Back to Journals » ClinicoEconomics and Outcomes Research » Volume 15
Cost-Effectiveness of Humidified High-Flow Therapy (HHFT) for COPD Patients on Long-Term Oxygen Therapy
Authors Groessl EJ , Tally SR, Hillery N
Received 8 December 2022
Accepted for publication 4 March 2023
Published 5 April 2023 Volume 2023:15 Pages 239—250
DOI https://doi.org/10.2147/CEOR.S400739
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Giorgio Colombo
Erik J Groessl,1,2 Steven R Tally,1 Naomi Hillery1
1Health Services Research Center, Herbert Wertheim School of Public Health and Human Longevity Science, University of California, San Diego, CA, USA; 2VA San Diego Healthcare System, San Diego, CA, USA
Correspondence: Erik J Groessl, University of California San Diego, Health Services Research Center, 9500 Gilman Drive, Mail Code 0994, San Diego, CA, 92037, USA, Tel +1 858 622 1771, Email [email protected]
Purpose: Chronic obstructive pulmonary disease (COPD) is the third leading cause of mortality, and is associated with significant respiratory impairment, decreased quality of life, and high health care costs. Recent evidence indicates significant clinical benefit results from adding humidified high-flow therapy (HHFT) to standard long-term oxygen therapy (LTOT) as a home-based therapy in persons with severe COPD. The objective was to evaluate the cost-effectiveness of adding HHFT to standard treatment of COPD patients using LTOT with US healthcare cost estimates.
Patients and Methods: A Markov state-transition model was developed using data from a prospective clinical trial of adding HHFT to standard therapy for persons with severe COPD using LTOT. The analysis was conducted from the US health care system perspective using a 5-year time horizon and 3% discount rate. QALYs and downstream healthcare costs were modeled. One-way and probabilistic sensitivity analyses were used to examine the impact of input parameters on the incremental net monetary benefit (NMB).
Results: Incremental QALYs accrued were 0.058 (2.047 vs 1.989 QALYs for HHFT and standard therapy groups respectively). Incremental total costs were -$3939 ($47,516 vs $51,455 for HHFT and standard therapy groups respectively). Thus, HHFT was the dominant treatment in the analysis, resulting on both better health and lower total costs. Varying utility and cost inputs individually never resulted in NMB approaching 0. Probabilistic analyses indicate that HHFT is cost-effective in 84% of simulations.
Conclusion: Our results indicate that the reductions in acute exacerbations of COPD (AECOPDs) that result from adding HHFT for persons with COPD on LTOT will produce both health benefit (QALYs) and cost savings. Cost savings occur because the HHFT device costs are more than offset by reductions in costly COPD exacerbations. Health care systems and payors can benefit from wider implementation of HHFT with existing treatments.
Keywords: COPD, acute exacerbations, cost-effectiveness, humidified high flow therapy
Corrigendum for this paper has been published.
Introduction
Chronic obstructive pulmonary disease (COPD) is now the third leading cause of death worldwide, estimated to account for 6% of all deaths according to the World Health Organization.1 Patients with COPD experience persistent respiratory symptoms such as dyspnea, cough, and/or sputum production due to abnormalities within the airway or alveoli.2 At the population level, COPD is associated with a decreased quality of life, decreased life expectancy, and higher healthcare costs.2 In the US, healthcare costs for COPD were second only to asthma among respiratory conditions, with spending estimated at over $34 billion.3 Furthermore, despite the large known impact on morbidity and mortality, it is estimated that COPD is underdiagnosed, as many cases are only identified during or after a severe episode.4
COPD is a complex disease, influenced by a multitude of factors such as genetics, environment, comorbidities, and lifestyle risk factors.5 Because of this heterogeneity, COPD can be difficult to treat, particularly in advanced stages of the disease. COPD identification and progression is often more easily characterized by symptoms and exacerbations.5 Exacerbations are defined by The Global Initiative for Chronic Obstructive Lung Disease (GOLD) as episodes of “acute worsening of respiratory symptoms that result in additional therapy”.6 These episodes are categorized as either mild, moderate, or severe, depending on the requisite treatment to stabilize the patient.6 Severe COPD exacerbations occur less frequently as compared with mild or moderate, but can be debilitating and life-threatening, thus requiring hospitalization.
Well-established treatments for COPD include pharmacologic therapy such as inhaled steroids or long-acting bronchodilators and pulmonary rehabilitation. Patients with chronic and/or severe symptoms may require long-term oxygen therapy (LTOT) to address severe resting chronic hypoxemia. LTOT is an established therapy to prevent hypoxemia as recommended in numerous medical guidelines.7,8 Patients started on LTOT are usually at an advanced stage of disease and thus more susceptible to acute exacerbation of COPD (AECOPD). In turn those who experience AECOPD are at greater risk of future exacerbations, hospitalization, and death.9,10 LTOT has been shown to help improve survival rates and improve quality of life for these high risk individuals.2
Several studies have shown that respiration in COPD with LTOT may be improved as part of home-based self-care with the addition of humidified high-flow therapy (HHFT) device due in part to increased mucus clearing and reduced resistance in respiration.11–14 HHFT devices produce warm, humidified respiratory gases with or without supplemental oxygen to the patient at a desired flow rate.15 Currently, HHFT is an established therapy in acute and critical care healthcare settings and is used mostly for treating acute hypoxemic respiratory failure. However, recent evidence indicates there may be additional long-term benefits of home-based HHFT for reducing the frequency of adverse symptoms and exacerbations in patients with advanced COPD.16,17 A recent prospective randomized controlled trial in people with COPD on LTOT compared standard treatment following GOLD guidelines to standard care plus HHFT.17 In this trial, 200 patients with COPD with chronic hypoxemic respiratory failure from four healthcare centers were randomized to one of the two groups and followed for one year. Data analyzed included total exacerbations and hospital admissions before and during the study as well as modified Medical Research Council (mMRC) scores, the St George’s Respiratory Questionnaire (SGRQ), forced expiratory volume in 1 second (FEV1), 6-minute walk test (6MWT) and arterial carbon dioxide (PaCO2). The study found that participants who used myAirvo™ Humidified High Flow Therapy (Fisher & Paykel Healthcare, Auckland, New Zealand) in addition to standard therapy had reduced episodes of AECOPD, reduced hospital admissions, and improvement in COPD symptoms. Given the solid results of this clinical trial and prior data supporting the benefits of HHFT, an important next step is further evaluation of the cost-effectiveness of this therapy.
The cost-effectiveness of HHFT has been evaluated in two prior studies. In 2014, Milne et al18 evaluated the cost-effectiveness of HHFT for persons with moderate or severe COPD, or bronchiectasis based on data from an initial trial published in 2010.16 The analysis examined health care costs from the New Zealand health care system perspective, and found that HHFT was a cost-effective treatment. A second cost-effectiveness analysis (CEA) of HHFT was based on a larger, more recent trial of HHFT for people with severe COPD using LTOT.17 That CEA used Danish health care costs and found an even more favorable incremental cost-effectiveness ratio (ICER) of about £3605 per QALY gained.
Although both of the prior analyses noted above appear scientifically rigorous, there are major differences between the US healthcare system and the Danish or New Zealand systems that make it important to examine the cost-effectiveness of HHFT devices from the US healthcare perspective.19 In the US healthcare system, high volumes and cost of care lead to high total health care costs, making system-specific cost-effectiveness information especially salient.20 Thus, the present study evaluated the incremental cost-effectiveness of adding HHFT for the treatment of COPD patients using LTOT with US healthcare cost estimates.
Methods
Study Design
A Markov model was developed to estimate the ICER associated with adding HHFT to standard therapy for advanced COPD in persons already using LTOT. Clinical effectiveness data were derived from a randomized clinical trial by Storgaard et al, 201817 which evaluated the effects of adding myAirvo™ Humidified High Flow Therapy (Fisher & Paykel Healthcare, Auckland, New Zealand) to LTOT. US healthcare cost estimates were applied to exacerbation events. Utilities were estimated using methods applied in previously published CEAs for COPD. The analysis was conducted from the US health care system perspective with costs adjusted for inflation to year 2021 US dollars. Research methods and analyses were aligned with recommendations of both the Second Panel on Cost-Effectiveness in Health and Medicine,21,22 and the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement.23 The study used only de-identified published data or publicly available estimates.
Model Structure
Although detailed disease-state models have been proposed for COPD,24,25 not all of the variables and parameters required for disease-state transition models are publicly available from some studies. In lieu of this data, some recent models have focused on rates of acute exacerbations as discrete events26,27 for which costs and health utilities can be more readily estimated. As noted above, AECOPDs are linked to lower quality of life, higher rates of hospitalization, and increased mortality.9,10 Thus, using available date on as COPD exacerbations, we modeled four health event/state options (no exacerbations, moderate exacerbation, severe exacerbations, or death) as discrete events for each cycle for hypothetical COPD patients on LTOT (or cohort of patients) as shown in Figure 1. A similar model structure has been used previously.27 When modeling discrete events, transition rates are assumed to be zero and monthly probabilities total to the count of annual events found in the clinical trial of interest.17 A 5-year time horizon was selected for the model with monthly Markov cycles. Monthly event rates, costs, and utilities were entered for each outcome option and QALYs and costs were summed over the 60 monthly cycles. The 5-year time horizon was chosen to align with the manufacturer’s 5-year estimated lifespan of the HHFT device and is consistent with recommended modeling practices.28 Longer time horizons appear of limited added value because of the high mortality rate in persons with COPD using LTOT. Costs and QALYs accumulated in months 13 to 60 were discounted at an annual rate of 3% following recommendations by Neumann et al.29 Device usage rates from the Storgaard trial were used to adjust device costs beginning in month 2. Because the trial exacerbation outcomes were obtained despite a device discontinuation rate of 14% in month 1, and 22% after 12 months, the model assumed a decelerating but ongoing decline in device usage. This is consistent with evidence of low burden of usage, improved quality of life17 and data from the use of similar devices.30 A traditional half-cycle correction was used. TreeAge Pro Healthcare 2021 software (https://www.treeage.com/) was used to conduct the analysis.
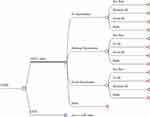 |
Figure 1 Markov model of event outcomes for HHFT + LTOT vs standard LTOT therapy. |
Exacerbation and Mortality Rates
Rates of moderate and severe exacerbations were obtained from a published clinical trial (n=200).17 The study was conducted at four outpatient clinics in Denmark and data presented in Table 1. Patients had all been diagnosed with COPD with chronic hypoxemic respiratory failure and prescribed LTOT at least three months prior to study initiation. All participants were receiving care following GOLD recommendations.6 Patients were randomized to receive either standard care according to GOLD recommendations (including LTOT) or the same recommended care plus HHFT. The primary study endpoints were AECOPD rates measured over the 12-month study period. In the LTOT + HHFT group, 86% of participants used HHFT throughout the 12 months for at least 78% of days (286 out of 365) and a mean of 7 hours per day. The study also reported that 14% of HHFT patient discontinued the device after 1 month and another 8% discontinued by the end of 12 months. These data were used to estimate a declining rate of device discontinuation in future years with adjustments to device costs and exacerbation rate differences. Total AECOPD rates for the HHFT group were significantly lower compared to the control group (3.12 vs 4.95 respectively; p < 0.001). When modeling usage of HHFT as a continuous measure, HHFT use was also associated with a significantly lower rate of hospital admissions (p < 0.001). For the model, hospital admissions were considered severe exacerbations, and all other exacerbations were considered moderate. Our analysis used rates of hospital admissions for all randomized participants following intent to treat principles. Baseline rates of exacerbations from the prior year were not considered in order to take a more conservative approach given higher baseline exacerbation rates in the HHFT arm.
 |
Table 1 Model Inputs |
Mortality rates observed in the Storgaard study were not significantly different between the study groups, and given the strong evidence showing that higher rates of exacerbations are associated with higher mortality, the model assumed a 12% annual mortality rate in both groups.17 This rate is very similar to the 11% used in a previous study.27
Health Utilities
Utility values for persons with COPD who experience exacerbations were derived from previous scientific studies. With our analysis based on a US healthcare system perspective, we estimated the utility of our sample to be 0.630 when no exacerbations were present using estimates from the National Health and Wellness Survey (NHWS),31 which are similar to recent Canadian estimates.32,33 Following conventions based on previous disease and cost-effectiveness modeling for COPD,34 and those used in other studies,35,36 utility decrements of 15% for moderate exacerbations and 50% for severe exacerbations resulted in monthly utility values of 0.045 and 0.026 as shown in Table 1. This translates to a monthly decrement of 0.008 and 0.027 for moderate and severe exacerbations, respectively. When compared to a similar analysis by Thanh et al27 that also used methods proposed by Samyshkin,37 the monthly decrements we use are smaller because the base value is considerably lower. Thus, these values appear conservative and appropriate for the population of interest.
Cost Inputs
A total estimated US cost of $7800 for the HHFT device and consumable accessories was provided by the device manufacturer.38 The total cost was amortized over an estimated device lifespan of 5 years or 60 months. Given the relatively high mortality rates in this population, we estimated the device to be in use for 50 of the 60 months, allowing 2 months per year or 10 months total for cleaning and reallocation, resulting in a $156 monthly cost (see Table 1). The initial physician specialist visit and consultation with a respiratory therapist were estimated to be $30039 and $10040 respectively, for a total initial visit cost of $400. The value for the respiratory therapist included salary, benefits, and overhead.
Increased health care costs associated with moderate and severe exacerbations were derived from a US study of costs associated with COPD exacerbations in emergency department (ED) and inpatient settings.41 The study reported mean ED and hospital admission costs from a large hospital administrative database. As shown in Table 1, mean ED costs were used as the input for moderate exacerbation costs and the mean across multiple types of inpatient admissions was used as the input for severe exacerbations. All costs have been adjusted and expressed in terms of 2020 US dollars. After inflationary adjustments, these estimates were found to be comparable to the estimates used in a recent study by Thanh et al when adjusting for different currency.27
Sensitivity Analyses
Univariate, one-way sensitivity analyses were performed using TreeAge software. Following methods used previously,26,27 exacerbation rates, device and initial visit costs, and utility estimates were varied 20% in either direction to examine the sensitivity of results to estimated model inputs. Given higher variability in health care costs, we varied healthcare costs for moderate and severe exacerbations 50% in each direction. Sensitivity analyses display model output in units of incremental net monetary benefit (NMB), which integrates QALYs and the willingness-to-pay (WTP) function (expressed in terms of Cost/QALYs).21,42 The incremental NMB represents the amount of additional monetary benefit per person provided by the dominant strategy given a WTP of $50,000. A WTP threshold of $50,000 is the most commonly used threshold in cost-effectiveness analysis42,43 and is considered conservative since it has not been adjusted for inflation for many years.
Probabilistic sensitivity analysis was used to vary multiple input variables across their estimated distributions to examine their simultaneous impact on costs, QALYs, and NMB Consistent with similar analyses,27 gamma distributions were used for health care costs. Following methods used in recent health economic analyses,44 triangular distributions were used to vary utility values and exacerbation rates 20% in each direction.
Results
The main results of the Markov decision tree model are presented in Table 2. Over the course of the 5-year time horizon, the model estimated that 2.058 QALYs accumulated in the HHFT group while 1.996 QALYs accumulated in the standard LTOT group. The incremental difference of 0.062 QALYs is substantial given the 12% annual mortality rate in this cohort, and about half the cohort would be assumed to be deceased after 4 years. In addition to experiencing better health over time, the HHFT group also accrued lower total costs. Total costs from our 5-year model were $50,409 for the group receiving HHFT and $53,304 for those on standard LTOT therapy. This indicates that the costs associated with fewer moderate and severe exacerbations of COPD more than offset the device cost. A meaningful ICER cannot be calculated because the HHFT group was dominant, accruing better health at a lower cost.
 |
Table 2 Incremental Costs and QALYs of LTOT + HHFT to LTOT Alone |
One-way sensitivity analyses that examined the impact of individual model parameters on the incremental NMB suggest that estimates of utility values and the health care costs for different levels of exacerbation were associated with the most variability in results (See Figure 2). However, when varied in the analysis, none of the variables approached an NMB=0 at which conclusions would begin to change. To better understand the robustness and variability of our model results using probabilistic sensitivity analysis, multiple inputs including device-related costs, exacerbation rates, health care costs associated with exacerbations, and utility values for exacerbation were varied simultaneously along relevant distributions. In Figure 3, we show 5000 simulations drawn from parameter distributions. Per convention, incremental costs are displayed on the vertical axis and incremental effectiveness is displayed on the horizontal axis. Results indicate that 72.3% of simulated outcomes were positioned in the lower right quadrant, indicating lower cost and greater health benefit. Another 11.5% of simulations were in the upper right quadrant but were below the WTP threshold of $50,000/QALY. Thus, the probability that LTOT + HHFT is a cost-effective strategy compared with LTOT alone is 84% as reflected in Figure 4, the cost-effectiveness acceptability curve in which WTP is varied.
 |
Figure 2 One-way sensitivity analysis – tornado chart. |
 |
Figure 3 Scatterplot of probabilistic sensitivity analysis. |
 |
Figure 4 Cost-effectiveness acceptability curve. |
Discussion
Growing research evidence supports the clinical effectiveness of HHFT use in persons with COPD,16,17,45–47 especially in those with chronic hypoxemic respiratory failure using LTOT.17 HHFT provides warm humidified airflow to persons with COPD, which reduces irritation to the airway and has been shown to reduce acute exacerbations. Clinical trials indicate that persons with COPD on LTOT using HHFT had fewer AECOPD, better lung function, and improved quality of life than those not using HHFT. Rates of adherence to HHFT were high and qualitative data show that patients found HHFT useful and easy to use.14 Given the strong emerging evidence for HHFT use in COPD, we sought to examine the cost-effectiveness of HHFT for persons with severe COPD who were on LTOT.
Our results indicate that LTOT + HHFT was both more effective and ultimately less costly than standard LTOT therapy. Using data from a recent clinical trial of 200 people with COPD on LTOT, HHFT had a greater health benefit because significantly fewer acute exacerbations occurred. Acute exacerbations are established as a major driver of reduced quality of life, higher mortality, and high healthcare costs in this population.2,5 In addition to the added health benefit, participants assigned to HHFT had lower total costs because costs related to device use were more than offset by reductions in healthcare costs linked to fewer acute exacerbations.
Our findings are similar to those of two recently published health economic studies of HHFT for persons with severe COPD conducted from the Danish48 and New Zealand49 healthcare perspectives using the same trial data. The Danish analysis found that the incremental cost-effectiveness ratio for LTOT + HHFT compared to LTOT alone was £3605/ QALY.48 The sensitivity analysis for that study found a high probability (84–92%) of HHFT being cost-effective in the Danish healthcare system. The budget impact analysis from a New Zealand healthcare perspective did not include effectiveness data but found a sizable healthcare cost savings in New Zealand currency.49
In comparison to the Danish analysis, our current study found a similar gain in QALYs using different estimation methods and a different source of data from the same clinical trial. Sorensen et al48 used a published algorithm50 to convert scores on the SGRQ to EQ-5D scores and then QALYs. Our study estimated QALYs using health utility values associated with acute exacerbations following methods used by previous cost-effectiveness analyses of COPD treatments.26,27 Costs were also calculated differently between the two studies because of major differences in the Danish and US healthcare systems, and different time horizons were used. Thus, the much higher healthcare costs in the US associated with COPD exacerbations resulted in a larger cost savings than the Danish analysis and 84% probability of cost-effectiveness.
The analysis from the New Zealand health care perspective did not include effectiveness, only capital expenses and hospital costs. However, like the analysis presented here, they used a 5-year time horizon and reported a discounted cost savings of NZ$16,934, or about US$9900. Our results reflect a more modest cost savings of $3939. Despite higher per day costs for hospital days, the different results can likely be explained by two factors. First, two different methods were used to estimate costs in the New Zealand and US perspectives. A second important difference is that our current analysis used a much more conservative estimate of ongoing device usage. Our model assumed continued mortality of 12% per year. In addition, the trial data and exacerbation outcomes were achieved with a device discontinuation rate of 14% in month 1, and 22% after 12 months. Given this decelerating but ongoing decline in device usage, we assumed a small amount of further discontinuation each month beyond 22%, which limited the effects and costs savings in future years. This more conservative approach may better estimate what would happen in a clinical setting.
One other previous CEA of HHFT for COPD used a broader disease population (moderate and severe COPD or bronchiectasis) and found HHFT to be cost-effective from a New Zealand healthcare perspective.20 Our current results are similar but stronger, supporting HHFT as a therapy that can reduce costs and improve health. These improved results are not surprising because we focused on data from a more impaired population with higher rates of acute exacerbations. In addition, our data come from the larger more recent trial. In summary, all four economic analyses of HHFT for COPD provide converging evidence of cost-effectiveness or cost-savings. Thus, when multiple analyses using different methodologies produce similar results, confidence in the estimated values and conclusions further increases. Cost-savings and cost-effectiveness was found using different methods to estimate QALYs and cost, across different time horizons, across different healthcare perspectives, and in both broad and more specific disease populations.
Given the increasing incidence of COPD worldwide, the added recent burden on pulmonary function resulting from the COVID-19 pandemic, and the enormous costs associated with treating moderate and severe AECOPD, HHFT appears to offer a promising treatment that benefits a variety of COPD patients and is easily adopted for use.12,13 Our current study provides important additional data that demonstrates that health care systems and third party payers may also benefit from wider implementation and use of HHFT devices in home care settings, especially among persons with COPD who are using LTOT.
Limitations
One possible limitation of the study is that results are primarily based on exacerbation rate data from a single clinical trial. Ideally, more trials (preferably multi-site) of HHFT for persons with COPD using LTOT will be conducted, strengthening overall effectiveness data and providing more robust estimates of the clinical effects for cost-effectiveness analysis. However, other trials of HHFT or similar therapies have been conducted in either broader, or slightly different populations, all of which demonstrate clinical benefit.45,47,51,52
Another possible limitation is that our current study uses outcomes data from a study conducted in Denmark to estimate cost-effectiveness of the device from the US healthcare system perspective. Our cost estimates are tied to the cost of US healthcare, but it is also possible that persons from another country would have different health care utilization or experience and react to COPD exacerbations in a systematically different way. Although cost per unit of care is expected to be higher in the US, there is also evidence that the volume of care delivered is typically higher in the US.53 Higher utilization at outset could potentially lead to greater savings in the US based on our analysis, but that remains unknown until more outcomes data are obtained within the US healthcare system. However, there is no reason to suspect a different disease course between the two countries and both countries have modern healthcare systems.
Conclusion
With growing scientific evidence suggesting that HHFT devices are easy to use, provide significant clinical health benefit, and have minimal side effects, it is important to evaluate the economic cost of providing these incremental benefits over existing treatments. The results of our cost-effectiveness analysis indicate that substantial reductions in AECOPD in persons with COPD on LTOT will lead to accumulating health benefit (QALYs) and cost savings. The cost savings occur because the HHFT device costs are more than offset by reductions in moderate and severe exacerbations that are very costly. Health care systems and payors can benefit from wider implementation of adding HHFT to existing treatment.
Ethics Statement
This analysis and research is based on published aggregate data only and does not contain any protected health information.
Acknowledgments
Funding for this analysis was provided by Fisher & Paykel Healthcare, Inc.
Disclosure
Dr Erik J Groessl reports personal fees from Fisher Paykel Inc., during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. World Health Organization. Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2019. World Health Organization; 2020.
2. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Respirology. 2017;22(3):575–601. doi:10.1111/resp.13012
3. Duan KI, Birger M, Au DH, Spece LJ, Feemster LC, and Dieleman JL. US health care spending on respiratory diseases, 1996–2016. Am J Respir Crit Care Med. 2022. doi:10.1164/rccm.202202-0238LE
4. Martinez CH, Murray S, Barr RG, et al. Respiratory symptoms items from the COPD assessment test identify ever-smokers with preserved lung function at higher risk for poor respiratory outcomes. An analysis of the subpopulations and intermediate outcome measures in COPD study cohort. Ann Am Thorac Soc. 2017;14(5):636–642. doi:10.1513/AnnalsATS.201610-815OC
5. Vogelmeier CF, Roman-Rodriguez M, Singh D, Han MK, Rodriguez-Roisin R, and Ferguson GT. Goals of COPD treatment: focus on symptoms and exacerbations. Respir Med. 2020;166:105938. doi:10.1016/j.rmed.2020.105938
6. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of COPD. 2022 Report. Global Initiative for Chronic Obstructive Lung Disease; 2022.
7. The global strategy for prevention, diagnosis and management of COPD (updated 2020). The GOLD Report. 2020. Available from: www.goldcopd.org.
8. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American college of physicians, American college of chest physicians, American thoracic society, and European respiratory society. Ann Intern Med. 2011;155(3):179–191. doi:10.7326/0003-4819-155-3-201108020-00008
9. Cardoso J, Coelho R, Rocha C, Coelho C, Semedo L, and Bugalho Almeida A. Prediction of severe exacerbations and mortality in COPD: the role of exacerbation history and inspiratory capacity/total lung capacity ratio. Int J Chron Obstruct Pulmon Dis. 2018;13:1105–1113. doi:10.2147/COPD.S155848
10. Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370(9589):786–796. doi:10.1016/S0140-6736(07)61382-8
11. Hasani A, Chapman TH, McCool D, Smith RE, Dilworth JP, and Agnew JE. Domiciliary humidification improves lung mucociliary clearance in patients with bronchiectasis. Chron Respir Dis. 2008;5(2):81–86. doi:10.1177/1479972307087190
12. Nishimura M. High-flow nasal cannula oxygen therapy in adults: physiological benefits, indication, clinical benefits, and adverse effects. Respir Care. 2016;61(4):529–541. doi:10.4187/respcare.04577
13. Roca O, Hernandez G, Diaz-Lobato S, et al. Current evidence for the effectiveness of heated and humidified high flow nasal cannula supportive therapy in adult patients with respiratory failure. Crit Care. 2016;20(1):109. doi:10.1186/s13054-016-1263-z
14. Storgaard LH, Weinreich UM, Laursen BS. COPD patients’ experience of long-term domestic oxygen-enriched nasal high flow treatment: a qualitative study. COPD. 2020;17(2):175–183. doi:10.1080/15412555.2020.1736998
15. Pilcher J, Eastlake L, Richards M, et al. Physiological effects of titrated oxygen via nasal high-flow cannulae in COPD exacerbations: a randomized controlled cross-over trial. Respirology. 2017;22(6):1149–1155. doi:10.1111/resp.13050
16. Rea H, McAuley S, Jayaram L, et al. The clinical utility of long-term humidification therapy in chronic airway disease. Respir Med. 2010;104(4):525–533. doi:10.1016/j.rmed.2009.12.016
17. Storgaard LH, Hockey HU, Laursen BS, Weinreich UM. Long-term effects of oxygen-enriched high-flow nasal cannula treatment in COPD patients with chronic hypoxemic respiratory failure. Int J Chron Obstruct Pulmon Dis. 2018;13:1195–1205. doi:10.2147/COPD.S159666
18. Milne RJ, Hockey H, Rea H. Long-term air humidification therapy is cost-effective for patients with moderate or severe chronic obstructive pulmonary disease or bronchiectasis. Value Health. 2014;17(4):320–327. doi:10.1016/j.jval.2014.01.007
19. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319(10):1024–1039. doi:10.1001/jama.2018.1150
20. Anderlini D. The United States health care system is sick: from Adam Smith to overspecialization. Cureus. 2018;10(5):e2720. doi:10.7759/cureus.2720
21. Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG. Cost-Effectiveness in Health and Medicine. Oxford University Press; 2016.
22. Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103. doi:10.1001/jama.2016.12195
23. Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Int J Technol Assess Health Care. 2013;29(2):117–122. doi:10.1017/S0266462313000160
24. Briggs AH, Baker T, Risebrough NA, et al. Development of the galaxy chronic obstructive pulmonary disease (COPD) model using data from ECLIPSE: internal validation of a linked-equations cohort model. Med Decis Making. 2017;37(4):469–480. doi:10.1177/0272989X16653118
25. Risebrough NA, Briggs A, Baker TM, et al. Validating A model to predict disease progression outcomes in patients with COPD. Value Health. 2014;17(7):A560–A561. doi:10.1016/j.jval.2014.08.1852
26. Khoudigian-Sinani S, Kowal S, Suggett JA, Coppolo DP. Cost-effectiveness of the Aerobika* oscillating positive expiratory pressure device in the management of COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2017;12:3065–3073. doi:10.2147/COPD.S143334
27. Thanh NX, Jacobs P, Suggett J, McIvor A, Kaplan A. Cost-effectiveness of the Aerobika(R) oscillating positive expiratory pressure device in the management of chronic obstructive pulmonary disease exacerbations in Canada. Can Respir J. 2019;2019:9176504. doi:10.1155/2019/9176504
28. Caro JJ, Briggs AH, Siebert U, Kuntz KM; Force I-SMGRPT. Modeling good research practices--overview: a report of the ISPOR-SMDM modeling good research practices task force-1. Value Health. 2012;15(6):796–803. doi:10.1016/j.jval.2012.06.012
29. Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG. Cost-Effectiveness in Health and Medicine.
30. McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1108–1114. doi:10.1164/ajrccm.159.4.9807111
31. Dhamane AD, Witt EA, Su J. Associations between COPD severity and work productivity, health-related quality of life, and health care resource use: a cross-sectional analysis of national survey data. J Occup Environ Med. 2016;58(6):e191–e197. doi:10.1097/JOM.0000000000000735
32. Chandra K, Blackhouse G, McCurdy BR, et al. Cost-effectiveness of interventions for chronic obstructive pulmonary disease (COPD) using an Ontario policy model. Ont Health Technol Assess Ser. 2012;12(12):1–61.
33. Lacasse Y, Bernard S, Martin S, Boivin M, Maltais F. Utility scores in patients with oxygen-dependent COPD: a case-control study. COPD. 2015;12(5):510–515. doi:10.3109/15412555.2014.995290
34. Borg S, Ericsson A, Wedzicha J, et al. A computer simulation model of the natural history and economic impact of chronic obstructive pulmonary disease. Value Health. 2004;7(2):153–167. doi:10.1111/j.1524-4733.2004.72318.x
35. Oostenbrink JB, Rutten-van Molken MP, Monz BU, FitzGerald JM. Probabilistic Markov model to assess the cost-effectiveness of bronchodilator therapy in COPD patients in different countries. Value Health. 2005;8(1):32–46. doi:10.1111/j.1524-4733.2005.03086.x
36. Rajagopalan K, Bloudek L, Marvel J, Dembek C, Kavati A. Cost-effectiveness of twice-daily indacaterol/glycopyrrolate inhalation powder for the treatment of moderate to severe COPD in the US. Int J Chron Obstruct Pulmon Dis. 2018;13:3867–3877. doi:10.2147/COPD.S177097
37. Samyshkin Y, Schlunegger M, Haefliger S, Ledderhose S, Radford M. Cost-effectiveness of roflumilast in combination with bronchodilator therapies in patients with severe and very severe COPD in Switzerland. Int J Chron Obstruct Pulmon Dis. 2013;8:79–87. doi:10.2147/COPD.S37486
38. Fisher Paykel, Inc. Cost of MyAirvo HFNC Device - Personal Communication. Fisher Paykel, Inc; 2021.
39. Medical Expenditure Panel Survey - Insurance Component. Agency for healthcare research and quality; 2018. Available from: https://meps.ahrq.gov/mepstrends/hc_use/.
40. US Department of Labor. Bureau of labor statistics. Occupational Outlook Handbook; 2020. Available from: https://www.bls.gov/ooh/healthcare/respiratory-therapists.htm.
41. Dalal AA, Shah M, D’Souza AO, Rane P. Costs of COPD exacerbations in the emergency department and inpatient setting. Respir Med. 2011;105(3):454–460. doi:10.1016/j.rmed.2010.09.003
42. King JT Jr, Tsevat J, Lave JR, Roberts MS. Willingness to pay for a quality-adjusted life year: implications for societal health care resource allocation. Med Decis Making. 2005;25(6):667–677. doi:10.1177/0272989X05282640
43. Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797. doi:10.1056/NEJMp1405158
44. Tice JA, Kumar V, Otounye I, et al. Cognitive and Mind-Body Therapies for Chronic Low Back and Neck Pain: Effectiveness and Value. Institute for Clinical and Economic Review; 2017.
45. Jing G, Li J, Hao D, et al. Comparison of high flow nasal cannula with noninvasive ventilation in chronic obstructive pulmonary disease patients with hypercapnia in preventing postextubation respiratory failure: a pilot randomized controlled trial. Res Nurs Health. 2019;42(3):217–225. doi:10.1002/nur.21942
46. Storgaard LH, Hockey HU, Weinreich UM. Development in PaCO2 over 12 months in patients with COPD with persistent hypercapnic respiratory failure treated with high-flow nasal cannula-post-hoc analysis from a randomised controlled trial. BMJ Open Respir Res. 2020;7:1. doi:10.1136/bmjresp-2020-000712
47. Tan D, Walline JH, Ling B, et al. High-flow nasal cannula oxygen therapy versus non-invasive ventilation for chronic obstructive pulmonary disease patients after extubation: a multicenter, randomized controlled trial. Crit Care. 2020;24(1):489. doi:10.1186/s13054-020-03214-9
48. Sorensen SS, Storgaard LH, Weinreich UM. Cost-effectiveness of domiciliary high flow nasal cannula treatment in COPD patients with chronic respiratory failure. Clinicoecon Outcomes Res. 2021;13:553–564. doi:10.2147/CEOR.S312523
49. Milne RJ, Hockey HU, Garrett J. Hospital cost savings for sequential COPD patients receiving domiciliary nasal high flow therapy. Int J Chron Obstruct Pulmon Dis. 2022;17:1311–1322. doi:10.2147/COPD.S350267
50. Starkie HJ, Briggs AH, Chambers MG, Jones P. Predicting EQ-5D values using the SGRQ. Value Health. 2011;14(2):354–360. doi:10.1016/j.jval.2010.09.011
51. Chao KY, Liu WL, Nassef Y, Tseng CW, Wang JS. Effects of high-flow nasal cannula with oxygen on self-paced exercise performance in COPD: a randomized cross-over trial. Medicine. 2021;100(51):e28032. doi:10.1097/MD.0000000000028032
52. Piquilloud L, Olivier PY, Richard JC, et al. High flow nasal cannula improves breathing efficiency and ventilatory ratio in COPD patients recovering from an exacerbation. J Crit Care. 2022;69:154023. doi:10.1016/j.jcrc.2022.154023
53. Squires DA. Explaining high health care spending in the United States: an international comparison of supply, utilization, prices, and quality. Issue Brief. 2012;10:1–14.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
