Back to Journals » ClinicoEconomics and Outcomes Research » Volume 11
Cost-effectiveness of secukinumab compared to other biologics in the treatment of ankylosing spondylitis in Finland
Authors Purmonen T , Puolakka K, Mishra D, Gunda P, Martikainen J
Received 25 October 2018
Accepted for publication 17 January 2019
Published 15 February 2019 Volume 2019:11 Pages 159—168
DOI https://doi.org/10.2147/CEOR.S192235
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Samer Hamidi
Timo Purmonen,1 Kari Puolakka,2 Dinesh Mishra,3 Praveen Gunda,3 Janne Martikainen4
1Novartis Finland Oy, Espoo, Finland; 2South Karelia Central Hospital, Lappeenranta, Finland; 3Novartis Product Lifecycle Services-NBS, Novartis Healthcare Private Limited, Hyderabad, India; 4School of Pharmacy, University of Eastern Finland, Kuopio, Finland
Aim: This study assesses the cost-effectiveness of secukinumab vs currently licensed biologics for the treatment of ankylosing spondylitis (AS) from the Finnish health care system perspective.
Methods: A semi-Markov model compared secukinumab with adalimumab, adalimumab biosimilar, certolizumab pegol, etanercept, etanercept biosimilar, golimumab, and infliximab in a biologic-naïve population over a lifetime horizon. The Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) was used to assess the treatment response. Efficacy inputs were obtained from the network meta-analysis, and other model inputs were obtained from the published literature and Finnish sources. Main study outcomes included quality-adjusted life years (QALYs) gained and incremental cost-effectiveness ratio in terms of cost per QALY gained. Robustness of results was confirmed by sensitivity analyses and alternative scenario analyses.
Results: Secukinumab achieved highest QALYs (13.1) at lowest expected lifetime cost (€279,872) vs other comparators in biologic-naïve AS patients in the base case analysis, thus it dominated other biologics. Golimumab had a second highest QALYs (12.9) at the total cost of €309,551. Results were sensitive to variation in BASDAI 50 response for secukinumab, baseline Bath Ankylosing Spondylitis Functional Index (BASFI) score across all drugs, change in BASDAI and BASFI scores, and discount rates as observed in the one-way sensitivity analyses. Secukinumab was either dominant or cost-effective treatment in different alternative scenarios.
Conclusion: Secukinumab presented itself to be the dominant (ie, less costly and more effective) treatment vs other comparators for the biologic-naïve patients with AS in Finland.
Keywords: radiographic axial SpA, secukinumab, cost-effectiveness, Finland, economic evaluation, health economics, IL-17, anti-TNF
Introduction
Ankylosing spondylitis (AS) is a chronic, systemic, inflammatory disease that affects primarily the sacroiliac joints and spine and leads to back pain, stiffness, discomfort, fatigue, impaired spinal mobility, and postural abnormalities.1 AS can also inflame peripheral joints and entheses2 and has extra-articular manifestation.3 The New York classification criteria for AS require radiographic sacroiliitis, which may take many years to develop, and hence the diagnosis as well as disease management is often delayed. The epidemiological data for AS are scarce in Finland. The annual incidence of AS or nonradiographic axial spondyloarthritis patients requiring advanced treatments beyond NSAIDs has been 17 per 100,000 adults during 2012–2014 in Finland.4
AS manifests itself usually in early adulthood (particularly during the third decade of life) and impacts patients for most of their life. AS is associated with decreased quality of life (QoL), increased mortality, and substantial health care-related costs, making it a burden to the patient and society.5 The AS-related costs were reported to vary across the European countries, and indirect costs contribute to the major component of the total costs ranging from 53.4% to 62%. Also, as the disease severity increases, direct costs increase two times while indirect costs increase almost four times.6
As per the recently updated treatment recommendations (2016) from ASAS and the European League Against Rheumatism,7 NSAIDs and physical therapy have been recommended as first-line treatment for AS; however, the disease becomes refractory to these agents over time.8–10 The use of anti-tumor necrosis factor (anti-TNF) biologics or IL-17A inhibitor is recommended in axial spondyloarthritis after the failure of NSAIDs. In Finland, however, biologic drugs are unfortunately not reimbursed until at least one traditional disease-modifying antirheumatic drug (DMARD) (sulfasalazine and methotrexate) has been tried or if DMARD is contraindicated.11
Despite major improvement in treatment results with the adoption of anti-TNFs, up to 40% of the patients do not respond sufficiently to or tolerate anti-TNFs or efficacy may reduce over time,12 indicating a significant unmet medical need in the treatment of AS patients. If patients are not responsive to initial biologic therapy, it is recommended to switch a second anti-TNF or secukinumab.7 These updated recommendations also include an overarching principle that addresses the cost issues with AS for the very first time. It highlights the need for “best care” along with the use of cost-effectiveness analyses results while making treatment decisions.
Secukinumab is the first and fully human recombinant antihuman IL-17A IgG1 monoclonal antibody, which is licensed for use in AS.8 Secukinumab was approved by European Medicines Agency (EMA) in 2015 for the treatment of adult patients with active AS who have responded inadequately to conventional therapy.13 Secukinumab is a highly efficacious treatment for AS providing sustained improvement in AS signs and symptoms, a rapid onset of action, and a favorable safety profile compared with placebo according to the results of multiple phase 3 clinical trials.14–17 Additionally, secukinumab has demonstrated superior efficacy in indirect comparison methods (matching-adjusted indirect comparison [MAIC],18 network meta-analysis [NMA]).19
This analysis reports the results of a cost-effectiveness study of secukinumab in patients with AS who have not been previously treated with any biologic (biologic-naïve) in Finland. Additionally, cost-effectiveness of secukinumab was also analyzed in mixed population (a combination of both biologic-naïve and biologic-experienced patients [patients who had been previously treated with biologics]) in the alternative scenario analysis.
Methods
Patient population and interventions
Adult AS patients (18 years or older) fulfilling the modified New York criteria for AS and having inadequate response to NSAIDs were included in the analysis. The patients who were naïve to biologic therapy were considered for the base-case analysis. For an alternate analysis, secukinumab 150 mg results were also analyzed in mixed population (a combination of both biologic-naïve and biologic-experienced patients). Population data inputs were obtained as weighted average across all patients from the MEASURE 1 and MEASURE 2 pooled trial data (Table S1).
The model evaluated the cost-effectiveness of subcutaneous (s.c.) secukinumab 150 mg compared with the currently licensed biologics (s.c. treatments adalimumab and its biosimilar, certolizumab pegol, etanercept and its biosimilar, golimumab and intravenous [i.v.] infliximab) for the treatment of AS from the Finnish health care system perspective. The list of dose and dosage frequencies of the treatments for this analysis is listed in Table S2. These are based on the EMA authorization approval.20
Model structure
A semi-Markov model was developed using Microsoft Excel (Figure 1). The semi-Markov model structure was chosen due to time-dependent probabilities associated with mortality, unlike standard Markov model where transition probabilities are constant over time. In the model, patients start a given treatment and response to the treatment was assessed at the end of 3 months by considering a 50% improvement from baseline in the initial Bath Ankylosing Spondylitis Disease Activity Index (BASDAI 50) response. Previous economic evaluations of anti-TNFs in AS considered similar model to assess the treatment response.21
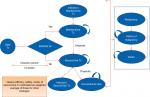  | Figure 1 Markov model structure with 3-month induction period. Abbreviations: Tx, treatment; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index. |
Patients transitioned to different health state based on the probabilities of BASDAI response rate, malignancy, chance of serious infection, dropout rate, and death (Figure 1). Adverse events (AEs) such as serious infection (including tuberculosis) and malignancy were included as separate health states to better track the associated costs and QoL effects. Patients who experienced infections were allowed to continue biologic treatment or switch to conventional care. In contrast, patients discontinued biologic treatment upon entering the “malignancy” health state. Patients in “malignancy” state were assumed to be at a higher mortality risk for 5 years into that state.
Patients withdrawing from initial biologic treatment were treated long-term with the subsequent biologic therapy. Considering data on the effectiveness of subsequent biologics were limited; average values of efficacy, treatment withdrawal rates, cost, and AE rates were used for all biologics. Patients receiving subsequent-line biologics either stayed on the treatment for rest of time horizon or could drop out to conventional care. Since there was no discontinuation rate available for patients dropping out from subsequent-line biologic to conventional therapy, it was assumed minimum of annual dropout rate from year 2 onward of all biologics (ie, annual dropout rate of 1.6% was applied for transition from second-line treatment to conventional care, see Table S3).
Model inputs
Clinical inputs
Comparative effectiveness data for treatments were obtained from a Bayesian fixed effects NMA,22 which included a total of seven trials with 1,361 biologic-naïve patients. The main clinical input was BASDAI 50 response at 3 months, which was used as primary response criteria (Table 1). Although the use of Ankylosing Spondylitis Disease Activity Score (ASDAS) as a primary response criteria measure was discussed when building the model, there are no sufficient data from trials available from different anti-TNFs to establish a sufficient data basis for a valid health economic model. Also, ASDAS is closely related to BASDAI, as three out of the six BASDAI items are used to calculate ASDAS and a study by Eder et al found that ASDAS was not superior to BASDAI in its ability to discriminate between high and low disease activity states in AS.23 Moreover, the use of BASDAI 50 is consistent with existing British Society of Rheumatology guidelines24 and the National Institute for Health and Care Excellence appraisals for AS.25,26 Additional clinical inputs included short-term changes in BASDAI and Bath Ankylosing Spondylitis Functional Index (BASFI) scores for responders and nonresponders (Table 2), long-term changes in BASFI captured through modified Stoke Ankylosing Spondylitis Spinal Score (Table 3), and biologic withdrawal rates (which were treatment specific and obtained for year 1 as well as year 2 and beyond) (Table S3).
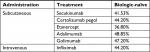  | Table 1 BASDAI 50 response at 3 months in biologic-naïve patients Notes: BASDAI 50 response data are from a Bayesian fixed effects network meta-analysis.22 Biologic-naïve data for certolizumab pegol and infliximab were not available and were assumed equivalent to average of other biologics in the network meta-analysis Abbreviation: BASDAI, Bath Ankylosing Spondylitis Disease Activity Index. |
  | Table 2 Short-term changes in BASDAI and BASFI in biologic-naïve patients Notes: Bayesian fixed effects network meta-analysis.22 aResponders are those who showed BASDAI 50 response. Change in BASDAI data for biologic-naïve patients was not available for SEC and CER P and was assumed to be equivalent to the average of other biologics in the NMA. Change in BASFI data for biologic-naïve patients for CER P was not available and was assumed to be equivalent to the average of other biologics in the NMA (excluding SEC). Abbreviations: ADA, adalimumab; AS, ankylosing spondylitis; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; CER P, certolizumab pegol; ETN, etanercept; GOL, golimumab; INF, infliximab; NMA, network meta-analysis; SEC, secukinumab. |
  | Table 3 Long-term changes in BASFI Notes: The long-term changes in BASFI were assessed by progression of radiographic disease measured by mSASSS score. aThis figure was calculated using the overall background progression rate of 0.98 units/year from the study by Ramiro et al (2015)50 and the MEASURE 1 week 104 mSASSS progression figure of 0.3. Abbreviations: BASFI, Bath Ankylosing Spondylitis Functional Index; mSASSS, modified Stoke Ankylosing Spondylitis Spine Score. |
Resource utilization and cost data
Four types of direct medical costs were incorporated in the model: drug acquisition costs, disease-related costs, medical support costs, and AE costs. Local unit costs were valued using public health care cost index, and where needed, costs were inflated per the 2017 exchange rate for Euro (Tables S4 and S6). The drug acquisition costs for secukinumab 150 mg and for brand comparators (Table 4) were obtained from the Finnish medicinal products and price database.27 Biosimilar pricing for etanercept and adalimumab was assumed to be 30% less than brand; as they were not, at the time of analyses, available in the market and were only used for sensitivity analysis. Additional drug administration costs were considered for i.v. administration of infliximab compared with other s.c. treatments.
  | Table 4 Drug acquisition costs for biologics in Finland Notes: Finnish medicinal products and price database.27 All prices exclude value added tax. Retail price for s.c. products and wholesale price for i.v. products are applied according to local guidelines. Infliximab price was calculated as a weighted average according to market shares of originator drug and biosimilars. Additionally, a cost of €382 (2016 value) was considered for each i.v. administration.51 Biosimilar pricing for etanercept and adalimumab was assumed to be 30% less than brand; they are not available in the market and thus, only used for sensitivity analysis. Abbreviations: i.v., intravenous; s.c., subcutaneous. |
Disease-related costs account for the AS disease management costs incurred. These costs are estimated based on an exponential BASFI regression model:28 Cost (€)=1,508.99 € × EXP (0.213 BASFI). Costs were calculated for 3 months according to the model cycle length.
Costs associated with medical support resources are presented in Table S4. Incidence of AEs and cost per event are available in supplementary materials (Tables S5 and S6).
Other inputs
Utility weight inputs were used to calculate quality-adjusted life years (QALYs) to reflect the improvement in QoL experienced by patients who achieve various levels of BASDAI and BASFI response. A linear mixed model was used to fit EuroQol 5-dimensions questionnaire utility score as a response variable, and BASDAI and BASFI scores, age, and sex as predictors. The coefficients for the regression equation were taken from the MEASURE 1 and MEASURE 2 pooled trial data (Table S7).
Three types of mortality risks were included in the analysis. Patients with AS have higher mortality rates compared with the general population. Disease-specific mortality was included as relative risks to account for patients having mortality vs the general population29 (Table S8). In addition, the increased risk of mortality due to AEs, such as serious infection or tuberculosis and malignancy, was obtained from the literature30 (Table S8). All-cause age-related mortality was estimated using National Life Tables for Finland (Statistics Finland, 2015).31
Base-case analysis
The primary effectiveness outcome was QALY. Total costs and QALYs were estimated for all treatments. First, it was checked if secukinumab is a dominant treatment option (having higher QALYs at a lower cost vs comparators). In case of nondominance, an incremental cost-effectiveness ratio was reported.32
The primary analysis was conducted in biologic-naïve population for 60 years of time horizon (lifetime) to account for the chronic nature of the disease. BASFI rebound was assumed to occur according to “natural history” for patients who discontinue biologic therapy. Annual discount rates were applied at 3% for both costs and outcomes, from the second year onward. The base-case analysis was done from a Finnish payer perspective; hence, only direct costs were included.
Sensitivity analysis
Three different sensitivity analyses (probabilistic, deterministic, and alternative scenario analyses) assessed the impact of changes in the input parameters on outcomes.
In the one-way sensitivity analysis, one parameter is varied at a time using lower and upper bounds of 95% CI, and their effects on results were estimated. Inputs and distribution used for each parameter in base-case population are shown in Table S9.
A probabilistic sensitivity analysis evaluated the impact of simultaneous variation in clinical outcome and resource utilization parameters on the model results. The results of the probabilistic sensitivity analysis were presented using cost-effectiveness acceptability curves calculated from the net monetary benefit statistic across a wide range of willingness-to-pay (WTP) thresholds. Also, it allows for the comparison of all treatment regimens simultaneously for each scenario.
Various alternative scenarios were analyzed using following alternative settings or input values: mixed population (biologic-naïve and experienced), alternative BASFI rebound assumption, AE disutilities, utilities sourced from McLeod et al,33 inclusion of indirect costs, and exclusion of disease-specific costs.
Results
Base-case results
Secukinumab 150 mg was compared with the other biologics to assess its cost-effectiveness in the biologic-naïve population. Patients treated with secukinumab 150 mg achieved highest expected QALYs of 13.1 at lowest expected total cost (€279,872) in comparison with all other comparators when considering 60 years of time horizon (Table 5). In addition, patients treated with secukinumab had better treatment outcomes; they spent more time (27.9 years) in the BASDAI 50 response state than patients in other biologics. Time spent in the BASDAI 50 response state corresponds to the time patients spend on maintenance treatment after exhibiting treatment response.
Apart from total costs, disaggregated cost components (drug costs, administration costs, disease-related costs, and AE-related costs) that contribute to the total costs for each treatment are also presented in supplementary materials (Table S10). Post-discontinuation costs associated with drug, disease, administration, and monitoring were much lower for patients who started on secukinumab vs other biologics, leading to the lower total costs for secukinumab when compared with other biologics.
Overall, secukinumab was less costly and more effective than the comparators and presented itself to be a dominant treatment for biologic-naïve patients with AS in Finland. The QALYs gained and corresponding total direct costs of other biologic treatment options considered are presented in Table 5.
Sensitivity analysis
One-way sensitivity analysis
In one-way sensitivity analysis, BASDAI 50 for secukinumab, baseline BASFI score for nonresponders across all drugs, change in BASDAI and BASFI scores in responders, and discount rates had the highest impact on incremental NMB across all biologics at WTP of €30,000 (Figure S1).
Probabilistic sensitivity analyses
In the probabilistic sensitivity analysis, secukinumab 150 mg had the highest probability of being cost-effective vs other comparators at different WTP thresholds in the biologic-naïve population (Figure 2). The probability of secukinumab being cost-effective was 98% at WTP levels of €20,000 per QALY gain. Additionally, secukinumab had 95%, 87%, and 75% probability of being cost-effective at other WTP thresholds of €30,000, €50,000, and €100,000 per QALY gained, respectively (Figure 2). The means of the total QALYs, total cost, and NMB are summarized in supplementary materials (Table S11). Comparing the means of costs and QALYs from probabilistic sensitivity analysis with base-case results showed minimal difference, indicating the robustness of the results and lower uncertainty in the parameters.
Scenario analyses
Results for the following scenarios are presented: overall population including a mix of biologic-naïve and experienced AS patients, alternative BASFI rebound assumption, an analysis with AE disutilities, an analysis assuming utilities sourced from McLeod et al, indirect costs, and disease-specific costs (Table 6).
  | Table 6 Alternative scenario analyses: cost-effectiveness results for secukinumab vs other biologics by varying base-case assumptions Notes: aSimilar to base-case results, means secukinumab 150 mg dominates all its comparator with higher QALYs and lower costs. bBASFI can be assumed to deteriorate in two ways: 1) rebound equal to gain: BASFI deteriorates by the same amount by which it improved when they responded to therapy; 2) rebound back to natural history: BASFI deteriorates to the level and subsequent trajectory it would have been had they not initially responded to therapy. Detailed results for all scenarios are available in supplementary materials (Tables S12–S17). Abbreviations: BASFI, Bath Ankylosing Spondylitis Functional Index; QALYs, quality-adjusted life years. |
In one of the alternative scenario analysis done on mixed population (naïve and experienced biologic users), secukinumab 150 mg dominated all its comparators with higher QALYs and lower costs. Similarly, in other alternative scenarios, except for the scenario including indirect cost, it was also observed that secukinumab dominated all its comparators with higher QALYs and lower costs (Table 6). In the scenario when indirect cost was included, secukinumab dominated all branded biologics and was cost-effective against biosimilars. Detailed results regarding alternative scenarios are available in supplementary materials (Tables S12–S17).
Discussion
Secukinumab 150 mg dominated all its currently licensed comparators (s.c. treatments adalimumab and its biosimilar, certolizumab pegol, etanercept and its biosimilar, golimumab, and i.v. infliximab) in the treatment of biologic-naïve AS patients in Finland.
The analysis was carried out from the perspective of the Finnish health care system. Secukinumab 150 mg had better health outcomes (calculated in terms of QALYs) at lowest total cost, which could be beneficial to the patient and health care system as a whole. Model uses multiple parameters like BASDAI 50, change in BASDAI, change in BASFI, discontinuation rates, utility values, and safety, and these parameters have different impact on the final results. Particularly, factors such as AEs and discontinuation rates have a strong impact on the lifetime QALYs, leading to higher QALYs for secukinumab vs other comparators over 60 years of time horizon. The robustness of the analyses was confirmed by one-way sensitivity analyses, probabilistic analyses, and scenario analyses.
This is among the first full-length studies assessing cost-effectiveness of secukinumab 150 mg compared with other biologics. However, other modeling studies have been conducted in different countries such as Canada,34 UK,35,36 Turkey,37 Colombia,38 Bulgaria,39 and Russia;40 which reported similar results. All these studies reported secukinumab to be dominant (more effective and less costly) over anti-TNFs in biologic-naïve as well as biologic-experienced AS population. Across multiple studies, secukinumab resulted in an additional 0.20–0.86 QALY gain at a total cost, which was €4,644 to €9,134 lower compared with other biologics.34,37,38 The current study is also the first to assess cost-effectiveness of secukinumab in a Finnish context. Thus, these findings add important evidence to support the use of secukinumab while making a comparison of this new class of biologic to anti-TNF agents.
Strengths and limitations
The cost-effectiveness model structure and many of its assumptions were based on models used in previous assessments of biologics in the treatment of AS. In the absence of direct comparative evidence from clinical trials, the relative efficacy of licensed treatments for AS was evaluated through a mixed treatment comparison using NMA, which is considered as standard modeling methodology in the field. We used a lifetime horizon (60 years) for base-case analysis, which is more appropriate to use for chronic disease like AS. Moreover, analyses included detailed cost details (such as costs for drug acquisition, hospitalization, AEs) to estimate economic burden of disease. We also established the robustness of our results through probabilistic and deterministic sensitivity analysis.
The current analysis has several limitations. The major limitations of this analysis may be related to the scarcity of data and the complexity coupled with the long-term duration of the disease. There might be a potential underestimation of long-term efficacy of secukinumab 150 mg as the effectiveness data are derived from NMA (with data up to week 16). However, an extension of MEASURE trial has established the significant clinical efficacy of secukinumab for >3 years in AS patients.41 In addition, secukinumab has shown statistically significant improvement in efficacy (in terms of ASAS20 and 40 responses) in comparison with adalimumab in MAIC for up to 52 weeks.18
Another limitation of this study is the lack of data in all subpopulations for available comparators and the lack of efficacy data for subsequent biologic treatment after withdrawal from first-line biologic. Thus, data for comparators that did not report all necessary subpopulations or outcomes were set to those of the average of other biologics in the NMA. Although a country-specific (Finland) cost-effectiveness model was built, the globally conducted clinical trials were used as a basis for efficacy parameters, which could be considered as one of the limitations of the present analysis.
The subsequent-line biologic treatment was modeled using average values of the efficacy, costs, and AE incidence rates across all biologics in the model. Patients with AS are reported to have a significant impairment in work productivity.42–44 The base-case analysis did not take into account any indirect costs (mainly in the form of productivity loss) associated with AS; however, indirect cost was included as a part of the alternative scenario analysis. In the real-world clinical practice, patients having disease remission or low disease activity can be managed by tapering the dose of biologics (by reducing the dose or increasing the interval between dosing frequency) to reduce the cost of treatment.45–49 However, for secukinumab, such dose tapering is not recommended (not part of drug label). We would need robust long-term clinical effectiveness data for all biologic treatments (including secukinumab) from real-world settings to enable comparison based on dose tapering. Once such data become available for all biologics, it can be included in the cost-effectiveness analyses. Currently, analysis considered drug withdrawal rates from clinical trials. In future, this analysis can also be rerun using drug withdrawal rates from long-term registries once such data for secukinumab become available.
Conclusion
Secukinumab 150 mg dominated all other biologics (more QALYs at lower costs) in the biologic-naïve population in Finland for the treatment of AS over a lifetime horizon. This analysis implied that secukinumab is the most cost-effective biologic option for the treatment of AS patients unresponsive to conventional therapy in Finland.
Acknowledgments
The authors thank Kavita Rodha and Niraj Modi (Novartis Healthcare Private Limited, Hyderabad, India) for editorial writing support. This study was funded by Novartis Pharma AG, Basel, Switzerland.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
TP is a full-time employee of Novartis Finland Oy, Espoo, Finland. KP receives honoraria from Abbvie, Bristol-Myers Squibb, Lilly, MSD, Novartis, Pfizer, Roche, Sandoz, and UCB; and congress trips from Abbvie and Pfizer outside the submitted work. DM and PG are full-time employees of Novartis Product Lifecycle Services-NBS, Novartis Healthcare Private Limited, Hyderabad, India. JM is a founding partner of ESiOR Oy, which provides health economic, outcome research, and market access services for pharmaceutical and medical companies, as well as hospitals and other health and social care providers. The authors report no other conflicts of interest in this work.
References
Spondylitis Association of America – Overview of Ankylosing Spondylitis. Available from: https://www.spondylitis.org/Ankylosing-Spondylitis. Accessed January 4, 2018. | ||
Schett G, Lories RJ, D’Agostino MA, et al. Enthesitis: from pathophysiology to treatment. Nat Rev Rheumatol. 2017;13(12):731–741. | ||
Wenker KJ, Quint JM. Ankylosing Spondylitis. Treasure Island (FL): StatPearls Publishing LLC; 2018. | ||
Rantalaiho V, Kautiainen H, Virta L, Puolakka K. FRI0560 the increase in the nationwide incidence of inflammatory rheumatic diseases in Finland during this millennium. Ann Rheum Dis. 2016;75(Suppl 2):643–644. | ||
Boonen A, van der Linden SM. The burden of ankylosing spondylitis. J Rheumatol Suppl. 2006;78:4–11. | ||
Blanch C, Comellas M, Prada C, Lizan L. Economic burden of ankylosing spondylitis in Europe. A systematic review of the literature. Value Health. 2016;19(7):A541–A542. | ||
van der Heijde D, Ramiro S, Landewé R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76(6):978–991. | ||
Braun J, Baraliakos X, Kiltz U. Secukinumab (AIN457) in the treatment of ankylosing spondylitis. Expert Opin Biol Ther. 2016;16(5):711–722. | ||
Busquets-Perez N, Marzo-Ortega H, Emery P. Emerging drugs for axial spondyloarthritis including ankylosing spondylitis. Expert Opin Emerg Drugs. 2013;18(1):71–86. | ||
Palazzi C, D’Angelo S, Gilio M, Leccese P, Padula A, Olivieri I. Pharmacological therapy of spondyloarthritis. Expert Opin Pharmacother. 2015;16(10):1495–1504. | ||
The Social Insurance Institution of Finland. Special reimbursement (281). Available from: https://www.kela.fi/laake281. Accessed April 24, 2018. | ||
Dougados M, Baeten D. Spondyloarthritis. Lancet. 2011;377(9783):2127–2137. | ||
NOVARTIS. Novartis receives two landmark European approvals for Cosentyx to treat patients with ankylosing spondylitis and psoriatic arthritis 2015. Available from: https://www.myscience.org/news/wire/novartis_receives_two_landmark_european_approvals_for_cosentyx_treat_patients_with_ankylosing_spond-2015-novartis. Accessed January 31, 2019 | ||
Braun J, Baraliakos X, Deodhar A, et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III measure 1 study. Ann Rheum Dis. 2017;76(6):1070–1077. | ||
Sieper J, Deodhar A, Marzo-Ortega H, et al. Secukinumab efficacy in anti-TNF-naive and anti-TNF-experienced subjects with active ankylosing spondylitis: results from the measure 2 study. Ann Rheum Dis. 2017;76(3):571–592. | ||
Marzo-Ortega H, Sieper J, Kivitz A, et al. Secukinumab and sustained improvement in signs and symptoms of patients with active ankylosing spondylitis through two years: results from a phase III study. Arthritis Care Res (Hoboken). 2017;69(7):1020–1029. | ||
Deodhar AA, Baeten D, Sieper J, Porter B, Richards H, Widmer A. Safety and tolerability of secukinumab in patients with active ankylosing spondylitis: pooled safety analysis of two phase 3, randomized, controlled trials. Arthritis Rheum. 2015;67:3478–3479. | ||
Maksymowych WP, Strand V, Nash P, et al. Comparative effectiveness of secukinumab and adalimumab in ankylosing spondylitis as assessed by matching-adjusted indirect comparison: an analysis based on all pivotal phase 3 clinical trial data. Arthritis Rheum. 2016;68 (Suppl 10). | ||
Lee YH, Song GG. Comparative efficacy and safety of secukinumab and adalimumab in patients with active ankylosing spondylitis: a Bayesian network meta-analysis of randomized controlled trials. J Rheum Dis. 2017;24(4):211–219. | ||
European Medicines Agency. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/cosentyx. Accessed January 31, 2019. | ||
Corbett M, Soares M, Jhuti G, et al. Tumour necrosis factor-α inhibitors for ankylosing spondylitis and non-radiographic axial spondyloarthritis: a systematic review and economic evaluation. Health Technol Assess. 2016;20(9):1–334. | ||
Baeten D, Mease P, Strand V, et al. SAT0390 secukinumab for the treatment of ankylosing spondylitis: comparative effectiveness results versus currently licensed biologics from a network meta-analysis. Ann Rheum Dis. 2016;75(Suppl 2):809.2–80810. | ||
Eder L, Chandran V, Shen H, Cook RJ, Gladman DD. Is ASDAS better than BASDAI as a measure of disease activity in axial psoriatic arthritis? Ann Rheum Dis. 2010;69(12):2160–2164. | ||
Keat A, Barkham N, Bhalla A, et al. BSR guidelines for prescribing TNF-alpha blockers in adults with ankylosing spondylitis. Report of a working Party of the British Society for Rheumatology. Rheumatology (Oxford). 2005;44(7):939–947. | ||
NICE. Golimumab for the treatment of ankylosing spondylitis. NICE Technology Appraisal 233. 2011; Available from: https://www.nice.org.uk/guidance/ta233. Accessed July 2017. | ||
NICE. TNF-alpha inhibitors for ankylosing spondylitis and non-radiographic axial spondyloarthritis: NICE technology appraisal; 2016. Available from: https://www.nice.org.uk/guidance/TA383. Accessed July 2017. | ||
Kustannus Oy Duodecim. Medicinal products and prices database. Available from: https://www.duodecim.fi/duodecim/. Accessed February 1, 2018. | ||
Corbett M, Soares M, Jhunti G, et al. TNF-alpha inhibitors for ankylosing spondylitis and axial spondyloarthritis without radiographic evidence of ankylosing spondylitis (including a review of technology appraisal 143 and technology appraisal 233) CRD/CHE Technology Assessment Group (Centre for Review and Dissemination/Centre for Health Economics) University of York. December 2014. Available from: https://www.nice.org.uk/guidance/ta383/documents/ankylosing-spondylitis-and-axial-spondyloarthritis-nonradiographic-adalimumab-etanercept-infliximab-and-golimumab-inc-rev-ta143-and-ta233-id694-appraisal-consultation-document2. Accessed February 7, 2018. | ||
Bakland G, Gran JT, Nossent JC. Increased mortality in ankylosing spondylitis is related to disease activity. Ann Rheum Dis. 2011;70(11):1921–1925. | ||
Abuabara K, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the UK. Br J Dermatol. 2010;163(3):586–592. | ||
Statistics Finland. Risk of dying during a year by age and sex (year 2015). Available from: http://www.stat.fi/index_en.html. Accessed August 15, 2017. | ||
Garrido-Castro JL, Medina-Carnicer R, Schiottis R, Galisteo AM, Collantes-Estevez E, Gonzalez-Navas C. Assessment of spinal mobility in ankylosing spondylitis using a video-based motion capture system. Man Ther. 2012;17(5):422–426. | ||
McLeod C, Bagust A, Boland A, et al. Adalimumab, etanercept and infliximab for the treatment of ankylosing spondylitis: a systematic review and economic evaluation. Health Technol Assess. 2007;11(28):1–158, iii–iv. | ||
Goeree R, Chiva-Razavi S, Gunda P, Jain M, Jugl SM. Cost-effectiveness analysis of secukinumab in ankylosing spondylitis from the Canadian perspective. J Med Econ. 2019;22(1):45–52. | ||
Marzo-Ortega H, Halliday A, Jugl S, et al. The cost-effectiveness of secukinumab versus tumour necrosis factor α inhibitor biosimilars FOR ankylosing spondylitis in the UK. Rheumatology. 2017;56(suppl_2):kex062.108. | ||
Emery P, van Keep M, Beard S, et al. Cost effectiveness of secukinumab for the treatment of active ankylosing spondylitis in the UK. Pharmacoeconomics. 2018;36(8):1015–1027. | ||
Sarioz F, Ozdemir O, Direk S, Cavusoglu Sezen S, Barutcugil M. Secukinumab is dominant vs. TNF-inhibitors in the treatment of active ankylosing spondylitis: results from a Turkish cost-effectiveness model. Value Health. 2017;20(9):A534. | ||
Romero Prada ME, Roa Cardenas NC, Serrano GY, Huerfano LM. Cost-utility analysis of secukinumab use versus TNF-α inhibitors, in patients with ankylosing spondilytis. Value Health. 2017;20(9):A938–A939. | ||
Djambazov S, Vekov T. Incremental cost-effectiveness analysis of biological drug therapies for the treatment of ankylosing spondylitis in Bulgaria, 2016. ISPOR 22nd Annual International Meeting; May 2017; Boston, MA, USA. | ||
Fedyaev D, Derkach EV. Cost-effectiveness analysis of different biologic agents for ankylosing spondylitis treatment in Russia. Value Health. 2016;19(7):A539. | ||
Baraliakos X, Kivitz AJ, Deodhar AA, et al. Long-term effects of interleukin-17A inhibition with secukinumab in active ankylosing spondylitis: 3-year efficacy and safety results from an extension of the phase 3 measure 1 trial. Clin Exp Rheumatol. 2018;36(1):50–55. | ||
Haglund E, Bremander A, Bergman S, Jacobsson LT, Petersson IF. Work productivity in a population-based cohort of patients with spondyloarthritis. Rheumatology (Oxford). 2013;52(9):1708–1714. | ||
Ramonda R, Marchesoni A, Carletto A, et al. Patient-reported impact of spondyloarthritis on work disability and working life: the Atlantis survey. Arthritis Res Ther. 2016;18(1):78. | ||
de Hooge M, Ramonda R, Lorenzin M, et al. Work productivity is associated with disease activity and functional ability in Italian patients with early axial spondyloarthritis: an observational study from the space cohort. Arthritis Res Ther. 2016;18(1):265. | ||
Edwards CJ, Fautrel B, Schulze-Koops H, Huizinga TWJ, Kruger K. Dosing down with biologic therapies: a systematic review and clinicians’ perspective. Rheumatology (Oxford). 2017;56(11):1847–1856. | ||
Lorenzin M, Ortolan A, de Hooge M, et al. Lengthening the time intervals between doses of biological agents in psoriatic arthritis patients: a single-center retrospective study. Int J Immunopathol Pharmacol. 2015;28(4):479–487. | ||
Arends S, van der Veer E, Kamps FB, et al. Patient-tailored dose reduction of TNF-alpha blocking agents in ankylosing spondylitis patients with stable low disease activity in daily clinical practice. Clin Exp Rheumatol. 2015;33(2):174–180. | ||
Závada J, Uher M, Sisol K, et al. A tailored approach to reduce dose of anti-TNF drugs may be equally effective, but substantially less costly than standard dosing in patients with ankylosing spondylitis over 1 year: a propensity score-matched cohort study. Ann Rheum Dis. 2016;75(1):96–102. | ||
De Stefano R, Frati E, de Quattro D, Menza L, Manganelli S. Low doses of etanercept can be effective to maintain remission in ankylosing spondylitis patients. Clin Rhfeumatol. 2014;33(5):707–711. | ||
Ramiro S, Stolwijk C, van Tubergen A, et al. Evolution of radiographic damage in ankylosing spondylitis: a 12 year prospective follow-up of the OASIS study. Ann Rheum Dis. 2015;74(1):52–59. | ||
Soini EJ, Leussu M, Hallinen T. Administration costs of intravenous biologic drugs for rheumatoid arthritis. Springerplus. 2013;2(1):531. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


