research » Volume 11
Cost-effectiveness of sensor-augmented insulin pump therapy vs continuous subcutaneous insulin infusion in patients with type 1 diabetes in the Netherlands
Authors Roze S, Smith-Palmer J , de Portu S, Dalbaere A , de Brouwer B, de Valk HW
Received 3 September 2018
Accepted for publication 30 November 2018
Published 14 January 2019 Volume 2019:11 Pages 73—82
DOI https://doi.org/10.2147/CEOR.S186298
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Giorgio Colombo
Stephané Roze,1 Jayne Smith-Palmer,2 Simona de Portu,3 Alexis Delbaere,3 Bonnie de Brouwer,4 Harold W de Valk5
1HEVA HEOR, Lyon, France; 2Ossian Health Economics and Communications, Basel, Switzerland; 3Medtronic International Trading Sàrl, Tolochenaz, Switzerland; 4Medtronic Trading NL, Heerlen, the Netherlands; 5Leiden University Medical Center, Leiden, the Netherlands
Aim: The aim of this study was to perform a cost-effectiveness analysis to establish the cost-effectiveness of sensor-augmented pump therapy (SAP) with automated insulin suspension vs continuous subcutaneous insulin infusion (CSII) alone in patients with type 1 diabetes in the Netherlands.
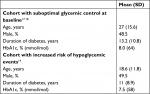
Patients and methods: The analysis was performed using the IQVIA CORE Diabetes Model (CDM) in two different patient cohorts: one with suboptimal glycemic control at baseline (mean age 27 years, mean baseline HbA1c 8.0% [64 mmol/mol]) and the other at increased risk of hypoglycemic events (mean age 18.6 years, mean baseline HbA1c 7.5% [58 mmol/mol]). Clinical input data were sourced from published literature, and the analysis was performed from the societal perspective.
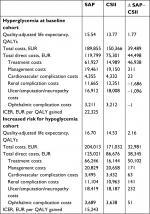
Results: In patients with suboptimal baseline glycemic control, SAP improved quality-adjusted life expectancy by 1.77 quality-adjusted life years (QALYs) vs CSII (15.54 QALYs vs 13.77 QALYs) with higher lifetime costs (EUR 189,855 vs EUR 150,366), resulting in an incremental cost-effectiveness ratio (ICER) of EUR 22,325 per QALY gained. In this cohort, sensitivity analyses showed that the influence of SAP on fear of hypoglycemia (FoH) and baseline HbA1c were key drivers of results. In patients at increased risk of hypoglycemia, the gain in quality-adjusted life expectancy with SAP vs CSII was 2.16 QALYs (16.70 QALYs vs 14.53 QALYs) with higher lifetime costs (EUR 204,013 vs EUR 171,032) leading to an ICER of EUR 15,243 per QALY gained. In this patient group, findings were most sensitive to changes in assumptions relating to the incidence of severe hypoglycemic events in the CSII arm.
Conclusion: For type 1 diabetes patients in the Netherlands who do not achieve target HbA1c levels or who experience frequent severe hypoglycemic events on CSII, switching to SAP is likely to be cost-effective.
Keywords: cost, cost-effectiveness, type 1 diabetes, the Netherlands, sensor-augmented insulin pump therapy
Introduction
The clinical and economic burden associated with type 1 and type 2 diabetes is substantial. In the Netherlands, there are currently an estimated 1.1 million people with diabetes, of whom approximately 9% (99,000 people) have type 1 diabetes.1 In 2011, the total direct cost of managing diabetes patients (type 1 and type 2 diabetes combined) was estimated at EUR 1.7 billion, which constitutes approximately 2% of total health care spending in the Netherlands.2 An analysis of temporal trends suggests that the incidence of type 1 diabetes in those aged ≤19 years is increasing; indeed, one study reported an annual increase in the age-adjusted incidence rate of type 1 diabetes of 3.7% per year over the period 1999–2011.3 In the Netherlands, type 1 diabetes patients are mostly treated within clinics in secondary care settings and all pediatric type 1 diabetes patients are treated by hospital-based pediatricians.4 The considerable clinical and economic burden as well as the humanistic burden of disease means that the evaluation of new technologies and interventions increasingly encompasses not only potential clinical benefits but also long-term cost-effectiveness and other aspects such as convenience and patient satisfaction, which may in turn influence patients’ compliance with using new technologies.
One such example is sensor-augmented pump therapy (SAP), which combines continuous glucose monitoring (CGM) with continuous subcutaneous insulin infusion (CSII). Some of the most recently introduced SAP devices are also equipped with advanced features such as low glucose suspend (LGS) or even predictive low glucose suspension (SmartGuardTM technology). With the LGS feature, insulin delivery is temporarily suspended if glucose levels drop below a predefined threshold level. Predictive LGS is the most recently introduced feature and a more sophisticated feature, whereby insulin delivery can be suspended based on the prediction of low glucose levels within the next 30 minutes and insulin delivery is automatically resumed once blood glucose levels start to recover. In the Netherlands, in 2008, there were an estimated 13,000 diabetes patients using insulin pumps, 90% of whom were type 1 patients.5 More recent data from Diabeter, a national diabetes center focusing on pediatric and adolescent diabetes, report that >50% patients aged 15–24 years and >60% patients aged ≥25 years treated within the Diabeter network were using insulin pumps.6 However, it should be noted that these data are sourced from a single specialist center and may therefore not be nationally representative.
Evidence from clinical studies has shown that SAP provides significant improvements in HbA1c and hypoglycemic event rate vs multiple daily injections (MDIs) and also vs standard CSII in patients with suboptimal glycemic control.7,8 Moreover, greater treatment satisfaction with SAP relative to CSII or MDI has also been reported in both pediatric patients and adults.9,10 There is also accumulating evidence suggesting that SAP incorporating an automatic insulin suspension feature provides even further incremental clinical benefits relative to SAP alone. For example, in the ASPIRE study, which compared SAP + LGS with SAP alone, the LGS feature was associated with a reduction in both the rate and the severity of nocturnal hypoglycemic events compared with SAP without suspension.11–13
In response to advances in insulin pump technology and the high rate of uptake of insulin pump use in the Netherlands, a cost-effectiveness analysis of SAP with automated insulin suspension vs CSII alone in two different groups of patients with type 1 diabetes was performed. The analysis was performed in two separate cohorts: one with suboptimal glycemic control at baseline and the other with increased risk of hypoglycemic events.
Patients and methods
Model description and outcomes
Cost-effectiveness analysis was performed using the IQVIA CORE Diabetes Model (CDM; IQVIA, Basel, Switzerland). The CDM is a published and validated cost-effectiveness model that can be used in either type 1 or type 2 diabetes.14–16 A comprehensive description of the CDM is provided by Palmer et al,14 but briefly, the CDM is based on a series of interdependent sub-models that simulate the progression of diabetes and diabetes-related complications. The sub-models have a semi-Markov structure and use time, time-in-state, and diabetes type-dependent probabilities to simulate long-term disease progression. Monte Carlo simulation using tracker variables is used to overcome the memory-less properties of a standard Markov model and allows for interconnectivity and interaction between individual sub-models. Outcomes from the CDM include life expectancy, quality-adjusted life expectancy, time to onset of diabetes-related complications, direct and indirect costs, and incremental cost-effectiveness ratios (ICERs).
Simulation cohorts and treatment effects
The cost-effectiveness of SAP vs CSII was investigated in two different patient cohorts. The first cohort was type 1 diabetes patients with suboptimal glycemic control at baseline (mean baseline HbA1c 8.0% [64 mmol/mol]), and the second one was type 1 diabetes patients at increased risk of hypoglycemic events. The rationale for this is that it permits independent assessment of the long-term outcomes with SAP in terms of improved glycemic control (in patients with poor glycemic control at baseline) and influence on hypoglycemic event rate (in patients at increased risk of hypoglycemic events).
Cohort characteristics of the patients with suboptimal glycemic control at baseline were sourced from an individual patient-level meta-analysis of randomized controlled trials by Pickup et al17 and, where necessary, supplemented with data from the Diabetes Control and Complications Trial (DCCT)18 (Table 1). A treatment effect in terms of HbA1c reduction of −0.42% was assumed for the SAP arm and −0.10% for the CSII arm. Treatment effects were based on formulae published by Pickup et al17 in an individual patient-level meta-analysis of the effects of CGM vs self-monitoring of blood glucose (SMBG) on glycemic control in patients with type 1 diabetes and accounted for age, baseline HbA1c, and sensor use. The treatment effect in the SAP arm was based on an assumed annual sensor usage of 43 sensors per year (each lasting for 6 days, ie, average sensor use of 4.95 days per week). Rates of severe hypoglycemic events were assumed to be the same in both treatment arms based on the findings of meta-analysis by Pickup et al17 (event rate of 2.6 events per 100 patient-years).
  | Table 1 Baseline cohort characteristics |
Baseline cohort characteristics and treatment effects for the cohort at increased risk of hypoglycemic events were from a randomized controlled trial of SAP vs CSII in 95 type 1 diabetes patients at increased risk of hypoglycemic events owing to impaired awareness of hypoglycemia (Table 1). In the base case analysis rates of severe hypoglycemic events of 2.2 events per 100 patient-months in the CSII arm and 0 events per 100 patient-months in the SAP arm were applied.19
Costs and utilities
For intervention costs, only the incremental cost of SAP relative to CSII was applied. The total incremental cost for SAP was EUR 2,713 and included 43 sensors per year (corresponding to 70% usage) and the sensor kit, which includes MiniLink™ transmitter, MiniLink™ charger, MiniLink™ tester, batteries, and Enlite™ serter™. The analysis also captured the reduced rate of SMBG in the SAP arm, based on the findings from an observational study,20 in which SAP was associated with an SMBG use of 4.35 strips per day compared with 7.11 strips per day in the CSII arm.
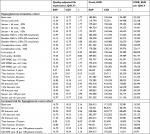
Direct costs for diabetes-related complications were sourced from published literature21–28 and inflated to 2014 EUR using the consumer price index published by the Centraal Bureau voor de Statistiek (Statistics Netherlands) (Table 2). Indirect costs due to lost productivity associated with diabetes-related complications were based on the human capital approach using findings from a study conducted in Denmark by Sørensen and Ploug.29 For indirect cost calculations, the mean age at first income was 25.2 years, the age at retirement was 65 years, and the number of working days was 228 days per year. Average salaries for males and females were sourced from the Centraal Bureau voor de Statistiek.
  | Table 2 Costs of diabetes-related complications Note: All costs are presented in 2014 EUR. |
For the cohort with suboptimal glycemic control at baseline, a utility benefit associated with reduced fear of hypoglycemia (FoH) was applied to the SAP arm. Findings from the INTERPET study showed that the SAP was associated with a 6.9 U decrease in the Hypoglycemia Fear Survey (HFS).30,31 Previous findings from another study reported that a 1 U increase in HFS score corresponds to a 0.008 U decrease in EQ-5Dindex score.32 Consequently, the 6.9 U decrease in HFS score associated with SAP was assumed to correspond to a utility benefit of 0.0552.
A quality-of-life (QoL) adjustment was also made for the cohort at increased risk of hypoglycemic events that considered the combined effect of the reduction in severe hypoglycemic event rate and reduced FoH with SAP based on the findings of a previous study by McBride et al.33 A utility benefit of 0.038 was applied to the SAP arm and a decrement of −0.035 was applied to the CSII arm.
Other model settings
The time horizon of the analysis was that of patient lifetimes. A discount rate of 4% per annum was applied to future costs, and a discount rate of 1.5% per annum was applied to clinical outcomes, in line with recommendations for the Netherlands.34 The base case analyses were performed from a societal perspective and therefore included both direct and indirect costs.
Sensitivity analyses
A series of one-way sensitivity analyses were performed for each patient group to determine the key drivers of outcomes in both patient groups. For both patient cohorts, sensitivity analyses were performed around time horizon and discount rate as well as perspective. The base case analysis was performed from the societal perspective (ie, incorporating both direct and indirect costs); therefore, a sensitivity analysis was performed in which only direct medical costs were included.
In the cohort with suboptimal glycemic control at baseline, sensitivity analyses were performed around the costs of the sensors and sensor kit as well as the number of sensors used annually. In the base case, it was assumed that 43 sensors per year were used; sensitivity analyses were performed in which the number of sensors used was increased to 48 and 61 per year. In this patient cohort, sensitivity analyses were also performed around baseline HbA1c, SMBG use, and FoH. In a series of sensitivity analyses, the effect of changing mean baseline to 7.5% (58 mmol/mol), 8.5% (69 mmol/mol), and 9.0% (75 mmol/mol) was investigated (compared with 8.0% [64 mmol/mol] in the base case). Similarly, the effect of reducing and negating the QoL benefit in terms of FoH was examined by performing simulations where, in the SAP arm, no FoH utility benefit was included and where the utility benefit was reduced to 0.0184 (compared with 0.0552 in the base case). The value of 0.0184 for the FoH utility benefit was based on the previously published findings by the Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group.35 Sensitivity analyses were also performed in which SMBG use in the SAP arm was changed to 7.11, 6.11, and 2.11 per day, compared with 4.35 per day in the base case.
In the cohort at increased risk of hypoglycemic events, sensitivity analyses were performed around the severe hypoglycemic event rate in the CSII arm, which was decreased to one event per 100 patient-months in one analysis and increased to eight events per 100 patient-months in a second analysis (compared with 2.2 events per 100 patient-months in the CSII arm in the base case).19
Results
Cohort with suboptimal glycemic control at baseline
In the patient cohort with suboptimal baseline glycemic control (mean baseline HbA1c 8.0% [64 mmol/mol]), the long-term use of SAP was associated with a gain in quality-adjusted life expectancy by 1.77 quality-adjusted life years (QALYs) relative to CSII (15.54 QALYs vs 13.77 QALYs; Table 3). Total lifetime costs (direct and indirect) for SAP were EUR 39,489 higher than those for CSII (EUR 189,855 vs EUR 150,366) resulting in an ICER of EUR 22,325 per QALY gained. SAP was also associated with a delay in the mean time to the onset of all diabetes-related complications included in the CDM. In particular, the onset of several complications including proliferative retinopathy, proteinuria, ulcer, and neuropathy was delayed by over 1 year.
If only direct costs were considered, the ICER was marginally higher at EUR 25,168 per QALY gained, which would still fall below the commonly cited willingness-to-pay threshold of EUR 30,000 per QALY gained. Indeed, in the base case analysis, at a willingness-to-pay threshold of EUR 30,000 per QALY gained, the likelihood of SAP being considered cost-effective vs CSII was 88.5% (Figure 1).
Sensitivity analyses showed that, in this cohort, the results were sensitive to changes in the assumptions around the QoL benefit associated with SAP use and also baseline HbA1c (Table 4). In the base case analysis, the use of SAP was assumed to be associated with an annual utility benefit of 0.0552 owing to a reduction in FoH. However, if this utility benefit was reduced to 0.0184, the gain in quality-adjusted life expectancy was reduced to 0.86 QALYs and the ICER was increased to EUR 46,147 per QALY gained. Similarly, if this utility benefit was negated entirely, the ICER was further increased to EUR 98,820 per QALY gained for SAP vs CSII. Sensitivity analyses also showed that SAP is like to be most cost-effective in those patients with the poorest glycemic control. If the mean baseline HbA1c was increased to 8.5% (69 mmol/mol), the ICER was reduced to EUR 19,595 per QALY gained. Moreover, at a mean baseline HbA1c of 9.0% (75 mmol/mol), the ICER for SAP vs CSII was further reduced to EUR 17,894 per QALY gained. These two sensitivity analyses may be the most relevant to the analyses as 8.0% (64 mmol/mol) is the threshold for the reimbursement of SAP in adults in the Netherlands.
Cohort at increased risk of hypoglycemic events
In the cohort at increased risk of hypoglycemic events, the incremental gain in quality-adjusted life expectancy associated with SAP use was 2.16 QALYs (16.70 QALYs with SAP vs 14.53 QALYs with CSII; Table 3). However, total lifetime costs (direct and indirect) were EUR 32,981 higher for SAP than for CSII (EUR 204,013 vs EUR 171,032). The combination of higher quality-adjusted life expectancy, but higher lifetime costs, resulted in an ICER of EUR 15,243 per QALY gained for SAP vs CSII. The analysis of the cost-effectiveness acceptability curve showed that, in this patient group, at a willingness-to-pay threshold of EUR 30,000, the likelihood of SAP being considered cost-effective compared with CSII was 99.5% (Figure 1). The use of SAP was also associated with a reduced cumulative incidence and delay to the onset of diabetes-related complications. The most pronounced delay was for neuropathy, the mean onset of which was delayed by 1.17 years with SAP compared with CSII alone.
In this patient cohort, sensitivity analyses showed that the cost-effectiveness of SAP vs CSII was strongly influenced by the rate of severe hypoglycemic events in the CSII arm (Table 4). In the base case analysis, an event rate of 2.2 events per 100 patient-months was assumed for the CSII arm; in a sensitivity analysis where this was decreased to one event per 100 patient-months, the ICER was increased to EUR 18,288 per QALY gained. Conversely, in a sensitivity analysis in which an event rate of eight events per 100 patient-months was assumed in the CSII arm, the ICER for SAP vs CSII was considerably lower at EUR 5,470 per QALY gained. The high direct costs and potentially severe clinical consequences associated with severe hypoglycemic events suggest that for patients who continue to experience severe hypoglycemic events on CSII, switching to SAP is likely to be cost-effective.
Discussion
The findings from cost-effectiveness analysis show that, for type 1 diabetes patients in the Netherlands, SAP with automated insulin suspension is likely to be cost-effective compared with CSII in patients with suboptimal glycemic control and patients who are at increased risk of hypoglycemic events. The findings presented in this study concur with those for type 1 diabetes in other Western European countries including the UK, France, Denmark, and Sweden.36–39
The uptake of insulin pump therapy in the Netherlands is considerably higher than many other European countries.6 Establishing the patient groups who are likely to benefit most from switching to more sophisticated technologies such as SAP, as well as the patient groups in which this switch is cost-effective, provides useful information for payers, policy makers, and clinicians in the Dutch setting. In the Dutch Diabeter clinic network, in which 93% patients have type 1 diabetes, at the end of 2014, 57% were on CSII and 8% patients were on SAP.40 Moreover, 87% of SAP patients, compared with 58% of CSII patients, had HbA1c<7.5%, suggesting that in routine clinical practice in the Netherlands, there is potentially a substantial proportion of CSII-treated patients who may derive clinical benefit from switching to SAP.40
In the cohort with hyperglycemia at baseline, the influence of SAP on FoH was a key driver of outcomes. In the sensitivity analysis in which the utility benefit associated with reduced FoH was negated the ICER increased to over EUR 98,000 per QALY gained, which would no longer be considered cost-effective using commonly cited willingness-to-pay thresholds. The Diabetes MILES, the Netherlands study,41 investigated FoH in type 1 diabetes patients in the Netherlands. Notably, mean FoH scores were lower than in other countries, which interestingly the authors partly attributed to the high uptake of insulin pump therapy in the Netherlands (54% of patients in the study used insulin pumps). It was also noted that high FoH owing to a history of severe hypoglycemic events may in instances lead to behavior modifications such as deliberately reducing insulin dose, resulting in suboptimal glycemic control, which can ultimately increase the risk of long-term complications. It is also important to note that the QoL impact of FoH in terms of utility values was derived from a single study (INTERPRET),30,31 where the main indications for SAP initiation were glycemic instability, persistently high HbA1c, recurrent hypoglycemia, hypoglycemia unawareness, and flexibility/lifestyle choice. The extent of FoH may vary between different patient populations and individuals, and conclusions relating to the impact of FoH may also be influenced by the accuracy with which its impact can be measured as different methods of eliciting utilities can result in different values, and for children and adolescents, patient-reported outcomes are sometimes determined using parents as proxies rather than the patients themselves. In terms of different individuals’ experiences of FoH, in the recently published multinational IO HAT study, FoH was assessed on a scale of 0–10, where 0= no FoH and 10= absolutely terrified. In this study, while the mean (SD) FoH score for patients with type 1 diabetes was 5.5 (3.3), 12.7% of patients reported a score of 0% and 17.1% reported a score of 10, illustrating that FoH may vary substantially from patient to patient.42
Baseline HbA1c was also a key determinant of cost-effectiveness in the cohort with suboptimal glycemic control at baseline. Sensitivity analysis around baseline HbA1c showed that SAP was most cost-effective in those with poorest glycemic control at baseline. In the base case analysis, the mean baseline HbA1c was 8.0% (64 mmol/mol). At a mean baseline HbA1c of 7.5% (58 mmol/mol), the ICER for SAP vs CSII was EUR 24,182 per QALY gained. However, in analyses where baseline HbA1c was 8.5% (69 mmol/mol) and 9.0% (75 mmol/mol), the ICER decreased to EUR 19,595 and EUR 17,894 per QALY gained, respectively. These sensitivity analyses are likely the most relevant to clinical practice in the Netherlands as the reimbursement of SAP in adults is limited to those with HbA1c>8.0% (64 mmol/mol). In addition, one study recently reported that >20% of type 1 diabetes patients aged 15–24 years and >5% of those aged ≥25 years had a HbA1c level ≥9.0% (75 mmol/mol).6
In the cohort at increased risk of hypoglycemic events, a major driver of the cost-effectiveness of SAP was the higher rate of severe hypoglycemic events in the CSII arm. Clinical input data for this patient cohort were based on a randomized controlled trial performed in type 1 diabetes patients with impaired awareness of hypoglycemia.19 Up to 30% of adults with type 1 diabetes have impaired awareness of hypoglycemia, which in turn increases the risk of severe hypoglycemic events.43 The automated insulin suspension functionality of some SAP may therefore be particularly beneficial for patients with impaired hypoglycemia and/or a history of frequent severe hypoglycemic events. In addition, there is evidence to suggest that in type 1 diabetes patients, the distribution of severe hypoglycemic events is highly skewed, indeed in one multinational study of hypoglycemia, 5% of patients accounted for 54% of all severe hypoglycemic events.44 As such, on an individual patient level, the use of SAP is likely to be highly cost-effective for the small proportion of patients who experience frequent severe hypoglycemic events. Notably, in the current analysis, only severe hypoglycemic events were accounted for, the QoL implications as well as the direct and indirect costs associated with minor hypoglycemic events were not included. While for many patients, minor events may be easily addressed and may not cause distress, for some patients, minor events may have a detrimental impact on everyday life. For example, hypoglycemia is known to lead to cognitive dysfunction in many patients, which may negatively influence the ability to perform everyday activities such as driving.45 In addition, even minor hypoglycemic events can have a considerable impact in terms of lost productivity. Indeed, in one multinational study, productivity loss due to non-severe hypoglycemic events was estimated at 8.3–15.9 hours (USD 15–93) per incident.46
The analysis performed in this study is associated with limitations. A limitation inherent to any health economic modeling analysis is the use of short-term clinical trial data to project outcomes over patient lifetimes. In the analysis presented for the cohort with the increase of hypoglycemic events, clinical input data were sourced from a clinical trial of 6-month duration. Similarly, input data for the cohort with suboptimal glycemic control at baseline were sourced from a meta-analysis of randomized controlled trials. However, large-scale long-term (>5 years) clinical and cost studies of SAP are currently lacking; therefore, health economic modeling represents a pragmatic alternative for the projection of long-term outcomes. The analysis did not account for the potential effect of adherence to SAP declining over time. In the base case analysis, an annual sensor use of 43 sensors was assumed (corresponding to mean sensor use of 71%). The findings of the SWITCH study showed that glycemic control was worse in patients with sensor use <70%;47 therefore, any waning of adherence over time may negatively influence the long-term efficacy of SAP. In addition, although the analysis was performed from a societal perspective and therefore captured indirect costs, costs associated with informal caregivers such as parents and partners, which may be substantial, were not captured in the analysis. Indeed, in a recent study in pediatric patients from Spain, the cost of informal care was estimated to account for 83% of total annual direct and indirect costs. As such, total costs in the current study, in both treatment arms, may be underestimated.48 A further limitation is that while the analysis was performed from a societal perspective, it did not capture the potential resource use costs associated with medical personnel training patients how to use SAP devices.
Conclusion
Overall, the findings from the current analysis suggest that for CSII-treated type 1 diabetes patients in the Netherlands, who have either suboptimal glycemic control or are at increased risk of hypoglycemia, switching to SAP with automated insulin suspension is likely to confer long-term clinical benefits and is likely to be good value for money. Further, SAP is likely to be most cost-effective in patients with the poorest glycemic control at baseline and in patients who experience frequent severe hypoglycemic events.
Disclosure
Funding for the analysis was provided by Medtronic International Trading Sàrl. AD and SdP are current employees of Medtronic International Trading Sàrl, which manufactures SAP devices. BdB is a current employee of Medtronic Trading NL. SR is a current employee of HEVA HEOR, which has received consulting fees from Medtronic International Trading Sàrl. JS-P is a current employee of Ossian Health Economics and Communications, which has received consulting fees from Medtronic International Trading Sàrl. HWdV has previously received consulting fees/honoraria from Medtronic International Trading Sàrl. The authors report no other conflicts of interest in this work.
References
Volksgezondheidenzorg.info [homepage on the Internet]. Prevalence of diabetes by age and sex. Available from: https://www.volksgezondheidenzorg.info/onderwerp/diabetes-mellitus/cijfers-context/huidige-situatie#node-aantal-nieuwe-gevallen-van-diabetes-huisartsenpraktijk. Accessed May 13, 2017. | ||
Volksgezondheidenzorg.info [homepage on the Internet]. Cost of care for diabetes mellitus. Available from: https://www.volksgezondheidenzorg.info/onderwerp/diabetes-mellitus/kosten/kosten#node-kosten-van-zorg-voor-diabetes-mellitus-naar-leeftijd-en-geslacht. Accessed May 13, 2017. | ||
Fazeli Farsani S, Souverein PC, van der Vorst MM, Knibbe CA, de Boer A, Mantel-Teeuwisse AK. Chronic comorbidities in children with type 1 diabetes: a population-based cohort study. Arch Dis Child. 2015;100(8):763–768. | ||
Spaans EAJM, Gusdorf LMA, Groenier KH, et al. The incidence of type 1 diabetes is still increasing in the Netherlands, but has stabilised in children under five (Young DUDEs-1). Acta Paediatrica. 2015;104(6):626–629. | ||
Hicks D. Why is the UK lagging behind in the pump usage stakes? Journal of Diabetes Nursing. 2008;12:105–108. | ||
Mcknight JA, Wild SH, Lamb MJ, et al. Glycaemic control of Type 1 diabetes in clinical practice early in the 21st century: an international comparison. Diabet Med. 2015;32(8):1036–1050. | ||
Hermanides J, Nørgaard K, Bruttomesso D, et al. Sensor-augmented pump therapy lowers HbA(1c) in suboptimally controlled Type 1 diabetes; a randomized controlled trial. Diabet Med. 2011;28(10):1158–1167. | ||
Šoupal J, Petruželková L, Flekač M, et al. Comparison of Different Treatment Modalities for Type 1 Diabetes, Including Sensor-Augmented Insulin Regimens, in 52 Weeks of Follow-Up: A COMISAIR Study. Diabetes Technol Ther. 2016;18(9):532–538. | ||
Hussain T, Akle M, Nagelkerke N, Deeb A. Comparative study on treatment satisfaction and health perception in children and adolescents with type 1 diabetes mellitus on multiple daily injection of insulin, insulin pump and sensor-augmented pump therapy. SAGE Open Med. 2017;5(1):205031211769493. | ||
Hommel E, Olsen B, Battelino T, et al. Impact of continuous glucose monitoring on quality of life, treatment satisfaction, and use of medical care resources: analyses from the SWITCH study. Acta Diabetol. 2014;51(5):845–851. | ||
Weiss R, Garg SK, Bode BW, et al. Hypoglycemia Reduction and Changes in Hemoglobin A1c in the ASPIRE In-Home Study. Diabetes Technol Ther. 2015;17(8):542–547. | ||
Garg S, Brazg RL, Bailey TS, et al. Reduction in duration of hypoglycemia by automatic suspension of insulin delivery: the in-clinic ASPIRE study. Diabetes Technol Ther. 2012;14(3):205–209. | ||
Bergenstal RM, Klonoff DC, Garg SK, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med. 2013;369(3):224–232. | ||
Palmer AJ, Roze S, Valentine WJ, et al. The CORE Diabetes Model: Projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin. 2004;20 (1):S5–S26. | ||
Palmer AJ, Roze S, Valentine WJ, et al. Validation of the CORE Diabetes Model against epidemiological and clinical studies. Curr Med Res Opin. 2004;20 (1):S27–S40. | ||
Mcewan P, Foos V, Palmer JL, Lamotte M, Lloyd A, Grant D. Validation of the IMS CORE Diabetes Model. Value Health. 2014;17(6):714–724. | ||
Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805. | ||
Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–2653. | ||
Ly TT, Nicholas JA, Retterath A, Lim EM, Davis EA, Jones TW. Effect of sensor-augmented insulin pump therapy and automated insulin suspension vs standard insulin pump therapy on hypoglycemia in patients with type 1 diabetes: a randomized clinical trial. JAMA. 2013;310(12):1240–1247. | ||
Lynch P, Attvall S, Persson S, Barsoe C, Gerdtham U. Routine use of personal continuous glucose monitoring system with insulin pump in Sweden [abstract 1052]. Diabetologia. 2012;55(Suppl 1):S432. | ||
de Vries FM, Denig P, Visser ST, Hak E, Postma MJ. Cost-effectiveness of statins for primary prevention in patients newly diagnosed with type 2 diabetes in the Netherlands. Value Health. 2014;17(2):223–230. | ||
Eefting F, Nathoe H, van Dijk D, et al. Randomized comparison between stenting and off-pump bypass surgery in patients referred for angioplasty. Circulation. 2003;108(23):2870–2876. | ||
Nederlands Huisarten Genootschap [homepage on the Internet]. Available from: https://www.nhg.org. Accessed May 20, 2017. | ||
Medicijnkosten.nl [homepage on the Internet]. Available from: https://www.medicijnkosten.nl/. Accessed May 20, 2017. | ||
Nederlandse Zorgautoriteit [homepage on the Internet]. Available from: https://www.nza.nl/regelgeving/tarieven/. Accessed May 20, 2017. | ||
Adarkwah CC, Gandjour A, Akkerman M, Evers SM. Cost-effectiveness of angiotensin-converting enzyme inhibitors for the prevention of diabetic nephropathy in The Netherlands – a Markov model. PLoS One. 2011;6(10):e26139. | ||
Cz.nl. Available from: http://www.cz.nl/~/media/actueel/voorwaarden/bijlagen-bij-basisverzekeringen/hulpmiddelen%20reglement.pdf. Accessed March 11, 2014. | ||
Redekop WK, Stolk EA, Kok E, Lovas K, Kalo Z, Busschbach JJ. Diabetic foot ulcers and amputations: estimates of health utility for use in cost-effectiveness analyses of new treatments. Diabetes Metab. 2004;30(6):549–556. | ||
Sørensen J, Ploug UJ. The cost of diabetes-related complications: registry-based analysis of days absent from Work. Economics Research International. 2013;2013(1):1–8. | ||
Nørgaard K, Scaramuzza A, Bratina N. Sensor-augmented pump therapy in real-life: patients reported outcomes results of the INTERPRET observational study. Abstract 1058, EASD Berlin. 2012. | ||
Nørgaard K, Scaramuzza A, Bratina N, et al. Routine sensor-augmented pump therapy in type 1 diabetes: the INTERPRET study. Diabetes Technol Ther. 2013;15(4):273–280. | ||
Currie CJ, Morgan CL, Poole CD, Sharplin P, Lammert M, Mcewan P. Multivariate models of health-related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin. 2006;22(8):1523–1534. | ||
McBride M, Eggleston AS, Jones T, Ly T. Health-Related Quality of Life in Patients with Type 1 Diabetes and Impaired Hypoglycaemia Awareness: The Role of Sensor-Augmented Insulin Pump Therapy with Automated Insulin Suspension. Value in Health. 2013;16(7):A448. | ||
College voor zorgverzekeringe. Guidelines for pharmacoeconomic research, updated version College voor zorgverzekeringen. 2006. | ||
Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Beck RW, Lawrence JM, et al. Quality-of-life measures in children and adults with type 1 diabetes: Juvenile Diabetes Research Foundation Continuous Glucose Monitoring randomized trial. Diabetes Care. 2010;33(10):2175–2177. | ||
Roze S, Smith-Palmer J, Valentine WJ, et al. Long-term health economic benefits of sensor-augmented pump therapy vs continuous subcutaneous insulin infusion alone in type 1 diabetes: a U.K. perspective. J Med Econ. 2016;19(3):236–242. | ||
Roze S, de Portu S, Smith-Palmer J, Delbaere A, Valentine W, Ridderstråle M. Cost-effectiveness of sensor-augmented pump therapy versus standard insulin pump therapy in patients with type 1 diabetes in Denmark. Diabetes Res Clin Pract. 2017;128:6–14. | ||
Roze S, Smith-Palmer J, Valentine W, et al. Cost-Effectiveness of Sensor-Augmented Pump Therapy with Low Glucose Suspend Versus Standard Insulin Pump Therapy in Two Different Patient Populations with Type 1 Diabetes in France. Diabetes Technol Ther. 2016;18(2):75–84. | ||
Jendle J, Smith-Palmer J, Delbaere A, et al. Cost-Effectiveness Analysis of Sensor-Augmented Insulin Pump Therapy with Automated Insulin Suspension Versus Standard Insulin Pump Therapy in Patients with Type 1 Diabetes in Sweden. Diabetes Ther. 2017;8(5):1015–1030. | ||
Diabeter: Our results, average HbA1c levels, international comparison data. Available from: https://diabeter.nl/en/our-care/our-results/#international_comparison. Accessed May 20, 2017. | ||
Nefs G, Bevelander S, Hendrieckx C, et al. Fear of hypoglycaemia in adults with Type 1 diabetes: results from Diabetes MILES – The Netherlands. Diabet Med. 2015;32(10):1289–1296. | ||
Emral R, Pathan F, Cortés CAY, et al. Self-reported hypoglycemia in insulin-treated patients with diabetes: results from an international survey on 7289 patients from nine countries. Diabetes Res Clin Pract. 2017;134:17–28. | ||
Yeoh E, Choudhary P, Nwokolo M, Ayis S, Amiel SA. Interventions That Restore Awareness of Hypoglycemia in Adults with Type 1 Diabetes: A Systematic Review and Meta-analysis. Diabetes Care. 2015;38(8):1592–1609. | ||
Pedersen-Bjergaard U, Pramming S, Heller SR, et al. Severe hypoglycaemia in 1076 adult patients with type 1 diabetes: influence of risk markers and selection. Diabetes Metab Res Rev. 2004;20(6):479–486. | ||
Rooijackers HM, Wiegers EC, Tack CJ, van der Graaf M, de Galan BE. Brain glucose metabolism during hypoglycemia in type 1 diabetes: insights from functional and metabolic neuroimaging studies. Cell Mol Life Sci. 2016;73(4):705–722. | ||
Brod M, Christensen T, Thomsen TL, Bushnell DM. The impact of non-severe hypoglycemic events on work productivity and diabetes management. Value Health. 2011;14(5):665–671. | ||
Battelino T, Conget I, Olsen B, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55(12):3155–3162. | ||
López-Bastida J, López-Siguero JP, Oliva-Moreno J, et al. Social economic costs of type 1 diabetes mellitus in pediatric patients in Spain: CHRYSTAL observational study. Diabetes Res Clin Pract. 2017;127:59–69. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.