Back to Journals » ClinicoEconomics and Outcomes Research » Volume 15
Cost-Effectiveness of Empagliflozin in Combination with Standard Care versus Standard Care Only in the Treatment of Heart Failure Patients in Finland
Authors Hallinen T, Kivelä S, Soini E , Harjola VP, Pesonen M
Received 10 October 2022
Accepted for publication 16 December 2022
Published 6 January 2023 Volume 2023:15 Pages 1—13
DOI https://doi.org/10.2147/CEOR.S391455
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Dean Smith
Taru Hallinen,1 Santtu Kivelä,1 Erkki Soini,1 Veli-Pekka Harjola,2 Mari Pesonen3
1ESiOR Oy, Kuopio, Finland; 2Emergency Medicine, University of Helsinki, Department of Emergency Medicine and Services, Helsinki University Hospital, Helsinki, Finland; 3Boehringer Ingelheim Ky, Helsinki, Finland
Correspondence: Taru Hallinen, ESiOR Oy, Tulliportinkatu 2 LT 4, Kuopio, FI-70100, Finland, Tel +358 50 568 1894, Email [email protected]
Purpose: Sodium-glucose cotransporter-2 (SGLT2) inhibitor empagliflozin has recently been shown to improve the outcomes of heart failure (HF) patients regardless of patient’s left ventricular ejection fraction by reducing the combined risk of cardiovascular death or hospitalization for worsening HF. The aim of this study was to assess the cost-effectiveness of adding empagliflozin to the standard care (SC) in comparison to SC only in the treatment of HF in Finland.
Patients and Methods: The assessment was performed in the cost-utility framework using two Markov cohort state-transition models, one for HF with reduced ejection fraction (HFrEF) and one for HF with preserved ejection fraction (HFpEF). The models have been primarily developed based on the EMPEROR-Reduced and EMPEROR-Preserved trials which informed the modelled patient characteristics, efficacy of treatments in terms of associated risks for heart failure hospitalizations, cardiovascular (CV) and non-CV death, treatment related adverse events (AE), and state- and event-specific health-related quality of life weights (EQ-5D). Direct health care costs were estimated from Finnish published references. Cost-effectiveness was assessed from health care payer perspective based on incremental cost-effectiveness ratio (ICER; cost per quality adjusted life-year [QALY] gained) and probability of cost-effectiveness (at willingness-to-pay [WTP] of 35,000 euros/QALY). The ICER was reported as the weighted (HFrEF, 43.5%; HFpEF, 56.5%) average result of the two models.
Results: Empagliflozin + SC treatment increased the average quality-adjusted life-expectancy, and treatment costs of HF patients by 0.15 QALYs and 1,594 euros, respectively, when compared to SC. An additional QALY with empagliflozin was thus gained at a cost of 10,621 euros. The probability of empagliflozin + SC being cost-effective compared to placebo + SC was 77.6% and 83.5% with WTP of 35,000 and 100,000 euros/QALY, respectively.
Conclusion: Empagliflozin is a cost-effective treatment for patients with HF in the Finnish health care setting.
Keywords: sodium-glucose cotransporter 2 inhibitor, cost-utility analysis, heart failure with reduced ejection fraction, heart failure with preserved ejection fraction
Introduction
Heart failure (HF) is a clinical syndrome caused by structural and/or functional cardiac abnormalities that impair ventricular filling or ejection of blood to the systemic circulation to meet the systemic needs.1 The overall prevalence of HF in the European adult population is approximately 1–2%.1,2 In a Finnish secondary care registry, HF prevalence increases from 3.7% among those aged 50 years or less to 15.3% among those aged 85 years or more.3 Due to the aging of the Finnish population, the number of patients with HF is expected to increase in the future years.
The main goals of HF treatment are to improve a patient’s clinical status, functional capacity, and quality of life as well as prevent hospitalizations and reduce mortality. Despite advancements in pharmaceutical treatments, the prognosis of HF remains poor with annual mortality of approximately 5–8% and 70% of the patients with hospitalization for acute HF dying within 5 years of the episode.2,4 Improvement in HF prognosis has been documented for beta blockers (BB) and drugs targeting the renin-angiotensin-aldosterone system: angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARB), mineralocorticoid receptor antagonists (MRA), and angiotensin receptor neprilysin inhibitors (ARNi).2,5 For most drugs, the evidence supports improved prognosis among HF patients with reduced ejection fraction (HFrEF), whereas only modest impact has been shown for patients with preserved ejection fraction (HFpEF).2
Recently, sodium-glucose cotransporter-2 (SGLT2) inhibitors dapagliflozin and empagliflozin have been shown to improve the outcomes of patients with HF.6–8 Empagliflozin was the first SGLT2 inhibitor with evidence of improved outcomes in the treatment of HF regardless of patient’s left ventricular ejection fraction (LVEF). Empagliflozin has been shown to reduce the combined risk of cardiovascular death or hospitalization for worsening HF in patients with HFrEF (LVEF ≤ 40)6 and HFpEF (LVEF >40%).8 Here, we assess the cost-effectiveness of empagliflozin administered in combination with the standard care (SC) to SC alone in the treatment of HF in the Finnish health care setting. Country-specific analyses are important because the transferability of economic evaluations across countries is limited by differences in eg, clinical practice patterns and relative prices. For simplicity, we use the HFrEF and HFpEF definitions in alignment with the EMPEROR-reduced and EMPEROR-preserved trials. Thus, the HFpEF group also includes patients who have HF with mildly reduced ejection fraction (HfmrEF, LVEF 41–49%) as defined in the current ESC guidelines.1
Materials and Methods
The assessment was conducted using two Markov cohort models that simulate the disease progression of HFrEF and HFpEF patients over their lifetime to capture all relevant costs and outcomes. Empagliflozin (10 mg/day) on top of standard care (SC) was compared to SC based on the comparative efficacy, event risk, and quality of life data available from the EMPEROR-Reduced6 and EMPEROR-Preserved8 trials and representative Finnish input data for health care resource use, costs, and background mortality. The assessment was performed from health care payer perspective with all costs and outcomes discounted at an annual rate of 3% in line with the national guidelines.9 Randomized controlled trials are considered as the preferred source for assessing treatment benefits in the national guidelines for health economic evaluations,9 and the time of the study EMPEROR-trials were the only randomized controlled clinical trials conducted for empagliflozin in patients with HFrEF and HFpEF.
The primary outcome measure for the analysis was the incremental cost-effectiveness ratio (ICER), given as cost per quality-adjusted life year (QALY) gained. The ICER for HF population was presented as weighted average of the HFrEF and HFpEF model results where 46.8% and 53.2% of patients were deemed to have HFrEF and HFpEF, respectively, based on the study by Huusko et al.10
Probabilistic sensitivity analyses (PSA) with 2000 simulations were performed to capture the uncertainty associated with model input values. Cost-effectiveness plane and cost-effectiveness acceptability curve were drawn based on the PSAs to illustrate the differences in costs and QALYs between compared treatments and the proportion of simulations considered cost-effective, respectively. The PSA results for the combined HF population were derived by weighting the results of each simulation as described for the base case analysis. Since there is no official Finnish threshold value for cost-effectiveness, we applied ICER-value of 35,000 €/QALY gained in the analysis similarly to other published Finnish cost-effectiveness analyses.11,12
Model
The modelled patient populations for HFrEF and HFpEF reflect the intention to treat population of the respective EMPEROR-trials6,8 with male preponderance (HFrEF: 76%; HFpEF: 55.3%), average age of 66.8 years and 71.9 years, and ischemic cause of HF in 52% and 35.4% of the patients at baseline. The standard care (SC) received by the patients consisted of appropriately titrated doses of ACEi (HFrEF: 24%; HFpEF: 40.2%), ARB (HFrEF: 24%; HFpEF: 38.7%), MRA (HFrEF: 71%; HFpEF: 37.5%), ARNi (HFrEF: 20%; HFpEF: 2.2%), BB (HFrEF: 95%; HFpEF: 86.3%) and ivabradine (HFrEF: 7%; HFpEF: 1.2%).
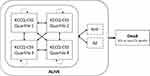
The models capture the disease progression of HFrEF and HFpEF patients based on the changes observed in patient’s clinical summary score (CSS) of Kansas City Cardiomyopathy Questionnaire (KCCQ) in the respective EMPEROR trials. KCCQ is an established and prognostically important measure of health status in HF patients.13–19 It quantifies patient’s perception of their health status, including HF symptoms (frequency and burden), limitations on physical and social function, and impact on health-related quality of life (HRQoL).16,20 The score ranges from 0 to 100, with higher scores indicating a better health status, lower symptom burden, and better HRQoL.
The model health states (Figure 1) were derived by classifying the trial patients into four equal-sized groups based on their baseline KCCQ-CSS scores (quartile 1 [0 to <55.2], quartile 2 [55.2 to <75.0], quartile 3 [75.0 to <89.6], quartile 4 [89.6 to 100]; HFpEF: quartile 1 [0 to <55.7], quartile 2 [55.7 to <74.0], quartile 3 [74.0 to <88.0], quartile 4 [88.0 to 100]) and death. Thus, at the model baseline, approximately a quarter of the patients reside in each of the KCCQ-CSS-based health states. During successive model cycles (with 1-month duration), the patients can then either remain in the same health state, transit to health states representing lower or higher disease burden or die from cardiovascular (CV) or non-CV reasons. In addition, the surviving patients are at risk of hospitalization for HF and adverse events (AE) at each cycle. The patients treated with empagliflozin + SC may also discontinue empagliflozin treatment and switch to SC until death or the end of the model time horizon.
The model estimates accrued quality-adjusted life years (QALY) and costs over time for each intervention to produce the incremental cost-effectiveness ratios (ICER).
Efficacy and Transitions
Transitions between the KCCQ-CSS-based health states were modelled based on treatment-specific transition probability matrices (Supplementary File, Tables S1 and S2) that were derived from the analyses of collected KCCQ-CSS data over the trial duration of the EMPEROR-Reduced and EMPEROR-Preserved trial data for three time periods: baseline to week 12 (months 1–3), week 12 to week 32 (months 4–8), and week 32 to week 52 (months 9+). Due to significant differences between empagliflozin + SC and SC, these transition matrices were derived separately for each treatment. The transition probability matrices were applied to the patients in the alive health states to calculate the state membership in the subsequent cycle.
The model estimates separately mortality due to CV and non-CV causes. CV-mortality was modelled based on the data from respective EMPEROR-trials (as described below), whereas non-CV mortality was modelled based on the difference between all-cause deaths and CV deaths in the trial. However, when the trial-based estimate for the probability of non-CV death was lower than the most recent age- and sex-specific probability of non-CV death for the general Finnish population,21,22 the latter was used.
Due to the limited trial duration, CV mortality, all-cause mortality, and empagliflozin treatment discontinuation were modelled based on applicable parametric distributions (Tables S3 and S4) to allow extrapolation of these outcomes beyond trial period. The parametric distributions were derived and chosen in line with the recommendations outlined in the NICE Decision Support United Technical Service Document.23 In the parametric survival analyses six commonly used parametric distributions (the Weibull, log-logistic, log-normal, the Gompertz, exponential, generalized Gamma) were fitted to the observed trial data. The model health states (KCCQ-CSS quartiles) were included in the analyses as time-varying predictors and treatment effect of empagliflozin as a coefficient (as no meaningful violations of the non-proportional hazard assumptions were identified). The most appropriate distribution for each outcome was identified based on the within-trial fit and clinical plausibility of the long-term extrapolation. The within-trial fit was assessed based on goodness-of-fit criteria (Akaike information criterion and Bayesian information criterion), diagnostic plots for each distribution type, and visual inspection of the fitted against the observed curve from the trial.
The risk of first and recurrent hospitalizations for HF (transient state) were modelled using a Poisson model (Tables S3 and S4) fitted to patient-level data with generalized estimating equations to account for the repeated measures on the patients. The possibility of experiencing treatment-related AEs with empagliflozin + SC and SC was modelled in alignment with the EMPEROR- trials assuming a constant hazard. For HFrEF, these include urinary tract infection (41.3 vs 37.6 per 1000 patient years), genital mycotic infection (13.8 vs 5.3 per 1000 patient years), acute renal failure (81.3 vs 90.2 per 1000 patient years), elevation of liver enzymes (34.3 vs 38.3 per 1000 patient years), volume depletion (92.6 vs 87.6 per 1000 patient years), hypotension (82.2 vs 76.9 per 1000 patient years), hypoglycemic event (12.0 vs 12.5 per 1000 patient years), and bone fracture (20.1 vs 18.9 per 1000 patient years). For HFpEF, these include urinary tract infection (55.6 vs 45.3 per 1000 patient years), genital mycotic infection (12.0 vs 3.9 per 1000 patient years), acute renal failure (68.7 vs 72.6 per 1000 patient years), elevation of liver enzymes (20.8 vs 28.4 per 1000 patient years), volume depletion (67.8 vs 53.8 per 1000 patient years), hypotension (58.8 vs 48.0 per 1000 patient years), hypoglycemic event (13.1 vs 14.1 per 1000 patient years), and bone fracture (24.3 vs 23.0 per 1000 patient years).
Quality of Life Estimates
QALYs were estimated based on time spent in the model health states, adjusted for disutilities associated with HF-related hospitalizations and AEs. For both HFrEF and HFpEF, the utility values (EQ-5D-3L) associated with model health states and disutilities associated with AEs and hospitalization for HF (Table 1) were primarily derived from the pooled analysis analyses of the ITT populations in the respective EMPEROR trials.
 |
Table 1 Utility, Disutility and Cost Inputs for the Health States and Clinical Events |
Because the trial-derived utility values for KCCQ-CSS quartile 4 (HFrEF 0.8581, HFpEF 0.8319) were higher than the utility of Finnish general population aged 64 to 74 years (0.776),24 the latter was used as the utility value for KCCQ-CSS quartile 4. The utilities for other KCCQ-CSS quartiles were then adjusted by the relative difference between the Finnish and trial values (HFrEF: −0.0957; HFpEF −0.0672). The impact of hospitalization for HF and AEs was captured as one-off decrements (over 1 month) to the proportion of patients experiencing the event.
Treatment Costs
The analysis included direct costs associated with drug acquisition, disease management, and clinical event management. Monthly drug acquisition costs (Table 2) for each drug class in SC (ie, ACEi, ARB, MRA, ARNi, and BB) were calculated as weighted average cost based on the prices and market shares of active substances within each class as reported in the official Finnish price tariff 3/2022 and sales data of Finnish Social Insurance Institution in 202025, respectively. The daily doses for each substance were based on appropriately titrated doses in accordance with the Finnish treatment practice.2 The monthly cost of SC was then calculated by weighting the costs for each drug class with the proportion of users in the EMPEROR-reduced and EMPEROR-Preserved trials.
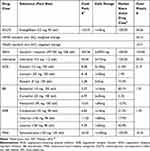 |
Table 2 Drug Costs and Daily Dosages |
The disease and event management costs applied in the analysis are summarized in Table 1. Disease management was modelled to consist of general practitioner (GP) visits, cardiologist visits, and emergency department referrals with monthly frequency of resource use separately defined for each KCCQ-quartile. Because directly applicable resource use data from Finland were unavailable from published references, resource use frequency was estimated indirectly. We assumed that the relative frequency of GP and cardiologist visits in modelled KCCQ-quartiles would be identical to those reported26 for HF patients in NYHA I–IV classes in Germany. Since the annual number of outpatient visits has been reported to be lower in Finland3 than Germany,26 the overall frequency (sum of GP and cardiologist visits) of resource use was adjusted to equal those reported in the Finnish retrospective registry study3 for patients with HFrEF (5.52 per year) and HFpEF (6.33 per year).
The clinical event costs associated with heart failure hospitalization, acute renal failure, hepatic injury, bone fracture, urinary tract infection, genital mycotic infection, volume depletion, hypotension, hypoglycemic event, and cardiovascular death were modelled based on the national Finnish unit costs27 without consideration for potential additional costs associated with eg, pharmaceutical outpatient treatments. A single outpatient visit was assumed to suffice for the treatment of the urinary tract infections, genital mycotic infections, volume depletion, hypotension, elevation of liver enzymes, and hypoglycemic event. The costs for acute renal failure, bone fracture, and deaths were modelled based on the national costs27 of diagnosis-related groups (DRG) associated with these events.
Sensitivity Analyses
We tested the impact of discounting, modelling timeframe, utility values, and health care cost as deterministic sensitivity analyses. In addition, we performed the analyses using localized estimates for the drug mix making up the SC for HFrEF and HFpEF (ARNi 10% and 2.2%; ivabradine 1% and 1.2%; ACEi 65% and 40.2%; BB 95% and 86.3%; ARB 25% and 38.7%; MRA 60% and 37.5%).
In the 2000 PSA simulations, the values for key model parameters were varied based on their probability distributions. The parameters included in the PSA were the rates of all-cause death, CV death, and hospitalization for heart failure (hHF), unit costs (except for drug costs), and the quality of life estimates associated with the health states and adverse events. Parameter draws utilized the observed standard error where available, whereas the standard error was assumed to be 10% around the mean value if the standard error was not known. All costs and utilities were varied using the gamma and beta distributions, respectively.
Results
Base Case Analysis
Empagliflozin + SC treatment increased life-expectancy, quality-adjusted life-expectancy, and treatment costs of patients with HFrEF compared to SC by 0.21 years (5.98 years in empagliflozin + SC, 5.77 years in placebo + SC), 0.22 QALYs and 1,552 euros, respectively. Among HFpEF patients, the corresponding increases were 0.05 years (7.03 years in empagliflozin + SC, 6.98 years in placebo + SC), 0.10 QALYs and 1,631 euros. Thus, an additional QALY with empagliflozin + SC was gained at a cost of 6,927 and 19,211 euros over patient’s lifetime in patients with HFrEF and HFpEF, respectively. The weighted average ICER for the whole HF population was therefore 10,621 euros/QALY gained.
Differences in LY and QALY gained between empagliflozin + SC and SC during the modelled lifetime horizon were mostly due to progression of HF, which influences hHF incidence and cardiovascular mortality. Cost differences between the compared regimens were mainly driven by the drug acquisition costs (3,860 vs 1,854 euros), but at the same time decreased clinical event management (3,941 vs 4,365 euros) and hHF costs (2,449 vs 2,866 euros) provided cost offsets for empagliflozin + SC. Differences between HFrEF and HFpEF were mostly due to higher mortality and higher risk of hHF among HFrEF patients. In line with this, less LYs were gained (HFrEF: 5.98 for empagliflozin + SC and 5.77 for SC; HFpEF: 7.03 for empagliflozin + SC and 6.98 for SC) and QALYs (HFrEF: 3.94 for empagliflozin + SC and 3.72 for SC; HFpEF: 4.73 for empagliflozin + SC and 4.65 for SC) and higher clinical event management costs were observed among HFrEF patients (HFrEF: 4,831 euros for empagliflozin + SC and 5,468 euros for SC; HFpEF: 3,158 euros for empagliflozin + SC and 3,393 euros for SC) (Table S5). Furthermore, lifetime costs for SC were over 2,000 euros higher in HFrEF patients due to the more frequent use of more expensive drugs as part of SC (ie, ARNI, MRA and ivabradine). Overall, the total costs over lifetime were similar for HFrEF and HFpEF, but they were accrued in a shorter timeframe in HFrEF population.
Sensitivity Analyses
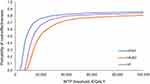
The cost-effectiveness plane illustrating differences between empagliflozin + SC and placebo + SC over 2000 model simulations is shown in Figure 2. Based on these simulations, the average total treatment costs were 17,680 euros for empagliflozin + SC and 16,197 euros for placebo + SC during patient’s lifetime. The respective average QALYs were 4.28 and 4.19. With WTP of 35,000 euros/QALY, the probability of empagliflozin + SC being cost-effective was 77.6% when compared to placebo + SC (Figure 3). The probability increased to 83.5% with the WTP of 100,000 euros/QALY.
The results of our analysis were insensitive to reasonable changes in the modelling assumptions and input values. The largest impact on ICER was associated with the applied utility values, discount rate, and modelling time horizon. When the applied utility values were categorically decreased and increased by 10%, the ICER was 11,669 euros/QALY and 9,548 euros/QALY, respectively. Exclusion of discounting decreased the ICER to 9,826 euros/QALY, whereas the restriction of the analyses to a 5-year timeframe increased the ICER to 14,699 euros/QALY. Empagliflozin remained cost-effective versus SC in all conducted deterministic sensitivity analyses (Table 3).
 |
Table 3 Results of Base Case and the Sensitivity Analyses |
Discussion
Empagliflozin treatment was shown to be a cost-effective treatment for HF patients in the Finnish setting in our modelling-based analysis. The obtained ICER-values in HFpEF population were higher compared to the HFrEF population in lifetime scenarios, suggesting that empagliflozin treatment is more cost-effective in HFrEF population. The differences in cost-effectiveness were mostly related to the differing prognosis of HFrEF and HFpEF patients since the benefit of empagliflozin in terms of additional QALYs gained compared to SC were more modest in HFpEF.
To our knowledge, our study is the first cost-effectiveness analysis assessing empagliflozin treatment in patients with both chronic HFrEF and HFpEF. Previously, empagliflozin’s cost-effectiveness for the treatment of HFpEF and the treatment of HF in T2D patients has been supported by assessments that were conducted in the United Kingdom28 and Australia.29 Similarly, to our analysis, empagliflozin treatment was associated with increased life-expectancy and quality-adjusted life expectancy at higher lifetime costs, with ICERs of 2093 £/QALY28 (ca. 2410 €/QALY) and 29,202 AUD$/QALY29 (ca. 18,890 €/QALY), respectively. A recent systematic review30 also concluded that another SGLT2 inhibitor, dapagliflozin, is cost-effective in the treatment of patients with HFrEF. In the review, European wide analyses based on DAPA-HF trial was included where reported ICERs were 9406 €/QALY in Spain, 5379 €/QALY in Germany and 5822 £/QALY in the United Kingdom.31 Primary outcomes in EMPEROR-Reduced trial (cardiovascular death or hospitalization for heart failure) and DAPA-HF trial (a composite of worsening heart failure or death from cardiovascular causes) produced similar hazard ratios (HR 0.75; 95% confidence interval [CI], 0.65 to 0.86, and HR 0.74; 95% [CI], 0.65 to 0.85, respectively)6,7 With broadly similar modelling approaches and patient populations the results of our cost-effective analyses for HFrEF were also congruent in the lifetime horizon with those reported31 for dapagliflozin + SC.
As always, there are certain key limitations in cost-effectiveness assessments that are based on modelling of clinical trial data. The patient populations and treatment practice in clinical trials may differ from the typical clinical practice, which may decrease the generalizability of the findings to standard care setting to some extent. Another key uncertainty is associated with the limited duration of clinical trials, which necessitates the extrapolation of observed outcomes beyond the trial time horizon. The key strength of our analysis is the fact that it covers the HF population regardless of LVEF and thus provides robust evidence for treating the larger patient population. However, the finding of differing ICERs in HFrEF and HFpEF also add to the recent discussion on whether indication-specific pricing policies should be developed to reflect differential clinical and economic value in each indication.32–34 Currently, the same price for a pharmaceutical product applies for all approved indications in Finland.
Empagliflozin was the first pharmaceutical treatment with a shown favourable impact on the prognosis of patients with HF regardless of LVEF. According to our analyses, empagliflozin in combination with SC is also cost-effective for the treatment of HF patients. Therefore, it is both clinically and economically plausible to initiate empagliflozin treatment early, in accordance with the updated AHA/ACC/HFSA treatment guidelines.35
Conclusion
Empagliflozin is a cost‐effective treatment for Finnish HF patients regardless of the left-ventricular ejection fraction status of the patients. The results are likely to be generalizable to countries that have a similar healthcare system and economy as Finland.
Funding
The study was supported and funded by Boehringer Ingelheim Ky, Finland. Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Disclosure
TH and ES are partners and employees of ESiOR Oy; SK is an employee of ESiOR Oy, which was commissioned by Boehringer Ingelheim Ky to perform this study. In these salaried positions, TH, ES and SK work with a variety of companies and are explicitly precluded from accepting any payment or honoraria directly from Boehringer Ingelheim Ky. ESiOR has carried out commissioned studies and health-economic analyses for several other pharmaceutical companies, food industry companies, device companies, research groups, health care organizations, and hospitals. VPH has received a consulting fee from Boehringer Ingelheim Ky, Finland. Mari Pesonen is an employee of Boehringer Ingelheim Finland Ky. The authors report no other conflicts of interest in this work.
References
1. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726. doi:10.1093/eurheartj/ehab368
2. Heart Failure. Current Care Guidelines. Working group set up by the Finnish Medical Society Duodecim and the Finnish Cardiac Society. Helsinki: The Finnish Medical Society Duodecim; 2017. Available from: https://www.kaypahoito.fi/hoi50113.
3. Huusko J, Kurki S, Toppila I, et al. Heart failure in Finland: clinical characteristics, mortality, and healthcare resource use. ESC Heart Fail. 2019;6(4):603–612. doi:10.1002/ehf2.12443
4. Koskinen J, Ukkonen H. Sydämen kroonisen vajaatoiminnan nykyhoito. Duodecim. 2019;135:37–44.
5. Malik A, Brito D, Vaqar S, Chhabra L. Congestive Heart Failure. Treasure Island (FL): StatPearls; 2021.
6. Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–1424. doi:10.1056/NEJMoa2022190
7. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. doi:10.1056/NEJMoa1911303
8. Anker SD, Butler J, Filippatos G, et al. EMPEROR-preserved trial investigators. empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–1461. doi:10.1056/NEJMoa2107038
9. Application instructions - health economic evaluation [https://www.hila.fi/]. Preparing a health economic evaluation to be attached to the application for reimbursement status and wholesale price for a medicinal product. Helsinki: Lääkkeiden hintalautakunta; 2019. Available from: https://www.hila.fi/content/uploads/2020/01/Instructions_TTS_2019.pdf.
10. Huusko J, Purmonen T, Toppila I, Lassenius M, Ukkonen H. Real-world clinical diagnostics of heart failure patients with reduced or preserved ejection fraction. ESC Heart Fail. 2020;7:1039–1048. doi:10.1002/ehf2.12665
11. Joensuu JT, Huoponen S, Aaltonen KJ, Konttinen YT, Nordström D, Blom M. The cost-effectiveness of biologics for the treatment of rheumatoid arthritis: a systematic review. PLoS One. 2015;10(3):e0119683. doi:10.1371/journal.pone.0119683
12. Hallinen T, Soini E, Asseburg C, et al. Cost-effectiveness of apixaban versus other direct oral anticoagulants and warfarin in the prevention of thromboembolic complications among Finnish patients with non-valvular atrial fibrillation. Clinicoecon Outcomes Res. 2021;13:745–755. doi:10.2147/CEOR.S317078
13. Napier R, McNulty SE, Eton DT, Redfield MM, AbouEzzeddine O, Dunlay SM. Comparing measures to assess health-related quality of life in heart failure with preserved ejection fraction. JACC Heart Fail. 2018;6(7):552–560. doi:10.1016/j.jchf.2018.02.006
14. Pokharel Y, Khariton Y, Tang Y, et al. Association of serial kansas city cardiomyopathy questionnaire assessments with death and hospitalization in patients with heart failure with preserved and reduced ejection fraction: a secondary analysis of 2 randomized clinical trials. JAMA Cardiol. 2017;2(12):1315–1321. doi:10.1001/jamacardio.2017.3983
15. Joseph SM, Novak E, Arnold SV, et al. Comparable performance of the Kansas City Cardiomyopathy Questionnaire in patients with heart failure with preserved and reduced ejection fraction. Circ Heart Fail. 2013;6(6):1139–1146. doi:10.1161/CIRCHEARTFAILURE.113.000359
16. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245–1255. doi:10.1016/S0735-1097(00)00531-3
17. Myers J, Zaheer N, Quaglietti S, Madhavan R, Froelicher V, Heidenreich P. Association of functional and health status measures in heart failure. J Card Fail. 2006;12(6):439–445. doi:10.1016/j.cardfail.2006.04.004
18. Parissis JT, Nikolaou M, Farmakis D, et al. Self-assessment of health status is associated with inflammatory activation and predicts long-term outcomes in chronic heart failure. Eur J Heart Fail. 2009;11(2):163–169. doi:10.1093/eurjhf/hfn032
19. Sullivan MD, Levy WC, Russo JE, Crane B, Spertus JA. Summary health status measures in advanced heart failure: relationship to clinical variables and outcome. J Card Fail. 2007;13(7):560–568. doi:10.1016/j.cardfail.2007.04.001
20. Medical device development tool (MDDT) qualification decision summary for Kansas City cardiomyopathy questionnaire (KCCQ). FDA’s medical device development tools (MDDT) program; 2016. Available from: https://www.fda.gov/medical-devices/science-and-research-medical-devices/medical-device-development-tools-mddt#Qualified_Tools.
21. Statistics Finland. Deaths by underlying cause of death (ICD-10, 3-character level), age and sex, 1998-2021. Helsinki: 2022. Available from: https://stat.fi/en/statistics/ksyyt.
22. Statistics Finland. Life table by age and sex, 1986-2021. Helsinki: 2022. Available from: https://stat.fi/en/statistics/kuol.
23. Latimer N; Undertaking survival analysis for economic evaluations alongside clinical trials - extrapolation with patient-level data [https://nicedsu.sites.sheffield.ac.uk]. Technical support document 14. Sheffield: NICE Decision Support Unit; 2011. Available from: https://www.sheffield.ac.uk/sites/default/files/2022-02/TSD14-Survival-analysis.updated-March-2013.v2.pdf.
24. Saarni SI, Härkänen T, Sintonen H, et al. The impact of 29 chronic conditions on health-related quality of life: a general population survey in Finland using 15D and EQ-5D. Qual Life Res. 2006;15(8):1403–1414. doi:10.1007/s11136-006-0020-1
25. Kelasto statistical database [https://www.kela.fi/web/en]. Reimbursements of medicine expenses: number of recipients and prescription data. Helsinki: The Social Insurance Institution of Finland; 2022. Available from: https://www.kela.fi/web/en/statistics-by-topic-dispensed-medicines-reimbursable-under-the-nhi-scheme.
26. Biermann J, Neumann T, Angermann CE, et al. Resource use and costs in systolic heart failure according to disease severity: a pooled analysis from the German Competence Network Heart Failure. J Public Health. 2012;20:23–30. doi:10.1007/s10389-011-0452-0
27. Mäklin S, Kokko P. Terveyden- ja sosiaalihuollon yksikkökustannukset Suomessa vuonna 2017 [Health and social care unit costs in Finland in 2017]. Työpaperi: 21/2020. Helsinki: Terveyden ja hyvinvoinnin laitos; 2021. Finnish. Available from: https://www.julkari.fi/bitstream/handle/10024/142882/URN_ISBN_978-952-343-493-6.pdf.
28. Reifsnider OS, Kansal AR, Franke J, et al. Cost-effectiveness of empagliflozin in the UK in an EMPA-REG OUTCOME subgroup with type 2 diabetes and heart failure. ESC Heart Fail. 2020;7(6):3910–3918. doi:10.1002/ehf2.12985
29. Zhou J, Liew D, Kaye DM, Zoungas S, Stub D. Cost-effectiveness of empagliflozin in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Qual Outcomes. 2022;15(10):e008638. doi:10.1161/CIRCOUTCOMES.121.008638
30. Wu M, Qin S, Wang L, et al. Economic evaluation of dapagliflozin in the treatment of patients with heart failure: a systematic review. Front Pharmacol. 2022;13:860109. doi:10.3389/fphar.2022.860109
31. McEwan P, Darlington O, McMurray JJV, et al. Cost-effectiveness of dapagliflozin as a treatment for heart failure with reduced ejection fraction: a multinational health-economic analysis of DAPA-HF. Eur J Heart Fail. 2020;22(11):2147–2156. doi:10.1002/ejhf.1978
32. Michaeli DT, Michaeli T. Overall survival, progression-free survival, and tumor response benefit supporting initial US food and drug administration approval and indication extension of new cancer drugs, 2003–2021. J Clin Oncol. 2022;40(35):4095–4106. doi:10.1200/JCO.22.00535
33. Michaeli DT, Mills M, Kanavos P. Value and price of multi-indication cancer drugs in the USA, Germany, France, England, Canada, Australia, and Scotland. Appl Health Econ Health Policy. 2022;20(5):757–768. doi:10.1007/s40258-022-00737-w
34. Michaeli DT, Mills M, Michaeli T, Miracolo A, Kanavos P. Initial and supplementary indication approval of new targeted cancer drugs by the FDA, EMA, Health Canada, and TGA. Invest New Drugs. 2022;40(4):798–809. doi:10.1007/s10637-022-01227-5
35. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895–e1032. doi:10.1161/CIR.0000000000001063
36. Statistics Finland. Price index of public expenditure, Local government finances by function area. Helsinki: 2022. Available from: https://stat.fi/en/statistics/jmhi.
37. Sullivan PW, Ghushchyan VH. EQ-5D scores for diabetes-related comorbidities. Value Health. 2016;19(8):1002–1008. doi:10.1016/j.jval.2016.05.018
38. Sullivan PW, Ghushchyan V. Preference-Based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26(4):410–420. doi:10.1177/0272989X06290495
39. Currie CJ, Morgan CL, Poole CD, Sharplin P, Lammert M, McEwan P. Multivariate models of health-related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin. 2006;22(8):1523–1534. doi:10.1185/030079906X115757
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.